Abstract
Plants can accumulate and release defensive chemicals by activating various signaling pathways when they are damaged by herbivores or pathogens. The jasmonic acid pathway is activated after damage by chewing herbivores. Here we used jasmonic acid (JA) as an exogenous elicitor to induce feral cabbage plants. In this study, the effects of root JA (RJA) and shoot JA (SJA) induction on the foraging behavior of Cotesia glomerata, a parasitoid of the large cabbage white butterfly Pieris brassicae, was investigated under semi-field conditions. In all combinations of differently induced plants (RJA, SJA and control plants), the percentages of shoot induced plants that were visited by at least one wasp were significantly higher than those of controls or root induced plants during 3 h of foraging. Consequently, parasitism rates of P. brassicae on shoot-JA induced plants were significantly higher than on plants induced with JA to the roots or control plants in all tests. However, this behavioral preference was not reflected in the allocation of offspring. The clutch sizes of C. glomerata eggs on control, root induced and shoot induced plants were not significantly different from each other in two-choice or three-choice experiments, but did differ with clutch size in the two-choice experiment of uninduced control plants versus SJA. This semi-field study helps to further understand the choice behavior and preferences of parasitoids in natural multitrophic communities in which plants induced with root or shoot herbivores occur together.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have evolved intricate direct and indirect defense systems during the course of their coevolution with herbivorous insects and pathogens (Turlings et al. 1995; Dicke and van Loon 2000). Indirect defenses, for instance, may consist of volatiles emitted by plants in response to damage from arthropod pests. These volatiles do not directly influence the performance of the herbivore but can serve as long-distance cues attracting parasitoids of the pests (Vet and Dicke 1992; Dicke 1999; Hilker and Meiners 2002). The emission of herbivore-induced plant volatiles (HIPV) is a general phenomenon. It has been demonstrated in many different plant species, and parasitic wasps, predatory insects and mites have been shown to use HIPV to locate their hosts or prey (Vet and Dicke 1992; Lou and Cheng 2003; Lou et al. 2005a, b). Most of the work on HIPV and their ecological roles have been performed on leaf herbivores and their aboveground natural enemies. However, belowground herbivores may also elicit HIPVs that attract specific natural enemies (Neveu et al. 2002, Ferry et al. 2007; Soler et al. 2007a, b). Interestingly, it was found that feeding activities of root herbivore Delia radicum on Brassica nigra may affect the plant preference in C. glomerata, an endoparasitoid of the leaf chewing herbivore Pieris brassicae, mediated by changes in plant volatiles (Soler et al. 2007a). This suggests that aboveground parasitoids are able to discriminate between aboveground and belowground induced plants.
Studies on the production of HIPV have demonstrated the role of herbivore-specific elicitors (Mattiacci et al. 1995; Alborn et al. 1997; Halitschke et al. 2001). These elicitors can activate various signaling pathways in the plant, resulting in the accumulation and release of specific HIPV bouquets by up-regulating a wide array of defense-related genes (Kessler and Baldwin 2002). Among the signaling pathways that can be activated by herbivores, the jasmonic acid (JA) pathway is the best studied. JA is a natural plant hormone that has been reported to play an important role in the induction of direct and indirect plant defenses after damage by chewing herbivores (Hopke et al. 1994; Dicke et al. 1999; Schmelz et al. 2003), especially for the induction of HIPV serving as cues for parasitoids and predators (Thaler et al. 2001; Moore et al. 2003). For example, exogenous application of JA or methyl-JA to tobacco and tomato plants increased the parasitism or predation rates of the herbivores in natural and agricultural fields (Kessler and Baldwin 2001; Thaler et al. 2001). In Lima bean plants, chemical and behavioral analyses have demonstrated that spider mite damage and JA treatment have similar, although not identical, effects on volatile induction (Dicke et al. 1999). Recently, JA has been used to mimic the induction by herbivores in above- and belowground multitrophic interaction studies (Qiu et al. 2009; van Dam et al. 2010). For studies analyzing the differences between root and shoot induction, the additional advantage of using JA is that the induction treatment is qualitatively and quantitatively more comparable for both organs than inductions with different species of real root and shoot herbivores (van Dam and Oomen 2008).
