Abstract
Two biological control practices are the use of suppressive growing media and the application of biological control agents (BCAs). The goals of this study were: (i) to screen 584 potential BCAs obtained from Fusarium wilt (FW) suppressive growing media; (ii) to evaluate in greenhouse conditions selected BCAs in three growing media with different degrees of suppressiveness of tomato FW. Two isolates selected after screening were identified as Fusarium solani (305) and Streptomyces sp. (A19). Results showed that tomato FW was reduced and total production was improved when both BCAs were applied to a conducive medium (coir fiber). In highly suppressive growing medium (grape marc compost), A19 and 305 inoculations did not improve suppressiveness. In moderately suppressive growing medium (cork compost), only A19 improved this compost to natural grape marc compost suppressiveness level. Therefore, compost suppressiveness of tomato FW depended on the nature of the compost and on the isolates applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato fruit, obtained from Solanum lycopersicum L. plants, has become the most consumed food worldwide (Borrero et al. 2006). Spain is one of the five main tomato-producing countries in Europe. Its production in 2009 was about 4604.8·103 tons, of which 41.3 % and 34.6 % were in Extremadura and Andalusia, respectively (Anonymous 2010). Fusarium wilt (FW) is one of the principal soilborne systemic diseases affecting tomato plants. Fusarium oxysporum f. sp. lycopersici W. C. Snyder & H. N. Hans (Fol) is the soilborne fungal plant pathogen responsible for this vascular wilt, which occurs throughout most tomato-growing areas and can devastate a crop (Larkin and Fravel 1998).
Management strategies are focused on preventive measures, including the use of resistant cultivars, effective soil fumigation treatments and biological control. Although the use of Fusarium-resistant tomato cultivars provides some degree of control, the occurrence and development of new pathogenic races is a problem (Larkin and Fravel 1998). In addition, the chemical control that is available for this disease is deficient, and there is concern over the rise of new fungicide-resistant pathogen species (Dekker 1981), the risk of non-target effects of pesticides and the damage caused to the environment. Therefore, there is an interest in technologies that reduce dependency on synthetic, chemical pesticides (Brimmer and Boland 2003). Biological control of plant diseases, using composts and biological control agents (BCAs), has received attention as an alternative to the intensive use of chemically synthesized products. This alternative is perceived to be safer and to have a minimal environmental impact (Brimmer and Boland 2003).
The capacity of certain composts to suppress plant diseases, minimize organic waste and reduce fertilizer and fungicide addition in crop production is widely known. Effectiveness of composts against plant diseases caused by a broad range of pathogens, including bacteria, fungi and nematode species, has been demonstrated in numerous studies (Litterick et al. 2004; Noble and Coventry 2005; Bonanomi et al. 2010; Avilés et al. 2011). Several composts have shown suppressiveness against tomato FW (Cotxarrera et al. 2002; Borrero et al. 2004, 2005, 2006; Castaño et al. 2011). Suppressive levels have been found to vary depending on the kind of compost used. For example, grape marc compost (GMC) and cork compost (CC) display high and moderate suppressive levels to tomato FW (Borrero et al. 2004, 2005, 2006), respectively, even when different batches are used (Castaño et al. 2011).
Another very common approach is the use of BCAs. These microorganisms are perceived to have advantages over synthetic fungicides, as they have fewer environmental negative effects, are effective against fungicide-resistant pathogens, reduce probability of resistance development and can be used in organic farming, where use of synthetic fungicides is restricted (Brimmer and Boland 2003). However, only a few BCAs are available on the European market. Some of these are selected from suppressive soils or growing media for their efficacy in controlling diseases and developing a biological control product (Nelson et al. 1983; Alabouvette et al. 2006). Rhizosphere microbes may exert specific plant growth promotion or biocontrol effects, which could be of great advantage to the selection of possible BCAs (Hartman et al. 2009). Effective fungi (Punja and Utkhede 2003), bacteria and actinomycetes (de Boer et al. 2003; El-Tarabily and Sivasithamparam 2006) have been used as BCAs against plant diseases. Compost manufacturers should evaluate the economic viability of each compost in relation to addition of a BCA (Trillas et al. 2006).
The goals of this study were: (i) selection of potential BCAs against tomato FW isolated from suppressive growing media, (ii) evaluation in greenhouse conditions of the selected BCAs in combination with conducive coir fiber (CF) and two composts with different degrees of natural suppression of FW: highly suppressive GMC and moderately suppressive CC (Borrero et al. 2004, 2006; Castaño et al. 2011).
