Abstract
Fusarium wilt of tomato is one of the most important diseases worldwide. The present study focused on comparing the efficacy of various control methods including Trichoderma harzianum, garlic extract and fungicide difenoconazole against the pathogen both in vitro and in planta. In a lab experiment, dual culture technique was used to test the efficacy of Trichoderma harzianum on inhibition of the pathogen growth. Garlic extract was tested at concentration of 15 ml/L of potato dextrose agar media while fungicide difenoconazole was used @ 100 ppm. The lowest colony diameter (1.8 cm) was observed when pathogen was grown in dual culture with Trichoderma harzianum. In a screen house experiment, these treatments were tested in planta under inoculated condition. Two weeks after the inoculation, plants were assessed for disease severity through visual observation. Inoculated plants receiving no treatment showed the highest disease severity (71%) while lowest disease severity (8.63%) was observed for bio-control followed by fungicide difenoconazole. Garlic extract proved less effective at concentration tested in this study. Area under disease progress curve was significantly lower for treated plants than inoculated control. A similar trend was evident for yield parameters. There was a strong negative correlation between disease severity and fruit size. A simple linear relationship (R2 = 0.99) was observed between disease severity and yield. The result indicates significance of the non-chemical control of tomato wilt to minimize the excessive use of fungicides. Work on effective delivery system in field and their efficacy over time is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum Mill.), a herbaceous solanaceous plant, became more popular due to its climatic adaptability and high nutritive value. It is available all over the year due to its overlapping growing season in various regions of the world. In Pakistan, the total cultivated area of tomato is 38,238 thousand hectares yielding 41,721 thousand tonnes. In Khyber Pakhtunkhwa (KP) Province, total production of tomato was 36.268 thousand tonnes and area under cultivation was 30.54 thousand hectares with an average yield of 11.87 tonnes per hectare (MINFAL 2020–2021). The low productivity of tomato in Pakistan is due to its susceptibility to diseases such as damping off, root rots, wilting, late and early blight. Fusarium wilt, caused by Fusarium oxysporum f. sp. Lycopersici is a major fungal disease in the region (Abdul-wahab 2004). The pathogen is soil borne, affecting plants at all stages of roots development, causing wilt and necrosis (Alam et al. 2011). It is a soil inhabitant; reside in contaminated soil for 10 years even without its host. Soil with temperature higher than 34 ℃ or less than 17 ℃ slows down pathogen’s activity (Di Pietro et al. 2003). Disease management in soil is difficult since the pathogen persists in infested soil for an extended period of time as resting spores, (chlamydospores) and mycelium in plant residues (Haware et al. 1996; Agrios 2005). Different management practices such as resistant varieties, cultural control and application of various types of fungicides are available for the elimination of the pathogen inoculum (Wharton and Kirk 2012). Excessive use of pesticides however, may cause environmental hazards and contamination of ground water. The use of disease resistant varieties is also a realistic option for diseases control, but sadly there are no standard resistant varieties available. Cultural practices such as crop rotation are not effective due to wide host range of the pathogen. Subsequently, there is need for some ecofriendly bio control agent which might help in resolving the above-mentioned problems. Biological management of soil-borne, plant pathogens is environmentally suitable, safe and cost-effective (Abdullah et al. 2016). Biological controls will also help to minimize the use of chemical fungicides and reduce production costs. Efficient biological control agents have been shown to colonies plant roots, occupy space, secrete cell wall degrading enzymes in response to the pathogen and produce antibiotics that destroy target species, thus raising plant growth and turning the plant defense mechanism on (Haggag and Mohammad 2007). Biological control and botanical extracts are thus regarded as long lasting, cheaper, non-hazardous, environment-friendly options for disease control in plants. Moreover, this will also address the problems associated with ground water contamination. In view of the above, the present study was conducted with the following objectives to compare the efficacy of Trichoderma harzianum, fungicide difenoconazole and garlic extract against Fusarium oxysporum both in vitro and in planta.
