Abstract
Development and reproductive traits of Tetranychus macfarlanei Baker & Pritchard (Acari: Tetranychidae) were investigated on kidney bean, Phaseolus vulgaris L., at eleven constant temperatures. Tetranychus macfarlanei was able to develop and complete its life cycle at temperatures ranging from 17.5 to 37.5°C. At 15 and 40°C, a few eggs (2–4%) hatched but further development was arrested. Development from egg to adult was slowest at 17.5°C and fastest at 35°C for both females and males. Using Ikemoto and Takai’s linear model, the estimated lower developmental thresholds for egg-to-female adult, egg-to-male adult and egg-to-egg development were 12.9–13.0°C. The thermal constants for the respective stages were 110.85, 115.99 and 125.32 degree-days (DD). The intrinsic optimum temperatures (T Φ) calculated by non-linear SSI model were determined as 24.4, 24.4 and 24.2°C for egg-to-female adult, egg-to-male adult and egg-to-egg development, respectively. The net reproductive rate (R 0) was highest at 25°C (167.4 females per female) and lowest at 17.5°C (42.6 females per female). The intrinsic rate of natural increase, r m, increased linearly with the rising of temperature from 0.102 at 17.5°C to 0.441 day−1 at 35°C. These values suggested that T. macfarlanei could be growing quickly in response to increasing temperatures from 17.5 to 35°C and provide a basis for predicting its potential geographical range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life processes are closely associated with environmental factors and of them temperature is the dominant abiotic factor for development and reproduction in poikilothermic arthropods; i.e., temperature affects essential population processes such as development rate, birth rate, death rate, fecundity and generation time, and as a result drives population growth traits of target species (Campbell et al. 1974; Roy et al. 2002; Gotoh et al. 2010; Ullah et al. 2011). Knowing the temperature requirements of the various life stages of a target species is instrumental in forecasts of its potential distribution and population dynamics. The ability of a pest to develop at different temperatures determines to a large extent of its survival under different climatic conditions (tropical, subtropical and temperate regions), which is important in predicting pest outbreaks. The intrinsic rate of natural increase (r m) is a common indicator to describe and evaluate the growth and adaptation of a population of arthropods to certain environmental conditions (Birch 1948). The r m-value can be determined from the life history components of organisms, such as developmental rate, ovipositional rate, survival rate and the proportion of females in the offspring (Sabelis 1985). The r m-value is a key demographic parameter used for predicting the potential severity of a pest species (Margolies and Wrensch 1996; Gotoh et al. 2004, 2010). Differences in r m-values among temperatures affect how rapid poikilothermic arthropods such as insects and mites can increase on their host plants. The validity of this general life history prediction can be tested by a comparison of r m-value, degree of polyphagy and pest status (Sabelis 1985, 1991; Gotoh et al. 2003).
The spider mite Tetranychus macfarlanei Baker & Pritchard has been reported from India, Madagascar, Mauritius and the Canary Islands, and can infest a wide range of crops in the families Malvaceae, Fabaceae, Cucurbitaceae, Convolvulaceae and Solanaceae (Jeppson et al. 1975; Bolland et al. 1998). Symptoms of infestation by T. macfarlanei first appear on leaves, as a pronounced yellowish hue, then the leaves wilt and finally drop, especially during dry periods (Moutia 1958). Tetranychus macfarlanei is new to Bangladesh, but it has spread throughout the country and causes serious damage to a variety of crops such as jute, bean, eggplant and bottle gourd (Haque et al. unpublished data). There are no published studies on the effects of temperature on development and reproduction of T. macfarlanei. Given this lack of information on the ecology of T. macfarlanei, we initiated a study of the effect of temperature on development and reproduction of T. macfarlanei, and disclosed its basic thermal requirements for development. Our purpose was to develop a comprehensive understanding of the effects of temperature on the population dynamics of T. macfarlanei.