Materials and methods
Selection of possible BCAs from growing media formulated with composts
To obtain potential BCAs, growing media made from composts of agricultural industry waste were assayed for suppressiveness against F. oxysporum f. sp. lycopersici (FOL) and dianthi (FOD) in tomato and carnation, respectively. The composts used, derived from turned piles, are described elsewhere (Trillas et al. 2002; Carmona et al. 2004). These growing media were: (1) GMC from the alcohol distilling industry (grape skins, seeds and stems), (2) CC from cork (Quercus suber L.) transformation, (3) olive oil husk + cotton gin trash, 1:1 v/v, composted and mixed with rice husk (1:1 v/v) (OC + R) and (4) spent mushroom compost (Recomsa, Quintanar del Rey, Spain) mixed with amended light peat (Klasmann, Valimex, Palleter, Spain) (1:1 v/v) (SM + P). These composts were not phytotoxic when used as growing media for tomato or carnation plants. At the end of these assays, 584 microorganisms were isolated from Fusarium-infested rhizospheres of healthy plants.
Microorganisms were isolated by dilution plating of aqueous extractions of rhizospheres on different semi-selective culture media, according to Tuitert et al. (1998) with modifications (Borrero et al. 2005). In culture media for isolation of Bacillus spp. (nutrient agar), fluorescent Pseudomonas spp. (King B), oligotrophic (nutrient agar 0.01) and cellulolytic (cellulose agar) bacteria and actinomycetes, 100 μg ml−1 of cycloheximide was substituted for 10 μg ml−1 of benomyl (Energía e Industrias Aragonesas, S.A., Madrid) and 0.3 μl ml−1 of Previcur (propamocarb, 72.2 %, Schering, Alcácer, Spain). Fungi were isolated on potato dextrose agar amended with 1,000 ppm of Tergitol-7 (Fluka Chemie AGB, Buchs, Switzerland) and 50 μg ml−1 of oxytetracycline hydrochloride (Sigma Chemical Company, St Louis, MO, USA) (Chen et al. 1988). Fusarium spp. were isolated on Komada’s medium (Komada 1975) and Talaromyces spp. and Trichoderma spp. were isolated on semi-selective media (Chung and Hoitink 1990; Dhingra and Sinclair 1995).
The disease-suppression effects of these isolates were measured with two kinds of efficacy assays against FW using tomato plants cultured in CF (Cocopeat, Projar, Valencia, Spain). These assays had randomized three-block designs. In the first kind of assay, isolates were inoculated in groups of four. In the second kind of assay, isolates previously tested in groups of four, which significantly reduced the disease severity compared to FOL-infested treatment alone, were tested individually. All isolates (584) were evaluated with the first assay and 112 isolates were evaluated with the second kind of assay. For these assays, each pot (330 ml) had four tomato seedlings. Plants were grown in a growth chamber (25 °C, 16:8 h light:dark photoperiod and 27 °C). Plants were irrigated as needed and fertilized with a nutrient solution containing: 0.5 g l−1 Peter’s foliar feed (27 + 15 + 12; N + P2O5 + K2O; and micronutrients; Scotts, Heerlen, The Netherlands), 0.6 g l−1 CaCl2, 0.7 g l−1 MgSO4·7H2O and 0.3 g l−1 urea (pH 6.1).
Bacterial and actinomycete isolates were grown on tryptic soy agar (50 %) and were inoculated at ~106 bacteria cm−3 substrate. Fungal isolates were grown on malt agar and were inoculated at 105 conidia cm−3 substrate. Inocula were quantified with aid of a haematocytometer (counting chamber depth: 0.1 mm for fungi and 0.02 mm for bacteria and actinomycete isolates). These inoculations were performed five days after sowing.
FOL (isolate FN2) was grown for seven days in AMAP culture media containing 10 g l−1 agar, 10 g l−1 malt extract (Difco, Le Pont de Claix, France), 2 g l−1 asparagine (Difco, Le Pont de Claix, France) and 0.5 g l−1 Peter’s foliar feed. Five ml of sterile distilled water (SDW) were added to each culture plate. The surface of the culture was scraped with a sterile spreader. The concentration of conidia was determined with aid of a haemocytometer. CF was infested with FN2 (4 · 104–105 conidia cm−3 growing medium) 4–5 days after potential BCAs inoculation. Pots of growth medium without FN2 were prepared to assess adequate agronomic conditions in the assays.
Disease severity was monitored at 2–3 day intervals and was scored based on a symptom severity scale (Borrero et al. 2004). In each disease assessment, the mean of disease severity per pot was calculated. The area under the disease progress curve standardized (AUDPCs) per pot was calculated by disease severity integrated between the onset of symptoms and assay completion and by dividing by the total duration (days) of the epidemic in each assay, in order to compare the various assays, which had a variety of epidemic durations (Campbell and Madden 1990). Therefore, AUDPCs data can range from 0 to 1.
Identification of the selected isolates by DNA sequencing
After selection assays two isolates were chosen for their severity reduction (see “Results”). To identify them total genomic DNA from two isolates selected, A19 (cellulolytic actinomycete) and 305 (Fusarium-like fungus by microscope-observed morphology), was extracted from lyophilized cultures according to Cassago et al. (2002) with minor modifications.