Materials and Methods
Cultures of Fusarium oxysporum f. sp. lycopersici and Trichoderma harzianum
Cultures of the pathogen Fusarium oxysporum f. sp. lycopersici and the bio-control agent, Trichoderma harzianum were obtained from the culture bank of the Department of Plant Pathology, The University of Agriculture Peshawar, Pakistan. The stock cultures were grown on fresh plates containing potato dextrose agar (PDA) media and maintained for further studies (Fig. 1). Identity of the pathogen was confirmed based on morphology of spores (Fig. 2), using identification key of Nelson and Tousson (1983).
Plant Extracts and Fungicide
Garlic bulbs and fungicide difenoconazole were obtained from local market in Peshawar. Extracts were prepared by using crushed garlic paste. Crushed garlic paste of 250 g was mixed with 500 ml of double distilled water in a beaker to obtain a homogenous mixture through stirring. The macerated biomass was kept for four days at ambient temperature for exudation of bio-chemicals. The biomass was later filtered with muslin cloth to remove any impurities and stored as stock solution. The liquid extracts were used to assess their antimicrobial activity against the pathogen as described by Shetty et al. (2013). The extracts were used @ 15 ml/L of potato dextrose agar media. Fungicide (difenoconazole) was used @ 100 ppm during the course of the experiment.
In vitro Assay
An experiment was set-up to determine the effect of biological control agent, garlic extract and fungicide difenoconozole on F. oxysporum f. sp. lycopersici. In one of the treatments T. harzianum and the pathogen were allowed to grow in the same petri plate using dual culture method (Skidmore and Dickinson 1976). The pathogen was placed in the center of petri plate while three plugs of the bio-control agent were placed at equal distance from the pathogen. Similarly, in treatments containing fungicides or plant extract, poisoned food technique (Grover and Moore 1962) was used. PDA medium was amended with either fungicide (difenoconozole) @ 100 ppm or garlic extract @ 15 ml/L of potato dextrose agar following sterilization. Plates without bio-control agent, fungicides or bio extract were used as control. A 5 mm plug of the pathogen was placed aseptically in the center of each plate, wrapped with parafilm and incubated at 27 ± 2 ℃ for one week.
The plates were examined on daily basis. The inhibition zones and/or fungal growth were measured until the colony diameter in the control plates reached 90 mm (Verma et al. 2007). Eight replicates for each treatment were maintained. Data were recorded on radial growth which was measured after one week along two perpendicular lines using a scale, and then taking the mean of two measurements. The results were compared with control. Percent decrease over control was then calculated according to the following formula
where
- PI:
-
= Per cent inhibition over control
- R1:
-
= Growth of the pathogen in the control
- R2:
-
= Growth of the pathogen in the treatments
The experiment was laid as Completely Randomized Design (CRD) with four treatments and eight replicates.
Screen House Experiment
Nursery Raising
Tomato nursery (var. Riogrande) was raised in plastic trays containing a sterilized mixture of sand, clay and farm yard manure (1: 1: 1). The trays were maintained on a screen house bench for one month to allow the plants to germinate. One-month old individual seedlings were then transplanted in plastic pots at a depth of 5 cm.
Preparation of Inoculum and Inoculation
Cultures of the pathogen were grown on PDA media at 27 ℃ for one week. Mycelial mats were scrapped with spatula and suspended in sterile distilled water (SDW) to obtain spore suspension. The concentration of the spore suspension was adjusted to 1 × 104 conidia ml−1 with a haemocytometer. Plants were then inoculated with 5 ml spore suspension of Fusarium oxysporium f. sp. lycopersici @ 1 × 104 spores ml−1, near the crown region by using a hypodermic syringe. Before inoculation, the roots were slightly wounded by inserting a needle to ensure pathogen penetration. Inoculation was performed during morning hours to enhance the chances of disease onset.
Biological control agent was applied as a soil drench (Patil et al. 2011) soon after inoculation of the pathogen. In the soil drenching method, 5 ml of fungal suspension (i.e. water containing 1 × 104 conidia ml−1) was applied to each of the plant by drenching the soil around the root zone. Control pots were sprayed with SDW to counter placebo effect. Fungicides difenoconazole @ 100 ppm and garlic extract @ 15 ml/L were prepared and sprayed on disease onset by using hand sprayer. Characteristic symptoms observed were stunting, choloris, wilting that ultimately resulted in the death of tomato plant (Fig. 3). Disease severity was assessed through visual observation. The experiment was laid as CR design with four treatments and eight replications.
