Abstract
Jasmonate signaling pathway plays an important role in induced plant defense against herbivores and pathogens, including the emission of volatiles that serve as attractants for natural enemies of herbivores. We studied the volatiles emitted from rice plants that were wounded and treated with jasmonic acid (JA) and their effects on the host-searching behavior of the rice brown planthopper, Nilaparvata lugens (Stål), and its mymarid egg parasitoid Anagrus nilaparvatae Pang et Wang. Female adults of N. lugens significantly preferred to settle on JA-treated rice plants immediately after release. The parasitoid A. nilaparvatae showed a similar preference and was more attracted to the volatiles emitted from JA-treated rice plants than to volatiles from control plants. This was also evident from greenhouse and field experiments in which parasitism of N. lugens eggs by A. nilaparvatae on plants that were surrounded by JA-treated plants was more than twofold higher than on control plants. Analyses of volatiles collected from rice plants showed that JA treatment dramatically increased the release of volatiles, which included aliphatic aldehydes and alcohols, monoterpenes, sesquiterpenes, methyl salicylate, n-heptadecane, and several as yet unidentified compounds. These results confirm an involvement of the JA pathway in induced defense in rice plants and demonstrate that the egg parasitoid A. nilaparvatae exploits plant-provided cues to locate hosts. We explain the use of induced plant volatiles by the egg parasitoid by a reliable association between planthopper feeding damage and egg presence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that plants respond to attack by specific herbivore species and tailor their induced direct and indirect defenses accordingly (Karban and Baldwin, 1997). Chemical defenses that target the herbivore directly result in herbivore death or retarded development (Barbosa et al., 1991; Karban and Baldwin, 1997; Agrawal, 1999; Lou and Baldwin, 2003; Sznajder and Harvey, 2003), whereas indirect defenses increase herbivore mortality through the recruitment of parasitoids and predators with volatile signals (Thaler, 1999; Kessler and Baldwin, 2001). Indirect plant defenses have been intensively studied since the late 1980s, and to date, this phenomenon has been reported in more than 23 plant species (see reviews in Vet and Dicke, 1992; Dicke, 1999; Sabelis et al., 1999; Turlings and Wäckers, 2004). Studies on the mechanisms leading to the production of herbivore-induced plant volatiles have revealed the role of herbivore-specific elicitors (Mattiacci et al., 1995; Alborn et al., 1997; Halitschke et al., 2001). These elicitors can activate various signaling pathways in the plant, causing an up-regulation of a large array of defense-related genes through cross-talk and resulting in accumulation or release of defense chemicals (Kessler and Baldwin, 2002).
Among these signaling pathways, the jasmonic acid (JA) pathway is the best studied and has been reported to play an important role in induced plant direct and indirect defenses (Hopke et al., 1994; Boland et al., 1995; Dicke et al., 1999; Schmelz et al., 2003). In the wild tobacco plant Nicotiana attenuata, for example, exogenous application of MeJA increases the release of volatile organic compounds (Halitschke et al., 2000), which enhances the mortality rates of the herbivores by attracting the natural enemies of herbivores (Kessler and Baldwin, 2001). Moreover, antisense suppression of a lipoxygenase gene LOX3, a specific wound- and herbivory-induced isoform involved in JA biosynthesis in N. attenuata, results in decreases in release of volatiles and nicotine and trypsin protease inhibitor levels (Halitschke and Baldwin, 2003). Exogenous application of JA to tobacco and tomato plants promotes parasitism and predation of the herbivores by natural enemies in nature (Thaler, 1999; Kessler and Baldwin, 2001). Chemical and behavioral analyses demonstrate that spider mite damage and JA treatment have similar, although not identical, effects on volatile induction in Lima bean plants (Dicke et al., 1999). In maize, caterpillar-induced volatile emissions are positively correlated with increased JA levels (Schmelz et al., 2003). Ozawa et al. (2004) reported that maize plants treated with JA attract specialist parasitoids under laboratory conditions. In rice, the world’s most important food crop, the role of JA signaling has been mainly studied for direct defenses. Exogenous application of JA on rice plants elicits the productions of proteinase inhibitors, phytoalexins, PRs, and salt-induced proteins (Tamogamia et al., 1997; Rakwal and Komatsu, 2000; Rakwal et al., 2001; Kim et al., 2003), and it may increase the emission of volatiles (Obara et al., 2002).