Total DNA from isolate 305 was used for polymerase chain reaction (PCR) using the EF1 (5′-ATGGGTAAGGAGGACAAGAC-3′) and EF2 (5′-GGAAGTACCAGTGATCATGTT-3′) primers to amplify a portion of the Translation Elongation Factor 1-α gene (TEF-1α). These primers amplified a highly informative region for differentiating Fusarium spp. and many formae speciales within the F. oxysporum complex (Geiser et al. 2004). Total DNA from A19 was used for PCR with the 8f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1389r (5′-ACGGGCGGTGTGTACAAG-3′) primers to amplify a region of bacterial 16S rDNA (LaMontagne et al. 2003).
Genomic DNA was amplified in a solution containing Biotools Reaction Buffer 1× (10×), 3.75 mM MgCl2 (50 mM), 100 μM of each dNTP (10 mM), 0.5 μM of each primer and 1 U of Biotools Pfu DNA polymerase (Biotools, Madrid, Spain) in a final volume of 50 μl. The PCR cycling conditions consisted of an initial denaturation at 95 °C for 2 min, a first phase of ten cycles with an elongation phase at 72 °C for 2 min, and a second phase of 20 cycles, in which the elongation phase was increased by 4 s per cycle, starting from 2 min and maintaining the elongation temperature at 72 °C. During both phases of the PCR, elongation steps were preceded by a denaturation step (94 °C for 1 min) and an annealing step (54 °C for 30 s). Amplification was finished after a final extension phase for 5 min at 72 °C. PCR amplifications were performed on a GenAmp PCR system 9700 (Applied Biosystems, California, USA). Negative and positive controls were included in all experiments.
The PCR products were purified using Speedtools PCR Clean-up kit (Biotools, Madrid, Spain). Sequencing was performed by Secugen Sequencing Service Enterprises (Secugen, Madrid, Spain). Sequences were edited and then queried for similarity using NCBI GenBank database with the BLASTn alignment software tool for both isolates. Sequence homology identity was determined using the FASTA algorithm, in which the reference sequences most closely related to our sequences were found. The FUSARIUM-ID database (Geiser et al. 2004) was also used to confirm 305 sequence homology identity.
Preparation and quantification of pathogen and selected isolate inocula
Pathogen inoculation was performed with a monosporic isolate of F. oxysporum f. sp. lycopersici race 2 (isolate FN2). The pathogenicity of this isolate was confirmed in previous studies (Borrero et al. 2006; Castaño et al. 2011). It was recovered from stored inoculum (silica gel 4 °C) and grown in malt broth (10 g l−1) in a 10 l fermenter Biostat B (Braun Biotech-Sartorius, Aubagne, France). Isolate 305 was grown for seven days in AMAP culture media. Five ml of SDW was added to each culture plate. Plate surfaces were scraped with a sterile spreader. Concentrations of microconidia from isolate 305 and FN2 were determined with haemocytometer.
Isolate A19 was prepared from stored inoculum at −80 °C (Vibakstore Nirco, Barberà del Vallès, Barcelona, Spain). Stored microorganisms were grown in 2–3 plates with agar cellulose (AC) medium (Borrero et al. 2005) for seven days. Five ml of SDW were added to each culture plate and were placed in a sterile empty bottle. This suspension was raised to 50–80 ml using SDW. Then, 0.1 ml of this suspension was poured onto each AC culture plate (600–650) and their surfaces were scraped. Plates were grown at 25 °C in the dark. After 2–3 weeks, 5 ml of SDW were added to each culture plate and the surface was scraped. Suspension was sonicated for 30 min to avoid cellular aggregation. Concentration of A19 was determined by acridine orange direct counting (AODC) technique, which was performed by counting an A19 concentrated solution with an epifluorescence microscope (Kepner and Pratt 1994; Borrero et al. 2004). Three samples were analysed. Controls were also counted in sterile water.
Evaluation of suppressiveness of FW assays in greenhouse conditions
Two composts (GMC and CC) were evaluated in a tomato FW suppressiveness assay under greenhouse conditions. Composts were compared with CF. Physicochemical and biological properties of these growing media were evaluated in previous studies (Borrero et al. 2009; Castaño et al. 2011).
The assay was conducted in a greenhouse in Seville (Spain) at the beginning of 2008, with the susceptible tomato cultivar Roma. Tomato seeds were sown in peat and grown in a growth chamber (27 °C, photosynthetically active radiation intensity of 280 μE s−1 m−2 with a 16:8 h light:dark photoperiod). Plants were irrigated as needed and fertilized with 0.25 g l−1 Peter’s Foliar Feed, 0.15 g l−1 KCl and 0.6 g l−1 CaCl2. After 20 days, three plantlets (2–3 true-leaf stage) were placed in bags (1 m × 25 cm) with 30 l of growing medium. Each experimental unit consisted of two bags. The experimental design involved three blocks × three growing media × two inoculated with pathogen and non-inoculated × three inoculated with 305, A19 or not inoculated leading to 54 experimental units. The assays were performed in a randomized complete-block design with three replicates and repeated twice.