Normal agronomic practices were followed throughout the course of the experiment. Plants in each treatment were observed individually for disease scoring. Data on fruit yield were recorded at the termination of this experiment. This included data on fruit size, fruit weight and fruit number per plant.
Disease severity data were recorded on individual plants according to a 0–4 disease scale (Song et al. 2004) (Table 1). Disease severity was recorded at 15 days intervals for six weeks. Disease progress was thus assessed by calculating area under disease progress curve (AUDPC) by using following formula (Madden et al. 2007).
where
- n::
-
Total number of assessment
- Xi::
-
Infection shown in quantity at ith assessment
- Ti::
-
Time at ith assessment
Data regarding fruit weight was recorded on all full-size fruit/plant with a digital balance. Fruit size per plant was determined by water displacement method (Karges et al. 2003) and then averaged for each treatment separately. A graduated cylinder was filled with water to a certain extent and its initial volume (V1) was recorded by observing the lower meniscus of water. Tomato fruit was then placed in the cylinder and the second reading (V2) due to displacement of water was recorded. The difference between the two volumes (V2-V1) was calculated and taken as the fruit size (volume). The number of fruit per plant (replicate) were counted at each picking. The fruit numbers of all the plants (replicate) in a treatment was then averaged.
Data Analysis
Data were subjected to analysis of variance (ANOVA) using statistix 8.1 statistical package. Treatments showing significant differences were separated by least significant difference (LSD) test at 5% level of significance (Steel et al. 1997).
Results
In vitro Assay
Effect of Treatments on Colony Diameter (cm)
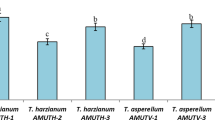
There was a highly significant difference (P = 0.00) between the treatments with regard to colony diameter of the pathogen (Fig. 4). Colony diameter of the pathogen ranged from 1.8–8.7 cm (Table 2). The lowest colony diameter of the pathogen was observed on PDA when grown in dual culture with Trichoderma harzianum (1.8 cm). The second lowest diameter of the pathogen was observed with the fungicides difenoconazole (2.5 cm). Considerably low growth of the pathogen was also observed in plates where PDA was amended with garlic extract (3.2 cm). Conversely, the highest growth of the pathogen was observed in the control plates where no fungicide was used (8.7 cm).
Percent Decrease in Fungal Growth
There were significant differences (P = 0.00) among the treatments with regards to percent decrease of fungal growth over control (Table 2). The values ranged from 63.2–79.3% when comparisons were maded on PDA. Plates having dual cultures of the pathogen and the biocontrol agent showed the highest % reduction (79.3%) in colony diameter of the pathogen which was closely followed by plates amended with fungicide difenoconazole (71.2%). However, the two treatments were statistically different from each other (P = 0.00). The least reduction in growth of the pathogen was observed in plates amended with garlic extracts (63.2%) when compared with control.
In vivo Study
Effect of Treatments on Disease Severity (%) After 15, 30 and 45 Days Post Inoculation
Analysis of data after application of treatments showed significant difference (P = 0.00) in terms of disease severity (Fig. 5).
After 15 days of post inoculation, disease severity recorded in pots treated with the biocontrol agent was 8.50% followed by those treated with difenoconazole (29.8%) and garlic extract (51%) respectively (Fig. 5). Maximum percent disease severity (71%) was observed in untreated control.
A similar trend was observed when data were recorded 30 days post inoculation. There were significant differences among the treatments (P = 0.00). T. harzianum treated pots showed disease severity of 22.12% followed by fungicide difenoconazole (51.87%) and garlic extract (67.5%) respectively (Fig. 5). The highest severity was observed in control pots (73.75%) which did not receive any treatments for disease control. There was a significant difference between garlic extract and untreated control over time after 30 days of inoculation.
The disease severity data revealed that there were significant difference (P = 0.00) among the treatments when disease severity data were recorded after 45 days of inoculation. Lowest disease severity (36%) was observed on tomato plants treated with T. harzianum followed by difenoconazole (66%) and garlic extracts (76%) while the highest disease severity (92%) was recorded in untreated control plants (Fig. 5).