Materials and methods
Spider mite
Tetranychus macfarlanei was initially collected on 27 August 2009 from a bean field (Lablab purpureus (L.) Sweet) at the Bangladesh Agricultural Research Institute, Bangladesh (24°00′N, 90°24′E). The T. macfarlanei strain was imported to Japan with the authorization of the Ministry of Agriculture, Forestry and Fisheries of Japan (No. 21Y421). All experiments with T. macfarlanei were conducted in a level P2 biohazard room under negative pressure hood and the residual individuals as well as trash were autoclaved before discarding. Laboratory stocks were maintained on leaf discs (ca. 16 cm2) of kidney bean, Phaseolus vulgaris L., placed on water-saturated polyurethane mats in plastic dishes (90 mm diameter, 20 mm depth) at 25 ± 1°C, 60–70% RH, and a photoperiod of 16: 8 h light: dark.
Immature development
Inseminated adult females obtained from stock cultures were transferred individually onto a leaf disc (2 × 2 cm) of kidney bean and kept at one of eleven constant temperatures, ranging from 15 to 40°C at a 2.5°C interval, under a long day photoperiod (16L: 8D) with 60–70% RH. Females were allowed to lay eggs for 24 h at 15–25°C, for 12 h at 27.5–35°C or for 6 h at 37.5–40°C. Only one egg was left and reared on the leaf disc, and the developmental stages were recorded at the same time every day until all individuals reached the adult stage. Some eggs did not hatch and some immatures drowned—they were included in the calculation of the survival rate, but did not include them in the calculation of developmental duration.
Reproduction and female longevity
When a female teleiochrysalis appeared in the developmental experiments, two adult males obtained from stock cultures were introduced onto the leaf disc for mating and then removed 24 h after emergence of the adult female. To determine the pre-oviposition period at the respective temperatures, we observed the leaf discs at 6- to 24-h intervals (depending on temperature—the shortest intervals were applied at the highest temperatures). Newly emerged females obtained from the above-mentioned experiments at 17.5, 20, 25, 30 and 35°C, were used to assess their reproductive traits and longevity. The number of eggs laid by a female was recorded daily throughout her life to determine oviposition period, total number of eggs laid per female, eggs laid per female per day, post-oviposition period and female longevity. Eggs laid were removed daily by means of tweezers. During the oviposition period, some adult females drowned, especially just after replacing the leaf discs, or were killed accidentally. These females were discarded from the analysis. Adult mites were transferred onto new leaf discs using a fine brush at 1- to 2-week intervals.
Hatchability, survivability and sex ratio
To calculate age-specific survival rate (l x ) and age-specific fecundity rate (m x ), we assessed egg hatchability, the survival rate of immature stages and the proportion of female offspring at 17.5, 20, 25, 30 and 35°C. To obtain these data, a single teleiochrysalis female and two adult males, which were maintained from eggs at the respective temperatures, were placed on a leaf disc (ca. 16 cm2) of kidney bean for copulation, and the females were allowed to lay eggs for five days after the pre-oviposition period. The eggs obtained from each female were maintained to determine the above-mentioned parameters after reaching adulthood.
Life-table parameters
Life table and fertility tables were calculated using the data obtained from the various experiments. The intrinsic rate of natural increase (r m) was estimated from the fecundity table according to the equation given by Birch (1948): \( \sum e^{-r_{m}x}l_xm_x = 1 \), where x is female age in days, l x is the age-specific survival rate [(proportion of females surviving at age x) × (rate of egg hatchability) × (survival rate of immature stages)] and m x is the expected number of daughters produced per female alive at age x [(age-specific oviposition) × (proportion of females)] (Sabelis 1985; Gotoh and Gomi 2003; Gotoh et al. 2010; Ullah et al. 2011). The net reproductive rate (R 0) is given by R 0 = ∑l x m x , the mean generation time (t G ) in days is given by t G = ln R 0/r m, the finite rate of increase (λ) is given by \( \lambda = e^{r_m} \), and the doubling time (t D ) in days is t D = ln 2/r m. After r m was computed from the original data (r all), the standard errors for the life-table parameters at different constant temperatures were estimated using the Jackknife method (Meyer et al. 1986; Maia et al. 2000). Briefly, one of the mites is omitted and r m (r i ) is calculated for the remaining mites (n − 1). Based on Meyer et al. (1986), the Jackknife pseudo-value (r j ) is computed for this subset of the original data according to the equation r j = n r all–(n − 1) r i . This process was repeated for all possible omissions of one mite from the original data set to produce pseudo-values which allowed for computing confidence limits for the parameter values.