At the beginning of the assay, plantlets were previously inoculated by root-dipping for 12 h using an inoculum suspension containing 103 microconidia (305) or cells (A19) ml−1. The inoculation dose in bag was 5 · 103 microconidia cm−3 and 105 cells cm−3 of growing media for 305 and A19, respectively. Inoculations were carried out on the same day of transplanting and were repeated at seven, 41 and 76 days after transplanting with the same doses. Pathogen inoculation was done two weeks after transplanting at 5 · 103 conidia cm−3 growing medium. A second pathogen inoculation was carried out after 70 days using 5 · 104 conidia cm−3 of growing media. Inoculum suspensions were applied by irrigating growing media with the help of syringes through dripper holes. Controls were irrigated with water.
During the assay, the fertirrigated nutrient solutions and the scale used to score disease severity in tomato plants were as described by Castaño et al. (2011). Disease severity was monitored twice a week. At each assessment, mean disease severity per experimental unit was calculated. The AUDPCs per experimental unit was calculated as previously. Duration of each assay was three months. Total production was scored as kg of tomato fruit harvested from each treatment during assays.
Quantification of Fusarium spp. and cellulolytic actinomycete populations in growing media
Population densities were determined by dilution plating on two semi-selective media using Komada’s medium for Fusarium spp. populations and AC medium for cellulolytic microbial populations. Samples were taken from growing media at the beginning and end of the assays. Growing media (5–10 g) were taken and mixed from two bags (an experimental unit) and suspended in 90 ml of Na4P2O7·10H2O 1 g l−1. The suspension was shaken and a tenfold dilution series was prepared with 0.1 % water agar (w/v). Suspensions were pipetted onto three plates per dilution. Four–five dilutions per series were placed on plates. Colony-forming units (CFU) were counted four days after plating and expressed as CFU cm−3 of growing media.
Isolate 305 and pathogen FN2 quantification were developed at the end of the assays in growing media, in which both microorganisms were inoculated, using Komada’s dilution plates. They were differentiated on the basis of colony characteristics and morphological differences under the microscope.
Statistical analysis
Disease severity from selection assays (AUDPCs) was analysed with one-way ANOVA. Disease severity (AUDPCs) in greenhouse conditions was analysed with two-ways ANOVA. Terms included in this model were: (A factor) growing media inoculated with pathogen × inoculated and non-inoculated with isolate 305 or A19 (6 levels), (B factor) blocks (three levels) and their interaction. Data from two assays were pooled for final analysis after finding no significant A factor × assay interaction in a preliminary analysis of variance. Significant means were compared by LSD test (P < 0.05). Data collected from populations (expressed as CFU cm−3 of growing media infested with FN2 per treatment at the beginning and the end of assay, n = 6) and total production (expressed as kg per treatment in growing media infested or non-infested with FN2 during assay, n = 6) were also analysed by two-way ANOVA. Data from populations and total production were pooled for final analysis after finding no significant A factor × assay interaction in a preliminary analysis of variance. All analyses were made with Statgraphics Plus (version 5.1; Statistical Graphics Corp., Rockville, MD, USA, 2002).
Results
Selection of possible BCAs from growing media formulated with composts and identification
Of all the isolates (584), isolates A19 (cellulolytic actinomycete) from CC and 305 (Fusarium spp.) from GMC showed excellent results, reducing disease severity in CF. Isolate A19 reduced AUDPCs by 95.6 % compared with controls, and isolate 305 by 96.6 %. Therefore, they were selected for further evaluation in efficacy assays in greenhouses.
Both strains showed strong sequence identity to known sequences. Isolate 305 showed a single mismatch with F. solani (99 %, FN689821.1) and isolate A19 showed a high identity with Streptomyces sp. (98 %, GU991351.1). Accession numbers are shown in Table 1.
Effects of growing media and selected isolates on suppressiveness to tomato FW in greenhouse conditions
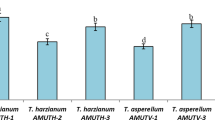
The onset of symptoms in CF appeared ~ ten days earlier than in GMC. The AUDPCs at the end of assays indicated that the two composts had significant suppressive effects on FW in comparison with CF, which showed the highest values of severity (Fig. 1). This disease was suppressed most effectively in GMC, followed by CC.