In this study, we investigated the effect of JA application to rice plants on the host-searching behavior of the rice brown planthopper Nilaparvata lugens and its mymarid egg parasitoid Anagrus nilaparvatae. N. lugens is one of the most important rice pests. It feeds on the plant’s phloem and causes a decrease in leaf area, plant height, dry weight, leaf and stem nitrogen concentration, chlorophyll contents, and photosynthetic rate, but an increase in free amino acids, sucrose, and leaf iron content (Rubia-Sanchez et al., 1999; Watanabe and Kitagawa, 2000). The parasitoid A. nilaparvatae is a major natural enemy of the rice planthoppers. Previous studies have shown that rice volatiles play an important role in host plant location by N. lugens (Liu et al., 2002), and the volatiles emitted from rice plants in response to N. lugens attack attract the parasitoid (Lou and Cheng, 1996; Lou et al., 2002). However, little to nothing is known about the effect of JA application on rice volatiles and in turn on host-searching behavior of N. lugens and the parasitoid.
To determine if JA induces emission of volatiles that affect the host-searching behavior of the herbivore and the parasitoid, we first measured their responses to JA-treated plants and control plants in the laboratory. In additional greenhouse and field experiments, we then tested if JA treatment of rice plants enhanced the parasitism of N. lugens eggs by the parasitoid. Finally, we collected and identified volatiles that were released from JA-elicited and the control plants.
Methods and Materials
Plant Growth
The rice variety used was TN1, which is susceptible to N.lugens (Lou and Cheng, 2003). Pregerminated seeds were sown in a greenhouse, and after 20–25 d, the seedlings were transplanted into small clay pots (8-cm diam × 10-cm height) each with one plant or big clay pots (16-cm diam × 10-cm height) each with three or six plants. For three plants per pot, they were arranged in an equilateral triangle 8 cm apart; for six plants per pot, they were arranged in two rows each with three plants and 8 cm between rows and 2 cm between plants. Plants were watered daily, and each pot was supplied with 10 ml of nutrient solution [Ca(NO3)2·4H2O, 0.5g/l; K(NO3)2·4H2O, 0.125g/l; MgSO4·7H2O, 0.125g/l; K2HPO4, 0.125g/l; FeCl2, 0.005g/l] every 3 d. All plants were placed in a controlled climate room that was maintained at 23 ± 2°C, 70% r.h., and 18 hr photophase (25,000 lx). The plants were used for experiments 25–30 d after potting. Plantings were continued at regular intervals so that enough plants of suitable age were available for experiments.
Insects
The N. lugens culture was originally obtained from the China National Rice Research Institute (CNRRI), Fuyang, Zhejiang, and maintained on TN1 rice plants in a greenhouse. Late instar nymphs of N. lugens were captured from the greenhouse and reared on potted TN1 rice plants, which were confined in plastic cages (11-cm diam × 40 cm high). The caged rice plants were maintained in a controlled climate room at 28 ± 2°C, 12-hr photophase, and 70–80% r.h. Newly emerged adults of N. lugens were collected daily and fed on potted fresh TN1 rice plants. Using this procedure, N. lugens adults of uniform age were obtained.
A laboratory colony of the egg parasitoid A. nilaparvatae was started from individuals trapped in rice fields in Hangzhou using TN1 rice plants with N. lugens eggs as bait. The colony was propagated on N. lugens eggs in rice shoots enclosed in glass tubes (2.5-cm diam × 20-cm height), which were kept in a controlled climate room at 28 ± 2°C, 12-hr photophase, and 70–80% r.h. Each day, the newly emerged wasps were collected into clean glass tubes (2.5-cm diam × 20-cm height), with access to both water and honey solution, and held for at least 2 hr to ensure mating. From the second generation onwards, female parasitoids were used in experiments less than 24 hr after emergence.
Plant Treatment
The potted plants were washed with running water and trimmed to leave one, three, or six plants for each pot. Plants were individually damaged with a needle at the lower and upper position of rice stems each with 200 pricks, and then each damage site was treated by applying 20 μl of 10 or 1 mM jasmonic acid in 50 mM sodium phosphate buffer (titrated with 1 M citric acid until pH 8) (JA). Control plants (BUF) were wounded the same way and treated with 20 μl of the buffer on each of the two damaged sites. Plants were treated at 1700 hr, and then the plants were placed in the controlled climate room that was maintained at 28 ± 2°C, 12-hr photophase, and 80% r.h. Fifteen hours after treatment, i.e., at 0800 hr the next day, plants were used for experiments.