Effect of temperature on mite developmental rate
The reciprocal of developmental time in days is denoted as developmental rate. These rates are used in linear and non-linear models. The thermal constant and lower threshold temperature were determined using the line-fitting method proposed by Ikemoto and Takai (2000). The data points that deviated from the straight line were not used to fit the linear model. The law of total effective temperature applied to the temperature-dependent development of arthropods is expressed by the equation:
where D is the duration of development (days), T is environmental (mean/isothermal) temperature (°C), t the lower threshold temperature, and k the thermal constant. The developmental rate, defined as the reciprocal of D, increases linearly with T according to:
The linear Ikemoto and Takai approximation enables to calculate two constants: the lower developmental threshold and the thermal constant or the sum of total effective temperature, within a limited temperature range. On the other hand, the non-linear thermodynamics model describes the developmental rate over a wide range of temperatures and estimates of optimum temperatures for development. The equation of the non-linear thermodynamics (Sharpe-Schoolfield-Ikemoto [SSI]) model can be expressed as follows (Ikemoto 2005, 2008; Shi et al. 2011):
where r represents the developmental rates (the dependent variables) at the absolute temperature ([T]) (the independent variable). All the other parameters are constants: [T L ], [T H ], and [T Ф ] represent absolute temperatures—[T L ] and [T H ] represent temperatures below (L) and above (H) an optimum temperature, at which an enzyme is 50% active –, ΔH A , ΔH L , and ΔH H represent enthalpy changes, R is the universal gas constant, and ρ is the development rate at [T Ф ]. [T Ф ] is the intrinsic optimum temperature for development that exhibits the minimum effects on enzyme inactivation related to development at low and high temperature (Ikemoto 2005) and it is expressed as follows:
Statistical analysis
Data were analyzed by means of analyses of covariance (ANCOVA), using temperature as the covariate. The purpose was to quantify relationships between the response variables (eggs/female, hatchability, survival rate, female ratio, oviposition period, female longevity, egg-to-adult developmental time, total oviposition, net reproductive rate, and intrinsic rate of natural increase, generation time and lambda) and predictor variables for egg-to-adult developmental time (gender and temperature) in order to identify which of the predictor variables contributes most to explain the variation in data. All models included a third-order term of temperature, but only significant terms were included in the final model. The full ANCOVA model for analyzing all response variables reads:
y = ß 0 + ß 1G + ß 2 T + ß 3 T 2 + ß 4 GT + ß 5 GT 2 + ε,
where y is the response variable, ß i is the parameter associated with the i-th term, G gender, T temperature, and ε the residual error. All response variables were analyzed by means of Generalized Linear Models (McCullagh and Nelder 1989) using PROC GENMOD (Enterprise Guide 4.1, SAS Institute 2006). The advantage of GENMOD is that it permits data with non-normal distributions. Proportions (female ratio, hatchability and survival rate) are likely to be binomially distributed, discrete numbers (eggs/female) to be Poisson or negative binomially distributed, whereas the continuous variables (developmental duration, oviposition period, adult longevity, R 0, r m, t G and λ) are likely to be normally distributed. When needed, continuous variables were subjected to a logarithmic (ln) transformation in order to stabilize the variance and to ensure that the back-transformed values were non-negative.
To test for differences in temperature responses between genders, we compared the deviance of the full model with the increase in deviance resulting from omitting genders from the full model. The difference was tested by means of Manly’s (1990) test:
where D 1 and D 2 denote the deviance of the reduced and the full model, respectively, p 1 and p 2 are the number of parameters of the reduced and full model, and N is the total number of observations in the data set. The degrees of freedom were calculated as ν 1 = p 2 − p 1 and ν 2 = N − p 2 − 1. Likewise, the effects of temperature and gender were assessed by comparing deviances of models with and without temperature and gender, respectively.