Effect of Treatments on Area Under Disease Progress Curve (AUDPC)
Disease symptoms apparently started simultaneously in treated and untreated pots, but disease progress was prompt in inoculated control which was evident from their maximum average AUDPC values (Fig. 6). Significantly different AUDPC values were observed in plants treated with different treatments when disease progress was monitored at bi-weekly intervals. The average values in various treatments ranged from 124.17 to 749.30% days. Minimum AUDPC values were recorded on plants treated with T. harzianum (124.17% days), followed by difenoconazole (460.73% days) and garlic extracts (641.95% days). Significantly, large AUDPC (749.30% days) was evident in control pots where no treatment was applied. Garlic extract and control treatments were statistically at par with each other.
Effect of Treatments on Yield
Fruit Size (mm)
There was highly significant difference between treatments with respective fruit size (P = 0.00). Fruit size ranged from 22.90–79.62 mm (Table 3). The highest fruit size (79.62 mm) was recorded in pots treated with the biocontrol. This was followed by the plants treated with the fungicide difenoconazole (63.10 mm). However the two treatments were significantly different from each other. The least fruit size was observed in control pots (22.90 mm) which did not receive any treatment for disease control.
Data in Fig. 7 show a strong negative correlation between the disease severity and fruit size (P = 0.00) with correlation coefficient values of −0.98. The result thus revealed a negative relationship between disease severity and fruit size thereby implicating that when severity increases the fruit size decreases proportionately.
Fruit Weight (g)
There were highly significant differences among the treatments (P = 0.00) for their effect on fruit weight (Table 3). The highest value (117.8 g) of fruit weight was observed when plants are inoculated with Trichoderma harzianum as a bio-control and the pathogen. This was followed by plants sprayed with difenoconazole (83.14 g) and garlic extracts (60.52 g). The two treatments were however significantly different from each other. The least fruit weight (29.63 g) was observed for plants kept without any treatment.
Regression analysis of disease severity and yield of tomato cultivar riogrande indicated that there was a simple linear relationship between the two variables (Fig. 8). The curve showed a good fit to the data (R2 = 0.99). The regression equation y = −1.38x + 127.21 indicated that in general, a unit (1%) increase in disease severity value decreased yield of the cultivar by 1.38 g.
Number of Fruits Per Plant
There was significant difference (P = 0.00) among the treatment with regard to number of fruits per plant (Table 3). The number of fruits produced in treated pots ranged from 3–4. However, the treatments were statistically at par with one another. The least number of fruit were observed in control pots (1.3) which were significantly different from the treated pots.
Discussion
In the present study effect of Trichoderma harzianum, garlic extracts and fungicide difenoconazole was assessed for management of fusarium wilt of tomato both in vitro and in planta. In lab study the colony development of the pathogen was strongly inhibited by T. harzianum followed by difenoconazole and garlic extracts. Treatments effect was also significantly different for disease severity, fruit size and fruit weight.
The superiority of T. harzianum can be attributed to a variety of ways in which it brings about disease control. The mode of action of Trichoderma spp. can be mycoparasitism, antibiotics production and competition for space and nutrients (Keswani et al. 2014; Mukesh et al. 2016). Alam et al. (2011) recognize the potentialities of Trichoderma spp, may be due to their ability to release hydrolytic enzymes that degrade cell wall of Fusarium oxysporum f. sp. Lycopersici.
It is a well-known fact that Trichoderma besides parasitizing fungal pathogens and producing antibiotics may have positive effects on plants: increasing growth and yield, fertilizer utilization efficiency, nutrient uptake, percentage and rate of seed germination and induced systemic resistance to plant diseases (Harman et al. 2004). Recently, workers have reported the application of trichompost in addition to other bio inoculants in tomato promoted yield (Akrami et al. 2011; Priyanka et al. 2014). Therefore, the antagonist T. harzianum may be the most promising bio-control agent for F. oxysporum f. sp. lycopersici (Rinu et al. 2013). Ahmed (2011) investigated the growth promoting ability of T. harzianum and a white sterile fungus (which did not fructify) on tomatoes plants grown in soil inoculated with Fusarium oxysporum. However, the antagonistic rhizosphere PGPF (plant growth promoting fungi) suppressed the deleterious soil microbes by competing at the active sites reduced the intensity of disease development and subsequently stimulated the growth and yield of plants.