Effect of JA Elicitation on Host Plant Choice by N. lugens
Pots with six plants each were used for this experiment. Three plants in one row were wounded and treated with either 10 or 1 mM JA, and plants in the other row were wounded and treated with the buffer, thus obtaining two types of treatment pairs: 10 mM JA-treated plants vs. the buffer-treated plants or 1 mM JA-treated plants vs. the buffer plants. The potted plants were then individually placed into a sleeve cage (25 cm long, 25 cm wide, 50 cm high) that was maintained in a controlled climate room at 28 ± 2°C, 12-hr photophase, 80% r.h. Fifteen hours after treatment, 30 macropterous N. lugens females (2 d old) were introduced into each cage. Subsequently, the number of N. lugens on JA-elicited and buffer-elicited plants were recorded 1, 2, 3, 4, 6, 12, 24, 36, 48, 60, and 72 hr after their release, respectively. Each of the two experiments was replicated five times.
Effect of JA Elicitation on Host-Searching Behavior of the Parasitoids
Olfactometer Test
Responses of A. nilaparvatae females to rice volatiles were measured in a Y-tube olfactometer. The olfactometer consisted of a Y-shaped glass tube of 1-cm diam. The base and the two arms of the Y tube were all 10 cm in length. Each arm was connected to an odor source container (a glass box, 10 × 10 × 30 cm). An air stream was generated and was divided in two, and each secondary air stream was led through a flowmeter, a tube with active charcoal, a humidifier bottle, and one of the odor containers. Subsequently, the two airstreams were led through the two arms of the Y-tube olfactometer at 150 ml/min. The Y-tube olfactometer was placed in a box painted white with an artificial light source consisting of a single 25-W lamp placed above the arms of the Y tube. All bioassays were conducted between 0900 and 1700 hr. During experiments, the temperature in the room was maintained at 25–28°C.
A. nilaparvatae females had the choice between odors from 1 mM JA-treated plants vs. the buffer-treated plants or 10 mM JA-treated plants vs. buffer-treated plants. To test for a possible effect of the treatment solutions per se, we added an experiment without plants, but with the solutions applied to filter paper (40 μl of 10 mM JA vs. 40 μl of the buffer). Fifteen hours after treatment, 10 plants that were individually planted in pots of each treatment were cut off at soil level, the cut stem was wrapped with wet cotton, and the entire plants were placed into one of the odor source containers. Mated female parasitoids were introduced individually into the base tube of the Y-shaped olfactometer and given 10 min to walk toward the end of one of the arms. Choice for an odor source was defined as a female crossing a line 7 cm after the division of the base tube and remaining there for at least 1 min. If a parasitoid did not make a choice within 10 min, this was recorded as no response. After testing two females, the olfactometer tube was washed with 98% alcohol and then was heated at 80°C for several minutes. To remove any asymmetrical bias, connections of the two arms of the olfactometer to the odor source containers were exchanged after testing two females, and the odor source containers were exchanged after testing eight females. The odor sources were replaced by a new set of 10 plants after testing 16 wasps, and for each odor source combination, at least 32 females were tested.
Greenhouse Experiment
Two plants, each with about 80–100 1-d-old N. lugens eggs, were transplanted into the center of a triangle of three plants that had been wounded 15 hr earlier and treated with either 10 mM JA, 1 mM JA, or with buffer. To obtain plants with N. lugens eggs, they were individually infested for 1 d with 10 gravid N. lugens females that were placed in two parafilm bags at the upper and lower position of the plant stems. After removal of the females, plants with 80–100 eggs were chosen. For each treatment, five pots were randomly placed into a cage (length 2.0 m, width 1.5 m, height 1.5 m) covered with nylon net into which 60 mated A. nilaparvatae females were introduced. The experiment was carried out in a greenhouse maintained at 24 ± 4°C. Two days later, the parasitoids were removed, and each pot was confined in a plastic cage (6.5 × 32 cm), all of which were placed into a controlled climate room at 28 ± 2°C, 12-hr photophase, and 80% r.h. Five days after placing them in the climate room, the plants were dissected, and the total of parasitized N. lugens eggs (the eggs become red) was recorded. The experiment was replicated five times.