The effects of JA-induced responses on the development of herbivores and their parasitoids have been investigated in the laboratory (van Dam and Oomen 2008; Qiu et al. 2009). For example, when the large cabbage white butterfly Pieris brassicae (Lepidoptera: Pieridae) and its parasitoid Cotesia glomerata (Hymenoptera: Ichneumonidae) are tested in a JA shoot and root induction system with Brassica oleracea as the host plant, both the herbivore and wasp developed much slower on shoot JA (SJA) induced plants than on root JA (RJA) induced or uninduced control plants (CON). Moreover, the mass of C. glomerata offspring that developed in P. brassicae on SJA plants was significantly less than from those developed from RJA and control plants (Qiu et al. 2009). Interestingly, choice experiments conducted in the greenhouse showed that shoot-induced plants were significantly preferred over RJA or control plants. This was closely correlated with the elevated production of mono-, sesqui- and homoterpenes in SJA plants, whereas in root JA plants only monoterpene levels were induced (van Dam et al. 2010). Under natural field conditions, however, these terpenes may rapidly degrade under the influence of ozone and sunlight (Holopainen 2004). Thus, it remains questionable whether the observed preference in the lab will also be shown in the field. Therefore, we also studied the attraction of JA induced plant volatiles to the parasitic wasp C. glomerata, a parasitoid of foliar herbivore P. brassicae, under semi-field conditions. In addition, we also analyzed the effect on the oviposition decisions of the wasps in terms of egg clutch size. Even if the wasps are very much attracted to shoot induced plants which support lower quality hosts (Qiu et al. 2009) they may decide to allocate fewer eggs under these conditions. So far, few studies have explored the influence of these JA induced volatiles on the foraging behavior and oviposition decisions of hymenopteran parasitoids in field or semi-field conditions (Thaler 1999). This lack of knowledge limits the potential to apply JA and related jasmonates as elicitors of volatiles that can enhance the efficiency of biocontrol agents in cropping systems (Powell and Pickett 2003).
Materials and methods
Plants and insects
The plant species used in this study was a feral strain of Brassica oleracea. Seeds of B. oleracea were initially collected from a road side population near Heteren, The Netherlands in 2000. A subset of these seeds were used to grow ten plants at NIOO-KNAW in Heteren for seed production in 2004. The latter seeds were sown in 1.3 l plastic pots containing soil-sand mixture (30% sand, 5% clay and 65% peat, Potgrond 4, Lentse Potgrond B.V., Lent, NL) in a greenhouse at 21°C (day) and 16°C (night), R.H. 60%, and plants were watered as needed. Natural daylight was supplemented with sodium lamps to maintain the minimum PAR (photosynthetically active radiation) at 225 μmol m−1 s−1 with a photoperiod of 16:8 (L:D).
P. brassicae second instar larvae were obtained from an insect culture maintained at the Laboratory of Entomology, Wageningen University, The Netherlands, where it is reared on B. oleracea (Brussels sprouts cv. Cyrus) plants. C. glomerata was obtained from cultures maintained at the Netherlands Institute of Ecology, Heteren, The Netherlands.
Plant JA induction
B. oleracea plants were used for experiments at four weeks of age when they had five to six fully expanded leaves. Plants were assigned to three treatment groups as described in van Dam and Oomen (2008), i.e., control (CON), root JA application (RJA) and shoot JA application (SJA). Here we used 500 μg JA (Sigma, St Louis, MO, USA) per plant to induce the test plants to mimic the response to chewing root and shoot herbivores. In the RJA group, 500 μg JA per plant was applied in 10 ml 0.1% Triton and 0.5% EtOH in demineralized water (pH 4.0) by injecting the solution in the soil near the root-shoot interface. SJA plants were treated by gently rubbing 0.25 ml of a 2.0 mg ml−1 JA solution in 0.1% Triton and 0.5% EtOH (pH 3.3) in water on the oldest two leaves. Plants in the control group received similar amounts of 0.1% Triton and 0.5% EtOH in acid water (pH 3.7 with HCl) on both roots and shoots. After JA application, each plant was supplied with 50 ml nutrient solution (0.5 Hoagland solution). When 500 μg JA is applied to these B. oleracea plants, the level of secondary compounds (e.g. glucosinolates) significantly increased in B. oleracea 3–14 days after induction (van Dam et al. 2004; van Dam and Oomen 2008) whereas volatile emissions increase within 2–3 days (van Dam et al. 2010).