Standardized areas under disease progress curve (AUDPCs) for tomato plants cultured in three growing media (GMC grape marc compost, CC cork compost, CF coir fiber) inoculated or not with isolate 305 or A19. All these treatments were artificially infested with F. oxysporum f. sp. lycopersici race 2 (FN2). Bars with the same letters were not significantly different (P ≤ 0.05) according to LSD test (F 5,18 = 11.10 and 14.16 and P = 0.0001 and <0.0001 for AUDPCs in 305 and A19 respectively). Analysis of variance was performed with arcsine (√x) transformed data. Error bars represent SE (n = 6)
No effects were observed on AUDPCs when isolates 305 and A19 were added to composts, not improving their naturally suppressive effect (Fig. 1). Moreover, CC inoculated with A19 obtained similar suppressiveness to natural GMC. However, isolates significantly reduced disease severity in CF (Fig. 1). This severity reduction was equivalent to that observed in naturally suppressive CC. None of the plants grown in the three non-infested control growth media inoculated or not with the BCAs showed signs of weaknesses or toxicity during cultivation and none developed symptoms of tomato FW.
Fusarium spp. population densities at the beginning and end of assays
At the beginning of assays, 305 and A19 behaved differently, depending on the growing media in which they were inoculated. Only Fusarium spp. density increased when 305 was inoculated in CC (Fig. 2). At the end of assays, Fusarium population density showed differences only when isolate 305 was applied to GMC, in comparison to GMC without 305 (Fig. 2). However, isolate 305 population was not statistically different from pathogen in GMC 305 when both microorganisms were scored (Table 2), and CC 305 and CF 305 growing media showed higher isolate 305 population than pathogen (Table 2). However, differences in suppressiveness were only achieved in CF 305 (Fig. 1).
Fusarium spp. population densities at the beginning and end of assays. Growing media were infested with F. oxysporum f. sp. lycopersici race 2 (FN2). GMC grape marc compost, CC cork compost, CF coir fiber; 305: inoculated with 305 isolate; A19: inoculated with A19 isolate. Bars with the same lower-case letter for the beginning of bioassays or upper-case letter for the end of bioassays were not significantly different (P < 0.05) according to LSD test (F 8,27 = 40.7 and 4.25 and P < 0.0001 and P = 0.0021 for Fusarium population densities at the beginning and the end of bioassays respectively). Analysis of variance was performed with transformed data as ln(x + 0.083). Error bars represent SE (n = 6)
Cellulolytic population densities at the beginning and end of assays
At the beginning of assays, cellulolytic populations were higher than in the control in inoculated CF (CF A19) (Fig. 3). At the end of assays, cellulolytic population densities were higher in GMC A19 and CF A19 than in GMC and CF, respectively. Differences in suppressiveness were only achieved in CF A19 (Fig. 1). However, no significant differences were observed in CC (Fig. 3).
Cellulolytic population densities at the beginning and end of assays. Growing media were infested with F. oxysporum f. sp. lycopersici race 2 (FN2). GMC grape marc compost, CC cork compost, CF coir fiber; 305 inoculated with 305 isolate; A19 inoculated with A19 isolate. Bars with the same lower-case letter for the beginning of bioassays or upper-case letter for the end of bioassays were not significantly different (P < 0.05) according to LSD test (F 8,27 = 37.85 and 67.51 and P < 0.0001 and P < 0.0001 for cellulolytic population densities at the beginning and the end of bioassays respectively). Analysis of variance was performed with transformed data as ln(x + 0.083). Error bars represent SE (n = 6)
Tomato production at the end of assays
Using the same fertirrigation in all growing media, A19 improved total tomato production over the natural growing medium in two situations: for CC in treatments not inoculated with the pathogen and in CF in treatments inoculated with the pathogen (Table 3). Isolate 305 did not improve or worsen total production in relation to natural growing media.
Discussion
The rhizodeposition of plants influences the surrounding soil and its microflora (Garbeva et al. 2011). Root exudates have selective and promoting effects on specific microbial populations, which are able to respond to chemotaxis and fast growth responses, but only a small part of the whole soil’s microbial diversity finally colonizes roots successfully (Hartman et al. 2009). If these microorganisms are obtained from healthy rhizospheres, they can probably collaborate in plant health, especially when the rhizosphere has high doses of a pathogen (Zheng et al. 2011). In this screening, we used selective culture media for some genera that are commonly involved in biocontrol (Bacillus, Pseudomonas, Fusarium, Talaromyces and Trichoderma). Additional culture media were used to obtain strains of other genera involved in suppressiveness that reflect the suppressive microbial diversity in the BCAs collection. After microorganism isolation, some authors screen microorganism collections with some biological and biochemical proofs such as antimicrobial compound production, mycoparasitism detection, siderophore production, enzyme activities related to biocontrol evaluation, etc. (Tondje et al. 2007; Roy et al. 2009; Paternoster et al. 2010; Taurian et al. 2010). In this study the selection criterion chosen was BCA efficacy against tomato FW, regardless of the mechanism of action.
The composts studied were evaluated in suppressiveness assays as in previous studies (Borrero et al. 2004, 2005, 2006, 2009; Trillas et al. 2006; Castaño et al. 2011), in which the authors discussed whether biocontrol mechanisms associated with natural suppression of composts may be associated partially with microbiostasis (Borrero et al. 2009). The high microbial diversity of these composts may favour a competitive state affecting pathogen and both isolates 305 and A19. This could explain why the application of isolates 305 and A19 to these composts, particularly GMC, did not lead to any significant advantage.