The effect of garlic extracts was also encouraging during the studies it has been reported that these plants contain antimicrobial compounds which restrict pathogen growth either by inducing systemic resistance or inhibit pathogen growth directly, resulting in control of the disease (Kagales et al. 2004). Allicin and ajeon are volatile bioactive compounds produced by garlic that inhibit spore germination of fungi (Curtis et al. 2004). Ajoene compound from garlic have stronger antifungal activity than alliicin. Ajoene damages the cell walls of fungi (Yoshida et al. 1987). The application of plant extracts should be made soon after the initial appearance of disease symptoms, like the application of fungicides. Similarly, the right concentration and frequency of application should be worked out for each plant extract preparation. Application should be carried out in clear weather condition since these may be washed off by run-off water.
Difenoconazole is a systemic fungicide that works by inhibition of demethylation during ergosterol synthesis. It is applied by foliar spray or seed treatment to control a wide range of leaf, seed and soil-borne diseases caused by Ascomycetes, Basidiomycetes and Deuteromycetes on a variety of crops. Previous studies have shown that systemic fungicides are more effective than protectant fungicide (Shtienberge et al. 1996). Amini and Sidovich (2010) reported that difenoconazole reduced mycelial growth of F. oxysporum f. sp. lycopersici at 10 ppm in vitro, while azoxystrobin and fludioxonil did not. Difenoconazole enhances yield and crop quality by foliar application or seed treatment. Generally concentration used in vitro is 1000–2000 ppm. A report suggested that fungicide @ 10 ppm is also effective. It was paradoxical as to how much concentration of the fungicide should be used in comparison with other treatments. Using higher concentration might overestimate the fungicide while lower concentration is expected to underestimate the efficacy of the fungicide. Keeping in view the above facts a concentration of 100 ppm was selected. Similarly in vivo, a concentration of 500 ppm was used which is not at par with the recommended dose of 1–2 g/L. Using higher amounts would have given a skewed data set while comparing with in vitro data. As such 500 ppm was used. The question whether efficacy of the fungicide was compromised cannot be estimated based on this data. The experiment needs to be repeated with various concentrations of fungicide in comparison with biological control agent to find out a plausible reason.
Conclusion
Bio-control Trichoderma harzianum was effective in controlling Fusarium wilt of tomato by reducing disease severity and increasing yield followed by fungicide difenoconazole. Garlic extracts were found less effective at concentrations tested. Bio-control agent could be exploited for sustainable disease management programs.
References
Abdul-wahab GM (2004) Integrated disease management of some root rot disease in tomato plant. Dissertation, Alexandria University Egypt
Abdullah BA, Mokni S, Khiareddine J, Nefzi H, Ramdi D (2016) Bio control of Fusarium wilt and growth promotion of tomato plant using endophytic bacteria isololated from Solanum elaegnifolium stems. J Phytopath 164:811–824
Agrios GN (2005) Plant Pathology. 5. ed. Academic Press, San Diego
Ahmed M (2011) Management of Fusarium wilts of tomato by soil amendment with Trichoderma harzianum and a white sterile fungus. Ind J Res 5:35–38
Akrami M, Golzary H, Ahmadzadeh M (2011) Evaluation of different combinations of Trichoderma species for controlling Fusarium rot of lentil. Afr J Biotechnol 10:2653–2658
Alam SS, Sakamoto K, Amemiya Y, Inubush K (2011) Biocontrol of soil-borne Fusarium wilt of tomato and cabbage with a root-colonizing fungus, penicillium. EU0013.19th World Con. Soil Sci. Proc. 20–22.