Experimental set-up
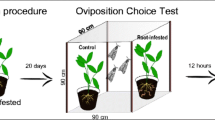
Experiments were performed outdoors (July 2008) in a semi-transparent tent (8 × 4 × 2.5 m) covered with fine-meshed nylon gauze (70 mesh). We considered the tents as semi-filed conditions, as most of the conditions (temperature, humidity, as well as photoperiod) are the same as those in the field, except light quality and the search range of the wasps. In the two-choice experiments (CON vs. RJA, CON vs. SJA, RJA vs. SJA), 12 plants from two treatments (six for each treatment) were alternately placed in the tents (Fig. 1a). In the three-choice experiments, 12 plants from three treatments (four for each treatment) were placed in the tent as depicted in Fig. 1b. The distance between two plants was approximately 0.8 m. All the plants were of similar height and shape to avoid any potential effects of plant morphological characteristics on parasitoid behavior.
Three days before the choice experiments, plants were induced by JA (as described above) in a greenhouse at 21°C (day) and 16°C (night), R.H. 60%, with a photoperiod of 16:8 (L:D). On the day of the experiment, the SJA, RJA and CON plants were moved to the test tents, and ten second instar larvae of P. brassicae were placed on the two untreated leaves of all plant (SJA, RJA and CON plants) at 8:00 am. They were allowed to feed on the plants for at least 3 h. From 11:00 am onwards the plants with the caterpillars were used for testing parasitoid preference. The experiments were repeated five times within five days and on each day the SJA, RJA and CON plants were tested simultaneously with new plants.
Parasitoid preference and oviposition decisions in semi-field tests
Parasitoid preference was investigated using experienced C. glomerata. To obtain experienced C. glomerata, a 3–6 days old female wasp was offered a neonate P. brassicae larva on a fine paint brush for an oviposition experience and then kept separate in a small plastic Petri dish until the experiment started. Two kinds of choice experiments were investigated in the current study: the first consisted of two-choice experiments (RJA-SJA, SJA-CON, and RJA-CON), and the second consisted of three-choice (SJA-RJA-CON) experiments, so parasitoids could select the preferred plants when two or three different treated plants were offered. At the start of the experiment, ten experienced C. glomerata females were released in the middle of a tent, 2 m above the ground. The wasps were allowed to forage freely during 3 h and were then recaptured. Subsequently, P. brassicae larvae were collected from the plants, and dissected to determine the number of parasitized and unparasitized larvae per plant. Based on these counts, we calculated (i) the percentage of RJA, SJA and CON plants selected by at least one parasitoid female among the 12 plants in total, (ii) the percentage of parasitized caterpillars on each plant among the ten larvae in total and (iii) the clutch sizes of eggs, i.e., the mean number of parasitioid eggs in each P. brassicae larva. Clutch sizes in C. glomerata were determined by counting the number of parasitoid eggs under a stereo-microscope. Briefly, five caterpillars were randomly selected and the wasp eggs in their body were determined in each replicate of SJA, RJA and CON treatments, and, as above, five replicates were performed.
Statistical analysis
For the statistical analyses we used Statistica 8.0 software (Statsoft Inc., Tulsa, OK, USA). The percentage of host plants that were visited by the wasps, and percentage parasitism in each treatment were analyzed with non-parametric Kruskal-Wallis ANOVA (Tukey unequal N HSD). The number of parasitioid eggs per host larva on the different plant treatments in each experiment was analyzed using two-way ANOVA (repeat × treatment) with SAS 8.2 software (SAS Institute, Cary, NC, USA). Means were separated using the Student–Newman–Keuls multiple range test at a significance level of α = 0.05.