Results
Immature development
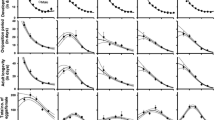
At temperatures ranging from 17.5 to 37.5°C, both the female and male of T. macfarlanei completed their development from egg to adult. Very few eggs of T. macfarlanei hatched at 15°C (2/96 eggs) and 40°C (4/96 eggs), but they died at the larval stage. These data were not used in any subsequent analysis. Developmental time from egg to adult was significantly affected by temperature (Tables 1, 2; Appendix 1). The developmental time from egg to adult was decreased with increasing temperatures until 35°C and then increased at 37.5°C (Fig. 1; Appendix 1). The developmental time differed significantly between females and males (F 3,795 = 31.468, p < 0.001).
Effect of temperature and/or sex on the developmental period from egg to adult in Tetranychus macfarlanei at various temperatures. See Appendix 1 for further information. Points show the sample averages with 95% confidence limits. The heavy line shows the predictions based on the generalized linear models after eliminating non-significant terms. Thin lines show the 95% confidence limits for the predicted line. The models as well as total sample sizes are given in Table 2
Ikemoto and Takai’s linear method, fitting the development rate values, gave a close fit for the 17.5–35°C range of temperatures (0.993 ≤ r 2 ≤ 0.996) (Table 3; Fig. 2; Appendix 1). The estimated lower threshold temperatures (T L = t) for egg-to-female adult, egg-to-male adult and egg-to-egg development were very similar: 12.9–13°C (Table 3). The thermal constants (k) for the respective stages of T. macfarlanei were 116.0, 110.9 and 125.3 degree-days (DD) (Table 3). The non-linear SSI model fitted the development rates closely in the temperature range between 17.5 and 37.5°C (0.0021 ≤ χ 2 ≤ 0.0024) (Table 3; Fig. 2). The intrinsic optimum temperature (T Ф ) for egg-to-female adult, egg-to-male adult and egg-to-egg development was 24.2–24.4°C (Table 3).
Ikemoto and Takai’s (2000) linear and thermodynamic (non-linear) SSI models fitted to the temperature-dependent development of Tetranychus macfarlanei. Circles show data points. Open circles indicate data points outside the range of the linear model. Lower squares show T L (=t), middle squares show intrinsic optimum temperature (T Ф ), and upper squares are T H of constant estimated by non-linear model (see also Table 3)
Reproduction
The pre-oviposition period decreased with increasing temperatures from 17.5 to 35°C (Table 4; Appendix 1). The oviposition period, post-oviposition period, adult longevity and daily egg production (eggs/female/day) were strongly affected by temperature (Tables 1, 2; Fig. 3). The oviposition period was lowest at 35°C. Adult longevity decreased with increasing temperatures from 17.5 to 35°C. Fecundity (eggs/female) was highest at 25 and 30°C (Fig. 3).
Effect on temperature on mean durations of oviposition period, female longevity and total fecundity in Tetranychus macfarlanei. See Appendix 2 for further information. Points show the sample averages with 95% confidence limits. The heavy lines show the predictions based on the generalized linear models after eliminating non-significant terms. Thin lines show the 95% confidence limits for the predicted line. The models as well as total sample sizes are given in Table 2
The effects of temperature on the number of eggs laid during the first five days of the oviposition period, their hatchability, the survival rate of immature stages and the proportion of female offspring were highly significant (p < 0.001; Table 1, Fig. 4; Appendix 3). Number of eggs laid during the first five days of the oviposition period increased with increasing temperatures from 17.5 to 30°C and then slightly decreased at 35°C. Hatchability and survival rate of immature stages increased with increasing temperatures and peaked at around 20–30°C followed by a small decline at 35°C. Offspring female ratio peaked at around 20–35°C. The models describing temperature responses are given in Table 2.