Field Experiment
The treatments for plants were the same as the greenhouse experiment. Both JA (10 or 1 mM)-elicited plants and the buffer-elicited plants were placed at 10 locations in a rice field (20 × 30 m) in October 2000 (Figure 1). The field was surrounded by rice fields with plants in the “heading” stage. Each location included three pots of plants, each pot with one of the three treatments. The three-pot groups were arranged in two rows, each included five groups placed 5 m apart. The distance between the two rows was 10 m (Figure 1). Two days after the plants were introduced into the rice field, the plants were transferred to the controlled climate room at 28 ± 2°C, 12-hr photophase, and 80% r.h., and each pot of plants was confined in an 11-cm diam × 40-cm-high plastic cage (herbivores, predators, and parasitoids on plants were all removed). Five days later, the plants were cut off at the soil level and dissected under a microscope to record the total, the parasitized, and the predated (sucked empty) N. lugens eggs. Parasitized eggs were carefully placed into petri dishes (6 cm in diam) that were lined with wet Whatman No. 1 filter paper. When the parasitoids were emerged, the species were identified.
Arrangement of the plants that were wounded and treated with 40 μl of either 10 mM JA in 50 mM sodium phosphate buffer (pH = 8), 1 mM JA in the buffer, or the buffer in a rice field. Numbers indicate locations, and each location includes three pots of plants, 10 mM JA-elicited plants, 1 mM JA-elicited plants, and the buffer-elicited plants.
Collection, Isolation, and Identification of the Volatile Compounds
The volatile collection system has been described in detail by Turlings et al. (1998). It consists of six vertically placed cylindrical glass tubes (9.5-cm i.d., 54 cm high). A split Teflon plate with a hole in the center at the base of a cylinder closed loosely around the stem of a plant, allowing the separation of the aerial part of a plant, in the cylinder, from the pot, which remained outside (Turlings et al., 1998). Purified and humidified air was pushed into each cylinder at a rate of 1 l/min and flowed over the plant. Around the base of each cylinder, just above the Teflon disk, eight openings served as ports that could hold the collection traps. Only one port was used during an experiment. For collections, air was pulled (0.8 l/min) through a Super-Q adsorbent trap (Heath and Manukian, 1994), whereas the rest of the air vented out through the hole in the bottom, thus preventing impure air from entering. The automated part of the collection system (Analytical Research System, Gainesville, FL, USA) controlled the flow through the trap. The climate chamber (CMP4030, CONVIRON, Winnipeg, Canada), in which the collection cylinders were housed, was kept at 17.5°C; because of the irradiation heat, the temperature inside the cylinders was 23 ± 3°C. During the light cycle, light intensity was about 20,000 lm/m2.
Volatiles emitted from nonmanipulated plants and plants that were wounded and treated with either 10 mM JA or the buffer were collected. We also collected the volatiles from a blank, only a pot of soil without plants, to check if the system is clean. Collections started immediately after lights went on, 15 hr after treatment. Each collection lasted 4 hr. After each collection, traps were extracted with 150 μl methylene chloride (Lichrosolv., Merck, Whitehouse Station, NJ, USA), and 200 ng of n-octane and nonyl acetate (Sigma, Switzerland) in 10 μl of methylene chloride was added to the samples as internal standards. Each treatment was replicated six times.
Analyses were carried out with an HP 6890 series gas chromatograph equipped with an automated on-column injection system (HP G1513 A) and a flame ionization detector. Of each sample, a 3-μl aliquot was injected onto an apolar SE-30 capillary column (30 m, 0.25-mm i.d., 0.25-μm film thickness, Alltech, Deerfield, IL, USA) preceded by a deactivated retention gap (5 m, 0.25-mm i.d.) and a deactivated precolumn (30 cm, 0.530 mm). Helium (24 cm/sec) was used as carrier gas. After injection, the column temperature was maintained at 40°C for 3 min, increased to 230°C at 8°C/min, and held at 230°C for 9.5 min. The detector signal was processed with HP GC Chemstation software.