Results
Plant preference of C. glomerata
When experienced C. glomerata wasps were initially released into the tents, all of them showed intensive searching behavior and high responsiveness. In the choices between CON versus SJA, RJA versus SJA and CON versus RJA versus SJA, the percentages of SJA plants that were visited by at least one wasp were 66.7, 56.7 and 80.0%, respectively, which were significantly higher than those of CON or RJA plants during the 3 h foraging (Fig. 2a, F 1,8 = 12.07, P = 0.0084; Fig. 2c, F 1,8 = 33.33, P = 0.0004 and Fig. 2d, F 2,12 = 13.29, P = 0.0009). In the RJA versus CON experiment, on average more RJA than CON plants were chosen by the C. glomerata wasps, but the difference was not statistically significant (43.3% vs. 56.7%, Fig. 2b, F 1,8 = 1.03, P = 0.3394).
Parasitism rates of P. brassicae on different plants
Dissection of the ten second instar P. brassicae larvae per plant showed that the percentage parasitism of P. brassicae on CON, RJA and SJA plants was different (Fig. 3). In both two-choice experiments (CON vs. SJA, RJA vs. SJA) and the three-choice experiment, parasitism rates of P. brassicae on SJA plants were similar, 55.2, 57.0 and 57.5%, respectively, and these rates were all significantly higher than those observed on CON and RJA plants (Fig. 3a, F 1,8 = 17.15, P = 0.0033; Fig. 3c, F 1,8 = 17.80, P = 0.0029; Fig. 3d, F 2,12 = 16.47, P = 0.0124). While in the CON versus RJA test, the parasitism rates were 45.4% and 50.8% on CON and RJA plants, respectively, which were not significantly different from each other (Fig. 3b, F 1,8 = 0.9016, P = 0.3702).
Clutch sizes of C. glomerata on different plants
C. glomerata females laid a mean of 36.4 eggs per host. The egg clutches laid by C. glomerata in P. brassicae larvae on the differently treated plants were not significantly different to each other in the two-choice of CON versus RJA, RJA versus SJA, nor in the three-choice experiment of CON versus RJA versus SJA (Table 1). However, significant differences were found in the clutch sizes of C. glomerata eggs in P. brassicae larvae on SJA plants compared with those on CON plants (F 1,40 = 4.52, P = 0.0398).
Discussion
The results of this study showed that the location of JA induction can influence the preference and oviposition behavior of an aboveground parasitoid also under semi-field conditions. In greenhouse experiments with the same species, we found significant differences in the induced volatile blends emitted by the plants (van Dam et al. 2010). In the current study, C. glomerata females also exhibited a similarly clear preference for SJA plants over RJA and CON plants under semi-field conditions as observed earlier in a flight-cage experiment under greenhouse conditions (Qiu et al. 2009; van Dam et al. 2010). Other than in the greenhouse (van Dam et al. 2010), the wasps in the field cages did not show a clear preference for RJA plants when they were next to control plants. However, Soler et al. (2007a) reported that, both in flight-cage and semi-field experiments with root-damaged and root-undamaged Brassica nigra (black mustard) plants, C. glomerata females were found to prefer plants without root induction. Even though it has been shown that root herbivore feeding may involve the induction of JA synthesis (Erb et al. 2009), the additional mechanical damage and the contact with regurgitant or microbes from the herbivore cannot be fully mimicked by JA application. This may explain the observed differences in volatile blends induced by JA or real herbivores. In a confined setting like the one that was used in this experiment, it is possible that all the plants in a test can be visited and every host can be parasitized if the experimental duration is prolonged beyond a certain temporal threshold (Soler et al. 2007a). In the present study, the rate of parasitism of P. brassicae on SJA plants was remarkably higher than those on CON and RJA plants in the time allowed for foraging (3 h). Our data showed that the foraging efficiency of experienced parasitoids on SJA plants was much higher than of wasps foraging in an environment consisting of RJA and CON plants.