Temperature effects on (top left) number of eggs laid per female during the first five days of oviposition, (top right) hatch rate from eggs to larvae, (bottom left) survival rate from hatching to the adult stage, and (bottom right) offspring sex ratio expressed as proportion. Points show the sample averages with 95% confidence limits. The heavy lines show the predictions based on the generalized linear models after eliminating non-significant terms. Thin lines show the 95% confidence limits for the predicted line. The models as well as total sample sizes are given in Table 2
Life-table parameters
The age-specific survival rate (l x ) started to drop at earlier ages as the temperature increased from 17.5 to 35°C (Fig. 5). The age-specific fecundity rate (m x ) peaked at earlier ages and the width of the peak, i.e. the oviposition period, was apt to become narrower as the temperature raised from 17.5 to 35°C. The first egg laying was recorded on days 28, 16, 12, 8 and 6 at 17.5, 20, 25, 30 and 35°C, respectively. Daily egg production reached a peak of 4.6 eggs on day 36 at 17.5°C, 9.4 eggs on day 23 at 20°C, 12.5 eggs on day 18 at 25°C, 16.6 eggs on day 13 at 30°C, and 14.1 eggs on day 9 at 35°C. Female adults started to die on days 43, 27, 24, 16 and 10 at the respective temperatures. At 17.5, 20, 25, 30 and 35°C, all females had died on days 72, 59, 46, 30 and 23 at the respective temperatures.
The net reproductive rate (R 0), the intrinsic rate of natural increase (r m), the mean generation time (t G ), and the finite rate of increase (λ) were significantly affected by temperature (Tables 1, 2; Fig. 6). The highest R 0-value was observed at 25°C, whereas the lowest value was found at 17.5°C (167.4 vs. 42.6) (Appendix 4). The r m and λ values increased with increasing temperatures from 17.5 to 35°C. The highest r m-value was observed as 0.441 day−1 at 35°C and the lowest value was observed as 0.102 day−1 at 17.5°C. Mean generation time (t G ) and doubling time (t D ) decreased with increasing temperatures: t G ranged from 37.0 days at 17.5°C to 9.5 days at 35°C. Likewise, t D declined from 6.8 days at 17.5°C to 1.6 days at 35°C (Appendix 4).
Effect of temperature on demographic parameters of Tetranychus macfarlanei. See Appendix 4 for further information. R 0, net reproductive rate (top left); r m, intrinsic rate of natural increase (bottom left); t G , mean generation time (top right); λ, finite rate of increase (bottom right). The heavy lines for R 0 and r m show the predictions, based on the generalized linear models after eliminating non-significant terms. Thin lines show the 95% confidence limits for the predictions. The models as well as total sample sizes are given in the Table 2
Discussion
Temperature had a highly significant effect on the development and reproduction of T. macfarlanei. Our results demonstrate that T. macfarlanei was capable of producing offspring across a wide range of temperatures. We found that temperatures higher than 35°C have a detrimental effect on the development and survival. Although 73.3–90.6% of immatures reached adulthood at temperatures ranging from 17.5 to 35°C, immature survival declined to 62.5% at 37.5°C. Changes in lipids, rate imbalances, perturbation of ionic activities, as well as desiccation have been identified as possible mechanisms of arthropod death due to high temperatures. It is also possible that at higher temperatures the arthropods were unable to replace the loss of water from their body, leading to higher mortality (Fields 1992). Ullah et al. (2011) showed that mortality of T. kanzawai and T. merganser increased sharply at temperatures higher than 35°C and a similar trend was also observed in T. tumidus (Liu and Tsai 1998). In the latter species, survival rates at 15 to 30°C were higher than 78.4%, but at 35°C, it was reduced to 56.4%. Insects have an optimum temperature range for development (Huffaker et al. 1999; Huang et al. 2008). Our results indicate that most T. macfarlanei eggs could not hatch at 15 and 40°C, and larval mortality reached 100% at both temperatures.