The highest disease suppression shown for natural GMC was difficult to improve with addition of one isolate. In this sense, the BCA Trichoderma asperellum T34 reduced 30 % of carnation FW severity in a moderately suppressive GMC formulated with peat (1:1 v:v) (Sant et al. 2010). In the present study CC (moderately suppressive compost) reached the same level of suppressiveness as GMC (highly suppressive compost) with A19. However, isolate T34 could not improve highly suppressive compost’s efficacy (Trillas et al. 2006), which was similar to results of isolates 305 and A19 for highly suppressive GMC. Therefore, this study suggests that it is difficult to improve with any BCA tomato FW control composts with the natural suppressiveness level of GMC.
A study by Larkin and Fravel (1998) reported that ranges of tomato FW reduction by BCAs in tomato plants were generally between 30–65 % and 40–66 % in cases of suppressive bacteria and fungi, respectively. Starting from 584 isolates obtained from suppressive rhizospheres, we found two potential BCAs, a strain of F. solani (isolate 305) and a Streptomyces sp. (isolate A19). Tomato FW severity was clearly reduced in CF by 305 and A19, providing the same level of suppression as the moderately suppressive CC. This conducive growing medium (CF) is employed worldwide, due to its good physical properties (Abad et al. 2002).
In agreement with this present study, the control of FW diseases by non-pathogenic Fusarium spp. has been demonstrated in several studies (Larkin and Fravel 1998, 1999, 2002; Weller et al. 2002; Alabouvette et al. 2009; Zheng et al. 2011). However, there are few reports on biocontrol by non-pathogenic Fusarium spp. belonging to species other than F. oxysporum. Two non-pathogenic F. solani (strains CS-1 and CS-6) were shown as BCAs for F. oxysporum f. sp. lycopersici, against niveum in watermelon, melonis in melon (Larkin and Fravel 1998) and basilici in basil (Larkin and Fravel 1999).
The best-documented biocontrol mechanisms between pathogenic and non-pathogenic F. oxysporum concern competition for nutrients, mainly carbon sources, and induced systemic resistance (ISR) (Larkin and Fravel 1999; Alabouvette et al. 2006). Therefore, a possible competition mechanism could be related to isolate 305 due to its higher population density in CF at the end of the assay in comparison with FN2. In contrast, strain CS-1 previously demonstrated evidence of ISR as a mechanism of biocontrol in watermelon and tomato (Larkin and Fravel 1998). Both action mechanisms confirm previous studies, in which two non-pathogenic F. oxysporum CS-20 and Fo47 have different control mechanisms against FWs (Larkin and Fravel 1998, 1999). In future studies, ISR action of isolate 305 could be evaluated.
Other studies using strains of actinomycetes such as BCA have been carried out with members of the genus Streptomyces (Doumbou et al. 2002; Coombs et al. 2004; Kim and Hwang 2007; Quecine et al. 2008). Furthermore, there are several examples using Streptomyces as BCAs against many plant diseases, such as Phytophthora root rot in alfalfa and soybean (Xia et al. 2002), FW in cucumber (Singh et al. 1999), drop disease caused by Sclerotinia minor in lettuce (El-Tarabily et al. 2000) and blast and sheath blight in rice (Prabavathy et al. 2006). Indeed, other non-streptomyces actinomycetes have been reported as potential BCAs (El-Tarabily and Sivasithamparam 2006).
Isolates 305 and A19 developed better in growing media from which they were not isolated. Severity in CC was reduced when isolate A19 was added, although there was no significant difference with natural CC. However, CC A19 showed similar suppressiveness to GMC. Possibly strain A19 (a cellulolytic actinomycete) in CC might improve severity reduction at an effective dose, because it is the origin of the growing medium CC, which liberates cellulose slowly (Trillas et al. 2006). Several studies reported the importance of dose application in relation with the mode of action of the BCAs (Larkin and Fravel 1998, 1999; Mandeel and Baker 1991; Alabouvette et al. 2006).
Disease reduction in CF due to A19 and 305 treatments increased total production but only A19 had significant production augment. Without pathogen the production increase in CC could indicate a plant-growth promoting effect. In this sense, some authors report that actinomycetes have a growth-promoting effect (Doumbou et al. 2002; El-Tarabily and Sivasithamparam 2006).
In conclusion, in highly suppressive growing medium (GMC), Streptomyces spp. (strain A19) and F. solani (strain 305) introduction did not improve suppressiveness. In moderately suppressive growing medium (CC), only A19 improved this compost to GMC suppressiveness level. In the conducive growing medium (CF), that is used worldwide, both isolates improved suppressiveness to the same level as CC.