To identify compounds, we collected volatiles emitted from 10 mM JA-elicited plants for 10 hr. A 3-μl aliquot from this sample was injected onto the same column and analyzed using the same temperature program. Volatiles were detected by a Hewlett-Packard 5973 mass selective detector (transfer line 230°C, source 230°C, quadrupole 150°C, ionization potential 70 eV, scan range 50–400 amu). Compounds were identified by comparison of GC retention times with those of authentic standards and by comparison of mass spectra with spectra of a NIST database.
Data Analysis
Differences in behavioral responses of the parasitoid to JA-induced rice volatiles and the buffer-induced volatiles were determined by chi-square tests, whereas differences in behavioral responses of female N. lugens adults were determined by t-tests. To test for differences in parasitism among the treatments, we used the Fieldman rank sum test. Comparison of the data on plant volatiles was analyzed by MANOVA after the data were log transformed. If the MANOVA analysis was significant (P < 0.05), univariate ANOVAs for the individual effects and Fisher LSD post hoc tests to detect significant differences between groups were conducted. Data were analyzed with Statistica (Statistica, SAS Institute Inc., Cary, NC, USA).
Results
Effect of JA-Elicited Plants on Host Preference of N. lugens
N. lugens female adults were recovered consistently more often from the JA-treated plants than from the buffer-treated plants, but this apparent preference was only significant for the 10 mM JA dose at 1 hr after the start of the experiment (Figure 2). The data may not suffice to conclude that JA induction renders the plants attractive to N. lugens females, but it can be concluded that the induced volatiles are not repellent.
Mean (±SE, N = 5) number of Nilaparvata lugens female adults on pairs of plants that were wounded and treated with 40 μl of 1 mM JA in 50 mM sodium phosphate buffer (pH = 8) (1 mM JA) vs. plants that were wounded and treated with 40 μl of buffer (Buf) (A), or that were wounded and treated with 40 μl of 10 mM JA in buffer (10 mM JA) vs. Buf (B), 1–72 hr after five replicated plant pairs were exposed to 30 insects. The exposure of the plants started 15 hr after the treatment. Asterisks indicate significant differences between members of a pair (JA vs. buffer, P < 0.05, t-test).
Effect of JA-Elicited Plants on Host-Searching Behaviors of the Parasitoids
In olfactometer tests, A. nilaparvatae preferred the volatiles emitted from JA-elicited plants to those emitted from the buffer-treated plants. JA itself did not attract the parasitoids (Figure 3).
Number of Anagrus nilaparvatae female adults attracted by volatiles released from either pairs of plants that were wounded and treated with 40 μl of 1 mM JA in 50 mM sodium phosphate buffer (pH = 8) (1 mM) vs. plants that were wounded and treated with 40 μl of the buffer (Buf), plants that were wounded and treated with 40 μl of 10 mM JA in the buffer vs. Buf, or a pair of chemicals, 40 μl of 10 mM JA vs. 40 μl of the buffer without plants [10 mM vs. Buf (NP)]. The plants were used 15 hr after the start of treatment. Asterisks indicate significant differences between members of a pair (JA vs. buffer, P < 0.05, chi-square test).
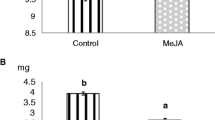
In the greenhouse experiment, parasitism of N. lugens eggs by A. nilaparvatae on plants that were surrounded by JA-treated plants was higher than on the buffer-treated plants (Figure 4A, Q = 8.40, P = 0.008), especially for the 10 mM JA-treated plants on which the parasitism of N. lugens eggs by A. nilaparvatae was 2.35-fold higher than those on the control plants.
Mean (±SE) parasitism rates (%) of N. lugens eggs by A. nilaparvatae in the greenhouse (A, N = 5) or field (B, N = 10) condition on rice plants surrounded by plants that were wounded and treated with either 40 μl of 1 mM JA in 50 mM sodium phosphate buffer (pH = 8) (1 mM), 40 μl of 10 mM JA in the buffer (10 mM), or 40 μl of the buffer (Buf). Fifteen hours after treatment, the plants were exposed to the parasitoid or the field for 2 d. The differences in parasitism among the treatments were determined by Fieldman rank sum test.