Development rate of T. macfarlanei increased almost linearly with an increase in temperature to 35°C, but did not increase further at higher temperature (37.5°C; Fig. 2). Similar trends were reported for other Tetranychus species such as T. tumidus (Liu and Tsai 1998), T. mcdanieli (Roy et al. 2003), T. evansi (Gotoh et al. 2010), T. kanzawai and T. merganser (Ullah et al. 2011). An excellent fit was obtained by Ikemoto and Takai’s (2000) linear model for egg-to-adult (female and male) and egg-to-egg development of T. macfarlanei, based on the obtained coefficient of determination (0.993 ≤ r 2 ≤ 0.996). In many temperature-dependent development studies of arthropods linear models were used to estimate the lower thermal threshold and the thermal constant (Howell and Neven 2000). Lower thermal threshold and thermal constant of an insect are indicators for forecasting its potential distribution and abundance (Campbell et al. 1974; Huang et al. 2008). The estimated lower thermal threshold (t) of T. macfarlanei was 12.9°C for egg-to-adult development (both female and male) (Table 3). The t value of T. macfarlanei is very close to that of seven strains of T. evansi (11.9–12.5°C; Gotoh et al. 2010) and T. merganser (12.2–12.3°C; Ullah et al. 2011), but slightly higher than that of T. okinawanus (11.6°C; Takafuji et al. 1996) and T. tumidus (11.9°C; Liu and Tsai 1998), which occur all in tropical and subtropical regions. In contrast, the t value of T. macfarlanei is higher than that of T. urticae (10.0 and 10.5°C; Herbert 1981; Bounfour and Tanigoshi 2001), T. pueraricola (10.8°C; Gotoh et al. 2004) and T. kanzawai (10.8°C; Ullah et al. 2011), which mainly occur in the temperate region. Tetranychid mites might escape the adverse temperature condition in winter of temperate regions by entering diapause, but as far as known T. macfarlanei has no diapause ability (Jose and Shah 1989). Therefore, temperate regions seem unsuitable for the development of T. macfarlanei.
We found that oviposition period and adult longevity of T. macfarlanei declined with increasing temperatures. Our results agree with the work of Bonato (1999) and Ullah et al. (2011), who observed that the oviposition period and adult longevity of T. evansi, T. merganser and T. kanzawai decreased in response to temperature increase. They found that lower temperatures extended the duration of the egg-laying period, which could be explained by reduced activity, less energy used for reproduction, or both (Papaj 2000; Carey 2001; Jervis et al. 2005, 2007; Berger et al. 2008). Fecundity was highest at 25–30°C. The lowest fecundity was obtained at 17.5°C followed by 35°C, which may indicate adverse effects of low and high temperatures on the biological processes of T. macfarlanei and explain why this species is prevalent in a wider part of the world.
Sabelis (1991) reviewed tetranychid life-table parameters and reported that r m-values for Tetranychus mites ranged from 0.219 to 0.336 day−1 near 25°C. The r m-value of T. macfarlanei falls within this range (0.275 day−1). The r m-value of T. macfarlanei was somewhat higher than that for T. cinnabarinus (0.164 day−1; Hazan et al. 1973), T. mcdanieli (0.201 day−1; Tanigoshi et al. 1975), T. tumidus (0.232 day−1; Liu and Tsai 1998), T. evansi (0.243 day−1; Bonato 1999) and T. marianae (0.172 day−1; Noronha 2006) at 25 ± 1°C, and it was similar to the r m-value reported for T. desertorum (0.28–0.29 day−1; Nickel 1960), T. evansi (0.264–0.277 day−1; Gotoh et al. 2010), T. kanzawai (0.187–0.283 and 0.282 day−1; Gotoh and Gomi 2003 and Ullah et al. 2011, respectively) and T. merganser (0.279 day−1; Ullah et al. 2011). The similarities of the r m-values between T. macfarlanei and other Tetranychus species suggest that T. macfarlanei has equal potential as T. desertorum, T. evansi, T. kanzawai or T. merganser to damage a variety of crops.
This study described the temperature-dependent development of the T. macfarlanei Bangladeshi strain under the broad range of temperatures generally prevailing in this region, and estimated its key bioclimatic parameters. Quantification of the thermal response of T. macfarlanei in a demographic context is critical for describing its population dynamics, and it may promote the understanding of adaptation to climatic conditions and predict how quickly its densities may change over time. The information gathered in this study will be important in the management of T. macfarlanei, by providing a better understanding of its life-table parameters and its ability to survive under different temperature regimes.