References
Abad M, Noguera P, Puchades R, Maquieira A, Noguera V (2002) Physico-chemical and chemical properties of some coconut coir dust for use as a peat substitute for containerised ornamental plants. Bioresour Technol 82:241–245
Alabouvette C, Olivain C, Steinberg C (2006) Biological control of plant diseases: the European situation. Eur J Plant Pathol 114:329–341
Alabouvette C, Olivain C, Migheli Q, Steinberg C (2009) Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol 184:529–544
Anonymous (2010) Avances de superficies y producciones agrícolas. Ministerio de Medio Ambiente y Medio Rural y Marino, Spain, p 28
Avilés M, Borrero C, Trillas MI (2011) Review on compost as an inducer of disease suppression in plants grown in soilless culture. In: Special Issue Compost III—dynamic plant, dynamic soil. Global Science Books. 5 (Special Issue 2), pp 1–11
Bonanomi G, Antignani V, Capodilupo M, Scala F (2010) Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol Biochem 42:136–144
Borrero C, Trillas MI, Ordovás J, Tello JC, Avilés M (2004) Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Phytopathology 94:1094–1101
Borrero C, Infantes MJ, González E, Tello JC, Avilés M (2005) Relation between suppressiveness to tomato Fusarium wilt and microbial populations in different growth media. Acta Hort 697:425–430
Borrero C, Ordovás J, Trillas MI, Avilés M (2006) Tomato Fusarium wilt suppressiveness. The relationship between the organic plant growth media and their microbial communities as characterised by Biolog®. Soil Biol Biochem 38:1631–1637
Borrero C, Trillas MI, Avilés M (2009) Carnation Fusarium wilt suppression in four composts. Eur J Plant Pathol 123:425–433
Brimmer T, Boland G (2003) A review of the non-target effects of fungi used to biologically control plant diseases. Agric Ecosyst Environ 100:3–16
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley, New York, USA
Carmona E, Avilés M, Domínguez I, Moreno MT, Pajuelo P, Ordovás J (2004) Exploitation of composted agricultural wastes as growing media. In: Bernal MP, Moral R, Clemente R, Paredes C (ed) Sustainable organic waste management for environmental protection and food safety, vol I. Ramiran 2004. Proceedings of the 11th international conference of the FAO ESCORENA network on the recycling of agricultural residues in agriculture, Murcia, Spain, pp 141–144
Cassago A, Panepucci RA, Tordella-Baião AM, Henrique-Silva F (2002) Cellophane based mini-prep method for DNA extraction from the filamentous fungus Trichoderma ressei. BMC Microbiol 2:14–17
Castaño R, Borrero C, Avilés M (2011) Organic matter fractions by SP-MAS 13C NMR and microbial communities involved in the suppression of Fusarium wilt in organic growth media. Biol Control 58:286–293
Chen W, Hoitink HAJ, Schmitthenner AF, Tuovinen OH (1988) The role of microbial activity in suppression of damping-off caused by Pythium ultimum. Phytopathology 78:314–322
Chung YR, Hoitink HAJ (1990) Interactions between thermophilic fungi and Trichoderma hamatum in suppression of Rhizoctonia damping-off in a bark compost-amended container medium. Phytopathology 80:73–77
Coombs JT, Michelsen PP, Franco MM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29:359–366
Cotxarrera L, Trillas-Gay MI, Steinberg C, Alabouvette C (2002) Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biol Biochem 34:467–476
De Boer W, Verheggen P, Klein Gunnewiek PJA, Kowalchuk GA, van Veen JA (2003) Microbial community composition affects soil fungistasis. Appl Environ Microbiol 69:835–844
Dekker J (1981) Resistance to fungicides in plant pathogens: abstracts of papers. Neth J Plant Pathol 87:233–255
Dhingra O, Sinclair J (1995) Basic plant pathology methods, 2nd edn. Lewis Publisher, Boca Raton, USA
Doumbou CL, Hamby Salove MK, Crawford DL, Beaulieu C (2002) Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection 82:85–102
El-Tarabily KA, Sivasithamparam K (2006) Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem 38:1505–1520
El-Tarabily KA, Soliman MH, Nassar AH, Al-Hassani HA, Sivasithamparam K, McKenna F, Hardy GESJ (2000) Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathol 49:573–583
Garbeva P, Gera Hol WH, Termorshuizen AJ, Kowalchuk GA, de Boer W (2011) Fungistasis and general soil biostasis—a new synthesis. Soil Biol Biochem 43:469–477
Geiser DM, Jiménez-Gasco MM, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’Donnell K (2004) Fusarium-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol 110:473–479
Hartman A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Kepner JR, Pratt JR (1994) Use of fluorochromes for direct enumeration of total bacterial in environmental samples: past and present. Microbiol Mol Biol Rev 58:603–615
Kim BS, Hwang B (2007) Microbial fungicides in the control of plant diseases. J Phytopathol 155:641–653