In the present study, we found that C. glomerata was able to recognize plants treated with jasmonic acid at different positions, as SJA plants were preferred over RJA plants and the controls by parasitoid females. Interestingly, the primary clutch size was not significantly different on CON, RJA and SJA plants in either two- or three-choice experiments although it did differ in the two-choice experiment with CON vs SJA plants. Hence, the female’s preferences did not always match with the hosts that yield the best performance for their offspring. Our earlier study has shown that the masses of both male and female adult wasps that developed from P. brassicae reared on SJA plants were significantly less than those that developed from P. brassicae on CON and RJA plants, thus hosts feeding on SJA plants are not the most profitable for C. glomerata (Qiu et al. 2009). Theoretical models predict that oviposition decisions by parasitoid females lead to the selection of the most profitable host for their offspring (van Alphen and Visser 1990; Godfray 1994), but C. glomerata exhibited the strongest preference for SJA plants which are the least profitable. Generally, parasitoids and especially females with oviposition experience, use herbivore induced-volatiles as cues to identify plants with potential hosts (Steidle and van Loon 2003). Only after landing on the plant and physically contacting the host are they able to assess its suitability as an oviposition site. Here, the caterpillars had been feeding on the plants for a very short time before parasitism (~3 h). Consequently, there may have been insufficient time for the plant to mount a defensive response, explaining why potential differences in host quality were not detected by the parasitoid. Qiu et al. (2009) reported that, although the overall development time of C. glomerata larvae in P. brassicae hosts on SJA plants was less than in wasps developing in P. brassicae hosts on RJA and CON plants, the parasitoid’s pupal stage was shortest on SJA plants. According to the slow-growth, high-mortality hypothesis (Benrey and Denno 1997), choosing a host on SJA plant may shorten the pupal stage period of C. glomerata, thus reducing the probability of being parasitized by secondary hyperparasitoids or predators. It has been reported that cocoons of C. glomerata harbor a diverse number of pupal hyperparasitoids, i.e. Gelis spp., Lysibia nana, Acrolyta nens and Pteromalus semotus (Tanaka et al. 2007; Harvey 2008; Harvey et al. 2009, 2011). The suitability of C. glomerata cocoons for the development of hyperparasitoids decreases linearly with time (Harvey 2008). If these higher trophic levels cause high mortality rates, fast pupal development may be advantageous.
In the field, plants are constantly being exposed to above- and below-ground herbivores, and it has become evident that root and shoot herbivores can influence each other through changes in the shared host plants (Bezemer and van Dam 2005). Other workers have suggested that jasmonates may be used as novel crop protectants enhancing the efficacy of biocontrol agents in cropping systems (Thaler 1999; Powell and Pickett 2003). Our study shows that the way that JA is applied determines to a large extent how JA influences the behavior of natural enemies such as parasitoids. Moreover, our results may help to explain the ecology and evolution of above- and below-ground interactions whilst increasing our understanding of decision-making processes and preferences of key players in communities over several trophic levels.
References
Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949
Benrey B, Denno RF (1997) The slow-growth-high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78:987–999
Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20:617–624
Dicke M (1999) Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp Appl 92:131–142
Dicke M, van Loon JJA (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249
Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25:1907–1922
Erb M, Lenk C, Degenhardt J, Turlings TCJ (2009) The underestimated role of roots in defense against leaf attackers. Trends Plant Sci 14:653–659
Ferry A, Dugravot S, Delattre T, Christides JP, Auger J, Bagneres AG, Poinsot D, Cortesero AM (2007) Identification of a widespread monomolecular odor differentially attractive to several Delia radicum ground dwelling predators in the field. J Chem Ecol 33:2064–2077
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125:711–717
Harvey JA (2008) Comparing and contrasting development and reproductive strategies in the pupal hyperparasitoids Lysibia nana and Gelis agilis. Evol Ecol 22:153–166