During the field experiment, only A. nilaparvatae wasp was observed parasitizing N. lugens eggs. As in the greenhouse experiment, JA treatments increased parasitism of N. lugens eggs by A. nilaparvatae (Figure 4B, Q = 12.80, P = 0.001). When 40 μl of 1 or 10 mM JA was applied to wounded plants, the parasitism of N. lugens eggs by A. nilaparvatae on plants that were surrounded by JA-treated plants were 1.39- and 1.85-fold higher than those on the control plants, respectively. Cyrtorhinus lividipennis Reuter (Hemiptera: Miridae) is the main predator of N. lugens eggs in the rice field. We found a tendency for predation rates of N. lugens eggs to be higher on JA-treated plants (1 mM JA 3.21 ± 1.00%; 10 mM JA 7.25 ± 5.07%; buffer 1.58 ± 0.66%), but the differences were not statistically significant (F = 0.944, df = 2,27, P = 0.402).
Analysis of Volatiles
Collection and analysis of the volatiles revealed that only small amounts were released by nonmanipulated rice plants, whereas the buffer- and JA-treated plants emitted 9.43 and 35.68 times larger amounts, respectively (Table 1; Figure 5). Most compounds were released in significantly larger amounts by JA-treated plants compared to buffer-treated plants: 2-heptanone, 2-heptanol, limonene, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene, (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, beta-caryophyllene, (E)-alpha-bergamotene, n-heptadecane, (E)-nerolidol, and two unknown chemicals (unknowns 1 and 2) (Table 1; Figure 5).
Typical chromatograms obtained from headspace collections from an empty glass container (a pot of soil without plants) (CK), untreated rice plants (TN1 H), or rice plants 15 hr after they were wounded and treated with either 40 μl of 50 mM sodium phosphate buffer (pH = 8) (TN1 BF) or with 40 μl of 10 mM JA in the buffer (TN1 JA). (1) 2-Heptanone; (2) 2-heptanol; (3) unknown 1; (4) unknown 2; (5) limonene; (6) unknown 3; (7) unknown 4; (8) linalool; (9) (E)-4,8-dimethyl-1,3,7-nonatriene; (10) methyl salicylate; (11) beta-caryophyllene; (12) (E)-alpha-bergamotene; (13) n-heptadecane; (14) (E)-nerolidol; (15) (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene.
Discussion
As reported for other plants, such as tobacco (Halitschke et al., 2000), maize (Schmelz et al., 2003), and lima bean (Dicke et al., 1999), wounding and application of JA to rice plants also resulted in an increase in volatiles emitted. The overall emission was almost fourfold higher than the emission of the buffer-treated plants. The increases involved aliphatic aldehydes and alcohols, monoterpenes, sesquiterpenes, methyl salicylate, n-heptadecane, and some unknown chemicals (Table 1; Figure 5). The composition of the odor blend was in part consistent with previous results reported for JA-treated rice plants (Obara et al., 2002). We too found a JA-mediated increase in the release of limonene, linalool, methyl salicylate, beta-caryophyllene, (E)-alpha-bergamotene, and several unknown chemicals. However, there are also some differences: the compounds 2-heptanone, 2-heptanol, n-heptadecane, (E)-4,8-dimethyl-1,3,7-nonatriene, (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, and (E)-nerolidol that we collected from the headspace of JA-treated plants were not reported by Obara et al. (2002). In turn, we did not detect the sesquiterpenes alpha-copaene, alpha-cadinene, alpha-humulene, and several others, which were identified by Obara et al. (2002). These differences may be due to treatment differences; they collected volatiles emitted from pieces of rice leaves (2.5 cm long) that were floating on a 0.5 mM JA solution for 7 or 48 hr. In maize, for example, it has been observed that excised leaves produced a 2.5- to 8.0-fold greater volatile in response to JA and the caterpillar produced elicitor volicitin than similarly treated intact plants (Schmelz et al., 2001). Herbivore- or elicitor-induced volatile releases also vary with time after treatment (Turlings et al., 1998), herbivore damage level (Gouinguene et al., 2003), and the applied elicitor amount (Halitschke et al., 2000). Compared to the nonmanipulated rice plants, wounding and application of the buffer also increased release of some volatiles (Figure 5), suggesting that wounding alone may be sufficient to induce a minor release of at least some of the compounds and that application of JA fortifies this effect.