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res 8:114–125
LaMontagne MG, Schimel JP, Holden PA (2003) Comparison of subsurface and surface soil bacterial communities in California grassland as assessed by terminal restriction fragment length polymorphisms of PCR-amplified 16S rRNA genes. Microb Ecol 46:216–227
Larkin RP, Fravel DR (1998) Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis 82:1022–1028
Larkin RP, Fravel DR (1999) Mechanisms of action and dose-response relationships governing biological control of Fusarium wilt of tomato by nonpathogenic Fusarium spp. Phytopathology 89:1152–1161
Larkin RP, Fravel DR (2002) Effects of varying environmental conditions on biological control of Fusarium wilt of tomato nonpathogenic Fusarium spp. Phytopathology 92:1160–1166
Litterick AM, Harrier L, Wallace P, Watson CA, Wood M (2004) The role of uncomposted materials, composts, manures, and composts extracts in reducing pest and disease incidence and severity in sustainable temperate agricultural and horticultural crop production—a review. Crit Rev Plant Sci 23:453–479
Mandeel Q, Baker R (1991) Mechanisms involved in biological control of Fusarium wilt of cucumber with strains of nonpathogenic Fusarium oxysporum. Phytopathology 81:462–469
Nelson EB, Kuter GA, Hoitink HAJ (1983) Effects of fungal antagonists and compost age on suppression of Rhizoctonia damping-off in container media amended with composted hardwood bark. Phytopathology 73:1457–1462
Noble R, Coventry E (2005) Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci Technol 15:3–20
Paternoster T, Défago G, Duffy B, Gessler C, Pertot I (2010) Selection of a biocontrol agent based on a potential mechanism of action: degradation of nicotinic acid, a growth factor essential for Erwinia amylovora. Int Microbiol 13:195–206
Prabavathy VR, Mathivanan N, Murugesan K (2006) Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biol Control 39:313–319
Punja ZK, Utkhede RS (2003) Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol 21:400–407
Quecine MC, Araujo WL, Marcon J, Gai CS, Azevedo JL, Pizzirani-Kleiner AA (2008) Chitinolytic activity of endophytic Streptomyces and potential for biocontrol. Lett Appl Microbiol 47:486–491
Roy AK, Mandal NL, Singh AN (2009) Screening of maize rhizobacteria against aflatoxigenic Aspergillus flavus strains in relation to siderophore and HCN production. Indian Phytopathol 62:440–444
Sant D, Casanova E, Segarra G, Avilés M, Reis M, Trillas MI (2010) Effect of Trichoderma asperellum strain T34 on Fusarium wilt and water usage in carnation grown on compost-based growth medium. Biol Control 53:291–296
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Taurian T, Anzuay MS, Angelini JG, Tonelli ML, Ludueña L, Pena D, Ibáñez F, Fabra A (2010) Phosphate-solubilizing peanut associated bacteria: screening for plant growth-promoting activities. Plant Soil 329:421–431
Tondje PR, Roberts DP, Bon MC, Widmer T, Samuels GJ, Ismaiel A, Begoude AD, Tchana T, Nyemb-Tshomb E, Ndoumbe-Nkeng M, Bateman R, Fontem D, Hebbar KP (2007) Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol Control 43:202–212
Trillas MI, Avilés M, Ordovás J, Bello A, Tello JC (2002) Using compost as a methyl bromide alternative. BioCycle 43:64–68
Trillas MI, Casanova E, Cotxarrera L, Ordovás J, Borrero C, Avilés M (2006) Composts from agricultural waste and the Trichoderma asperellum strain T-34 suppress Rhizoctonia solani in cucumber seedlings. Biol Control 39:32–38
Tuitert G, Szczech M, Bollen GJ (1998) Suppression of Rhizoctonia solani in potting mixtures amended with compost made from organic household waste. Phytopathology 88:764–773
Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Xia K, Kinkel LL, Samac DA (2002) Biological control of Phytophthora root rot on alfalfa and soybean with Streptomyces. Biol Control 23:285–295
Zheng Y, Xue QY, Xu LL, Xu Q, Lu S, Gu S, Guo JH (2011) A screening strategy of fungal biocontrol agents towards Verticillium wilt of cotton. Biol Control 56:209–216
Acknowledgments
This research was supported by grants from Ministerio de Educación y Ciencia (AGL2005-08137-C03-02), Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía (P06-AGR-02313) and Ministerio de Ciencia e Innovación (AGL2008-05414-C03-01) of Spain. We thank M.L. Castillo, S. Castillo and J. Rojo for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Monica Höfte
Rights and permissions
About this article
Cite this article
Castaño, R., Borrero, C., Trillas, M.I. et al. Selection of biological control agents against tomato Fusarium wilt and evaluation in greenhouse conditions of two selected agents in three growing media. BioControl 58, 105–116 (2013). https://doi.org/10.1007/s10526-012-9465-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-012-9465-z