References
Berger D, Walters R, Gotthard K (2008) What limits insect fecundity? Body-size and temperature-dependent egg maturation and oviposition in a butterfly. Func Ecol 22:523–529
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari: Tetranychidae). Brill Academic Publishers, Leiden, p 392
Bonato O (1999) The effect of temperature on life history parameters of Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 23:11–19
Bounfour M, Tanigoshi LK (2001) Effect of temperature on development and demographic parameters of Tetranychus urticae and Eotetranychus carpiniborealis (Acari: Tetranychidae). Ann Entomol Soc Am 94:400–404
Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M (1974) Temperature requirements of some aphids and their parasites. J Appl Ecol 11:431–438
Carey JR (2001) Insect biodemography. Annu Rev Entomol 46:79–110
Fields PG (1992) The control of stored-products insects and mites with extreme temperatures. J Stored Prod Res 28:89–118
Gotoh T, Gomi K (2003) Life-history traits of the Kanzawa spider mite Tetranychus kanzawai (Acari: Tetranychidae). Appl Entomol Zool 38:7–14
Gotoh T, Ishikawa Y, Kitashima Y (2003) Life-history traits of the six Panonychus species from Japan (Acari: Tetranychidae). Exp Appl Acarol 29:241–252
Gotoh T, Suwa A, Kitashima Y, Rezk HA (2004) Developmental and reproductive performance of Tetranychus pueraricola Ehara and Gotoh (Acari: Tetranychidae) at four constant temperatures. App Entomol Zool 39:675–682
Gotoh T, Sugimoto N, Pallini A, Knapp M, Hernandez-Suarez E, Ferragut F, Ho CC, Migeon A, Navajas M, Nachman G (2010) Reproductive performance of seven strains of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) at five temperatures. Exp App Acarol 52:239–259
Hazan A, Gerson U, Tahori AS (1973) Life history and life tables of the carmine spider mite. Acarologia 3:414–440
Herbert HJ (1981) Biology, life tables, and innate capacity for increase of the two spotted spider mite, Tetranychus urticae (Acarina: Tetranychidae). Can Entomol 113:371–378
Howell JF, Neven LG (2000) Physiological developmental time and zero development temperature of the codling moth (Lepidoptera: Tortricidae). Environ Entomol 29:766–772
Huang Z, Ren SX, Musa PD (2008) Effects of temperature on development, survival, longevity, and fecundity of the Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) predator, Axinoscymnus cardilobus (Coleoptera: Coccinellidae). Biol Control 46:209–215
Huffaker CB, Berryman A, Turchin P (1999) Dynamics and regulation of insect populations. In: Huffaker CB, Gutierrez AP (eds) Ecological entomology, 2nd edn. Wiley, New York, pp 269–305
Ikemoto T (2005) Intrinsic optimum temperature for development of insects and mites. Environ Entomol 34:1377–1387
Ikemoto T (2008) Tropical malaria does not mean hot environments. J Med Entomol 45:963–969
Ikemoto T, Takai K (2000) A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ Entomol 29:671–682
Jeppson LF, Keifer HH, Baker EW (1975) Mites injurious to economic plants. University of California Press, USA, p 614
Jervis MA, Boggs CL, Ferns PN (2005) Egg maturation strategy and its associated trade-offs: a synthesis focusing on Lepidoptera. Ecol Entomol 30:359–375
Jervis MA, Ferns PN, Boggs CL (2007) A trade-off between female life span and larval diet breadth at the interspecific level in Lepidoptera. Evol Ecol 21:307–323
Jose VT, Shah AH (1989) Carryover of spider mite, Tetranychus macfarlanei through alternate host plants in cotton-growing areas of south and central Gujarat, India. In: Channabasavanna GP, Viraktamath CA (eds) Progress in acarology, vol 2. Oxford and IBH, India, pp 29–31
Liu YH, Tsai JH (1998) Development, survivorship, and reproduction of Tetranychus tumidus banks (Acarina: Tetranychidae) in relation to temperature. Int J Acarol 24:245–252
Maia AHN, Luiz AJB, Campanhola C (2000) Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. J Econ Entomol 93:511–518
Manly BFJ (1990) Stage-structured populations: sampling analysis and simulation. Chapman & Hall, London, p 187