Amini J, Sidovich DF (2010) The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J Plant Prot Res 50:172–178
Curtis H, Noll U, Störmann J, Slusarenke A (2004) Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Eur J Plant Pathol 65(2):79–89. https://doi.org/10.1016/j.pmpp.2004.11.006
Di Pietro A, Madrid MP, Caracuel Z, Delgado-Jarana J, Roncero MIG (2003) Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol Pl Path 4:315–325
Grover RK, Moore JD (1962) Toximetric studies of fungicides against brown rot organisms, Sclerotinia-fructicola and s‑laxa. Phytopath 52(9):876
Haggag WM, Mohammad HAA (2007) Biotechnology aspect of microorganism used in plant biological control. Am J Eur J Sust Agric 1:7–12
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Micro 2:43–56
Haware MP, Nene YL, Natarajan M (1996) Survival of Fusarium oxysporum f. sp lycopersici. Cicero. Pl Dis 66:809–810
Kagales S, Marimuthu T, Thayumanavan B, Nadakumar R, Samiyappan R (2004) Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metal against Rhizoctonia solani and Xanthomonas ryzea pv. Oryzea. Physilog Molecul Plant Pathol 65:91–100
Karges JR, Mark BE, Stikeleather SJ, Worrell TW (2003) Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther 83(2):134–145
Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB (2014) Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. App Micro Biote 98:533–544
Madden LV, Hughes G, Van Den Bosch F (2007) The study of plant disease epidemics. APS Press, St. Paul
MINFAL (2020–2021) Fruit, Vegetables and Condiments Statistics of Pakistan, Government of Pakistan Ministry of Food, Security and Research (Economic Wing) Islamabad. pp 17–18
Mukesh S, Vipul K, Mohammad S, Sonika PA (2016) Trichoderma—a potential and effective bio fungicide and alternative source against notable phytopathogens: a review. Afr J Agri Res 11:310–316
Nelson PE, Tousson TA (1983) Fusarium species. An IIIustrated Manual for identification. University Press, Pennysylvania State, p 193
Patil S, Sriram S, Savitha MJ (2011) Evaluation of non-pathogenic Fusarium for antagonistic activity against Fusarium wilts of tomato. J Bio Cont 25:118–123
Priyanka M, Pooja S, Tripathi NN (2014) Evaluation of plant extracts against Fusarium oxysporum f. sp. lycopersici, wilt pathogen of tomato. Res J Microbiol 9:129–134
Rinu K, Sati P, Pandey A (2013) Trichoderma gamsii (NFCCI 2177): A newly isolated endophytic, psychrotolerant, plant growth promoting and antagonistic fungal strain. J Basic Microbiol 54(5):408–417. https://doi.org/10.1002/jobm.201200579
Shetty S, Thomas B, Shetty V, Bhandary R, Shetty RM (2013) An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens. AYU J Org 34:445–451
Shtienberge D, Blachinsky D, Ben-Hador G, Dinoor A (1996) Effect of growing season and Fungicide Type on the Development of Alternnaria solani and potato yield. Plant Dis 80:994–998
Skidmore AM, Dickinson CH (1976) Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Transac. Br Myco Soc 66(1):57–64
Song W, Zhou L, Yang C, Cao X, Zhang L, Liu X (2004) Tomato Fusarium wilt and its chemical control strategies and hydroponic system. Crop Prot 23:243–247
Steel RD, Torrie JH, Dicky DA (1997) Principles and procedures of statistics: a biomatrical approach, 3rd edn. McGraw Hill Book, New York
Verma M, Brar S, Tyagi R, Surampalli R, Valero J (2007) Antagonistic fungi, Trichoderma spp. panoply of biological control. J Biochem Eng 37:1–20
Wharton P, Kirk W (2012) Early blight of tomato disease. Michigan State University (http//www.tomatodisease.org/earlyblight.html)
Yoshida AS, Kasuga S, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa S (1987) Antifungal activity of ajoene derived from garlic. Appl Environ Microbiol 53:615–617
Acknowledgements
The authors acknowledge Department of Plant Pathology, The University of Agriculture Peshawar, Pakistan for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Ali, S. Hussain, A. Nadeem, S. Ullah and M. Yasin declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, M., Hussain, S., Nadeem, A. et al. Studies on Biological Management of Fusarium Wilt of Tomato. Gesunde Pflanzen 75, 1475–1483 (2023). https://doi.org/10.1007/s10343-022-00800-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00800-5