The volatiles emitted from JA-treated rice plants were attractive to the parasitoid (Figure 2) and enhanced parasitism of N. lugens eggs in the greenhouse and field (Figures 3 and 4). This result is consistent with results reported from other plants (Thaler, 1999; Kessler and Baldwin, 2001). We have previously shown that linalool is attractive to A. nilaparvatae (Lou et al., 1999). The higher linalool concentrations in the headspace of JA-induced plants compared to the buffer-induced plants might in part explain the difference in attractiveness. Which other compounds may be involved in the attraction remains to be elucidated.
We also studied the effect of treating rice plants with JA on the settling behavior of adult N. lugens females. A negative effect was expected, as JA is an important defense-related plant hormone. JA not only induces plants to release volatiles, but also elicits increases in many nonvolatile defense chemicals, such as phenolics, alkaloids, terpenoids, and proteinase inhibitors (Karban and Baldwin, 1997; Lou and Baldwin, 2003, 2004). These nonvolatile compounds are likely to affect the settling behavior of the planthoppers. Although numerous studies have shown that JA application results in reduced preference and performance of herbivores (Karban et al., 1997; Karban and Baldwin, 1997; Black et al., 2003), contrary effects have also been reported. For example, volatiles emitted by potato plants in responses to JA application enhanced the plant’s attractiveness to female Colorado potato beetles (Landolt et al., 1999). In wild radish, responses induced by JA application increased feeding by some herbivores (Agrawal, 2000; Agrawal and Sherriffs, 2001). In addition, N. lugens female adults tended to prefer 10 mM JA-elicited plants to the buffer. The fact that this difference was only apparent 1 hr after release suggests that JA-induced rice volatiles were slightly attractive to female N. lugens, whereas JA-induced nonvolatile chemicals had no particular effect on host preference. The latter is somewhat surprising as JA elicitation increases the production of proteinase inhibitors, phytoalexins, and PRs in rice plants (Rakwal and Komatsu, 2000; Rakwal et al., 2001; Kim et al., 2003). N. lugens female adults are attracted to limonene (Lou et al., unpublished data). Hence, a slightly stronger attraction of JA-induced rice volatiles to N. lugens compared to the control can be related to higher levels of this and/or other compounds.
Thaler (1999) was the first to report that inducing plants with jasmonic acid increases parasitism of caterpillar pests in an agricultural field. Subsequent studies confirmed that JA treatment enhances the attractiveness of plants for parasitoids of lepidopteran larvae (Ozawa et al., 2004) and predatory mites that use induced plant volatiles to locate spider mite prey (Gols et al., 2003). These studies hold promise that field application of JA may enhance the efficacy of parasitoids and predators as biological control agents. Our results indicate that this may also be the case for egg parasitoids. The plants with N. lugens eggs that were used in our experiments were not elicited with JA; they were just placed near JA-treated plants. The supposed attraction of the wasps to the JA-treated plants did not prevent them from visiting neighboring plants, where they parasitized eggs. We have previously shown that the wasp does not discriminate between the volatiles emitted from N. lugens-infested plants and those from JA-induced plants (Wang et al., 2005). Therefore, it is likely that wasp uses only few or a general blend of induced volatiles to locate plants that are potentially infested by its host, and that specific close-range attraction to the host is mediated by visual cues and/or kairomones released from N. lugens. Kairomones are attractive to the parasitoid at close range for all developmental stages of N.lugens (Lou and Cheng, 1994). A general attraction to induced plant volatiles is also evident from the fact that the volatile profiles from N. lugens-infested plants and JA-elicited plants are quite different, but both blends are attractive to wasps (Wang et al., 2005). In fact, the study by Wang et al. (2005) shows that N. lugens infestation does not trigger the JA pathway. However, some evidence is available showing the involvement of salicylic acid (SA) and ethylene in response to N. lugens feeding (Du et al., unpublished data). Hence, different defense pathways result in different volatile emissions, but all are attractive to wasps.
Egg parasitoids have been shown to respond to oviposition-induced plant volatiles in other studies, where herbivore feeding did not induce the volatiles attracting the egg parasitoids (Hilker et al., 2002; Hilker and Meiners, 2002; Meiners and Hilker, 2000). A. nilaparvatae females are equally attracted to volatiles emitted by rice plants infested by female N. lugens adults as to those from nymph-infested plants (Lou, unpublished data). Therefore, here we expect that the feeding damage is responsible for the induction. However, we do not rule out an effect of oviposition as well, especially because an ovicidal response of rice plants to oviposition by the rice white-backed planthopper Sogatella furciferra has been observed (Suzuki et al., 1996; Yamasaki et al., 2003). As the rice brown planthopper feeds and oviposits on the same plant (Cheng and He, 1996), the indirect association of feeding with egg presence is reliable and thus adaptive.