Margolies DC, Wrensch DL (1996) Temperature-induced changes in spider mite fitness: offsetting effects of development time, fecundity, and sex ratio. Entomol Exp Appl 78:111–118
McCullagh P, Nelder JA (1989) Generalized linear models. Monographs on statistics and applied probability 37, 2nd edn. Chapman & Hall, Boca Raton, p 511
Meyer JS, Ingersoll CC, McDonald LL, Boyce MS (1986) Estimating uncertainty in population growth rates: Jackknife vs Bootstrap techniques. Ecology 67:1156–1166
Moutia LA (1958) Contribution to the study of some phytophagous acarina and their predators in Mauritius. Bull Entomol Res 49:59–75
Nickel JL (1960) Temperature and humidity relationships of Tetranychus desertorum Banks with special reference to distribution. Hilgardia 30:41–101
Noronha ACS (2006) Biological aspects of Tetranychus marianae McGregor (Acari, Tetranychidae) reared on yellow passion fruit (Passiflora edulis Sims f. flavicarpa Deg.) leaves. Rev Bras Zool 23:404–407
Papaj DR (2000) Ovarian dynamics and host use. Anu Rev Entomol 45:423–448
Roy M, Brodeur J, Cloutier C (2002) Relationship between temperature and development rate of Stethorus punctillum (Coleoptera: Coccinellidae) and its prey Tetranychus mcdanieli (Acarina: Tetranychidae). Environ Entomol 31:177–187
Roy M, Brodeur J, Cloutier C (2003) Effect of temperature on intrinsic rates of natural increase (rm) of a coccinellid and its spider mite prey. BioControl 48:57–72
Sabelis MW (1985) Reproductive strategy. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier, Amsterdam, pp 265–278
Sabelis MW (1991) Life-history evolution of spider mites. In: Schuster R, Murphy PW (eds) The acari: reproduction, development and life-history strategies. Chapman & Hall, London, pp 23–49
SAS (2006) SAS Enterprise Guide 4.1. SAS Institute Inc. SAS Campus Drive, Cary, NC, USA
Shi P, Ikemoto T, Egami C, Sun Y, Ge F (2011) A modified program for estimating the parameters of the SSI model. Environ Entomol 40:462–469
Takafuji A, Yokotsuka T, Goka K, Kishimoto H (1996) Ecological performance of the spider mite, Tetranychus okinawanus Ehara (Acari, Tetranychidae), a species newly described from Okinawa island (1). J Acarol Soc Jpn 5:75–81
Tanigoshi LK, Hoyt SC, Browne RW, Logan JA (1975) Influence of temperature on population increase of Tetranychus mcdanieli (Acarina: Tetranychidae). Ann Entomol Soc Am 86:972–986
Ullah MS, Moriya D, Badii MH, Nachman G, Gotoh T (2011) A comparative study of development and demographic parameters of Tetranychus merganser and Tetranychus kanzawai (Acari: Tetranychidae) at different temperatures. Exp Appl Acarol 53:1–18
Acknowledgments
We thank to Dr. Y. Kitashima and Mr. D. Moriya, for their kind help in this research. We also thank Dr. T. Ikemoto for providing his SSI model program and kind suggestions on the draft.
Author information
Authors and Affiliations
Corresponding author
Additional information
In a recent paper, Ali et al. (2011; insectscience.org/11.167) reported that Tetranychus macfarlanei occurs in Bangladesh, India, Madagascar, Mauritius, Thailand, Japan, Taiwan, USA, Malaysia, and the Canary Islands, based on ‘personal communication’. However, to date this species is reported only from India, Madagascar, Mauritius and the Canary Islands, and in the current paper we newly add Bangladesh. Tetranychus macfarlanei is not known from Japan and its geographical distribution is fabricated in the paper by Ali et al. Mr. Ali confessed to TG that voucher specimens of T. macfarlanei were not deposited, simply because the authors did not collect the species in Japan or any of the other countries mentioned.
Appendices
Appendix 1
See Table 4.
Appendix 2
See Table 5.
Appendix 3
See Table 6.
Appendix 4
See Table 7.
Rights and permissions
About this article
Cite this article
Ullah, M.S., Haque, M.A., Nachman, G. et al. Temperature-dependent development and reproductive traits of Tetranychus macfarlanei (Acari: Tetranychidae). Exp Appl Acarol 56, 327–344 (2012). https://doi.org/10.1007/s10493-012-9523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9523-3