Harvey JA, Wagenaar R, Bezemer TM (2009) Life-history traits in closely related secondary parasitoids sharing the same primary parasitoid host: evolutionary opportunities and constraints. Entomol Exp Appl 132:155–164
Harvey JA, Gumovsky A, Gols R (2011) Effect of host-cocoon mass on adult size in the secondary hyperparasitoid wasp, Pteromalus semotus (Hymenoptera: Pteromalidae). Insect Sci (in press)
Hilker M, Meiners T (2002) Induction of plant responses to oviposition and feeding by herbivorous arthropods: a comparison. Entomol Exp Appl 104:181–192
Holopainen JK (2004) Multiple functions of inducible plant volatiles. Trends Plant Sci 9:529–533
Hopke J, Donath J, Blechert S, Boland W (1994) Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid. FEBS Lett 352:146–150
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Ann Rev Plant Biol 53:299–328
Lou YG, Cheng JA (2003) Role of rice volatiles in the foraging behaviour of the predator Cyrtorhinus lividipennis for the rice brown planthopper Nilaparvata lugens. BioControl 48:73–86
Lou YG, Du MH, Turlings TCJ, Cheng JA, Shan WF (2005a) Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J Chem Ecol 31:1985–2002
Lou YG, Ma B, Cheng JA (2005b) Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J Chem Ecol 31:2357–2372
Mattiacci L, Dicke M, Posthumus MA (1995) Beta-glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA 92:2036–2040
Moore JP, Paul ND, Whittaker JB, Taylor JE (2003) Exogenous jasmonic acid mimics herbivore-induced systemic increase in cell wall bound peroxidase activity and reduction in leaf expansion. Funct Ecol 17:549–554
Neveu N, Grandgirard J, Nenon JP, Cortesero AM (2002) Systemic release of herbivore-induced plant volatiles by turnips infested by concealed root-feeding larvae Delia radicum L. J Chem Ecol 28:1717–1732
Powell W, Pickett JA (2003) Manipulation of parasitoids for aphid pest management: progress and prospects. Pest Manag Sci 59:149–155
Qiu B-L, Harvey JA, Raaijmakers CE, Vet LEM, van Dam NM (2009) Non-linear effects of plant root and shoot jasmonic acid application on the performance of Pieris brassicae and its parasitoid Cotesia glomerata. Funct Ecol 23:496–505
Schmelz EA, Alborn HT, Banchio E, Tumlinson JH (2003) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673
Soler R, Harvey JA, Kamp AFD, Vet LEM, van der Putten WH, van Dam NM, Stuefer JF, Gols R, Hordijk CA, Bezemer MT (2007a) Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant-volatile signals. Oikos 116:367–376
Soler R, Bezemer TM, Cortesero AM, van der Putten WH, Vet LEM, Harvey JA (2007b) Impact of foliar herbivory on the development of a root-feeding insect and its parasitoid. Oecologia 152:257–264
Steidle JLM, van Loon JJA (2003) Dietary specialization and infochemical use in carnivorous arthropods: Testing a concept. Entomol Exp Appl 108:133–148
Tanaka S, Nishida T, Ohsaki N (2007) Sequential rapid adaptation of indigenous parasitoid wasps to the invasive butterfly Pieris brassicae. Evolution 61:1791–1802
Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399:686–688
Thaler JS, Stout MJ, Karban R, Duffey SS (2001) Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecol Entomol 26:312–324
Turlings TCJ, Loughrin JH, McCall PJ, Rose USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92:4169–4174
van Alphen JJM, Visser ME (1990) Superparasitism as an adaptive strategy for insect parasitoids. Ann Rev Entomol 35:59–79
van Dam NM, Oomen WAT (2008) Root and shoot jasmonic acid applications differentially affect leaf chemistry and herbivore growth. Plant Sign Behav 3:91–98
van Dam NM, Witjes L, Svatos A (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161:801–810
van Dam NM, Qiu B-L, Hordijk CA, Vet LEM, Jansen JJ (2010) Identification of biologically relevant compounds in aboveground and belowground induced volatile blends. J Chem Ecol 36:1006–1016
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol 37:141–172
Acknowledgments
The authors thank Roel Wagenaar and Gregor Disveld (NIOO-KNAW) for their assistance with plant rearing. NM van Dam was funded by a NWO VIDI grant, no. 864-02-001, the Netherlands Organization for Scientific Research, and BL Qiu is granted by the China National Natural Science Foundation (30871678) and China Scholarship Programs no. 2006-3036.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Torsten Meiners
Rights and permissions
About this article
Cite this article
Qiu, BL., van Dam, N.M., Harvey, J.A. et al. Root and shoot jasmonic acid induction differently affects the foraging behavior of Cotesia glomerata under semi-field conditions. BioControl 57, 387–395 (2012). https://doi.org/10.1007/s10526-011-9410-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-011-9410-6