References
A. A. Agrawal (1999) ArticleTitleInduced responses to herbivory in wild radish: Effects on several herbivores and plant fitness Ecology 80 1713–1723
A. A. Agrawal (2000) ArticleTitleSpecificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars Oikos 89 493–500 Occurrence Handle10.1034/j.1600-0706.2000.890308.x
A. A. Agrawal M. F. Sherriffs (2001) ArticleTitleInduced plant resistance and susceptibility to late-season herbivores of wild radish Ann. Entomol. Soc. Am. 94 71–75
H. Alborn T. C. J. Turlings T. H. Jones G. Stenhagen J. H. Loughrin J. H. Tumlinson (1997) ArticleTitleAn elicitor of plant volatiles from beet armyworm oral secretion Science 276 945–949 Occurrence Handle10.1126/science.276.5314.945
P. Barbosa P. Gross J. Kemper (1991) ArticleTitleInfluence of plant allelochemicals on the tobacco hornworm and its parasitoid, Cotesia congregata Ecology 72 1567–1575
C. A. Black R. Karban L. D. Godfery J. Granett W. E. Chaney (2003) ArticleTitleJasmonic acid: a vaccine against leafminers (Diptera: Agromyzidae) in celery Environ. Entomol. 32 1196–1202
W. Boland J. Hopke J. Donath J. Nüske F. Bublitz (1995) ArticleTitleJasmonic acid and coronatin induce odor production in plants Angew. Chem., Int. Ed. Engl. 34 1600–1602
J. Cheng J. He (1996) Rice Insect Pests China Agricultural Press Beijing
M. Dicke (1999) Specificity of herbivore-induced plant defences D. J. Chadwick J. Goode (Eds) Insect–Plant Interactions and Induced Plant Defence. Novartis Foundation Symposium 223 Wiley Chichester, United Kingdom 43–59
M. Dicke R. Gols D. Ludeking M. A. Posthumus (1999) ArticleTitleJasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants J. Chem. Ecol. 25 1907–1922
R. Gols M. Roosjen H. Dijkman M. Dicke (2003) ArticleTitleInduction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation J. Chem. Ecol. 29 2651–2666 Occurrence Handle10.1023/B:JOEC.0000008010.40606.b0 Occurrence Handle14969353
S. Gouinguene H. Alborn T. C. J. Turlings (2003) ArticleTitleInduction of volatile emissions in maize by different larval instars of Spodoptera littoralis J. Chem. Ecol. 29 145–162 Occurrence Handle10.1023/A:1021984715420 Occurrence Handle12647859
R. Halitschke I. T. Baldwin (2003) ArticleTitleAntisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata Plant J. 36 794–807 Occurrence Handle10.1046/j.1365-313X.2003.01921.x Occurrence Handle14675445
R. Halitschke A. Kessler J. Kahl A. Lorenz I. T. Baldwin (2000) ArticleTitleEcophysiological comparison of direct and indirect defenses in Nicotiana attenuata Oecologia 124 408–417 Occurrence Handle10.1007/s004420000389
R. Halitschke U. Schittko G. Pohnert W. Boland I. T. Baldwin (2001) ArticleTitleMolecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses Plant Physiol. 125 711–717 Occurrence Handle10.1104/pp.125.2.711 Occurrence Handle11161028
B. Heath A. Manukian (1994) ArticleTitleAn automated system for use in collecting volatile chemicals released from plants J. Chem. Ecol. 20 593–608 Occurrence Handle10.1007/BF02059600
M. Hilker T. Meiners (2002) ArticleTitleInduction of plant responses towards oviposition and feeding of herbivorous arthropods: a comparison Entomol. Exp. Appl. 104 181–192 Occurrence Handle10.1023/A:1021232319226
M. Hilker O. Rohfritsch T. Meiners (2002) The plant’s response towards insect egg deposition M. Hilker T. Meiners (Eds) Chemoecology of Insect Eggs and Egg Deposition Blackwell Berlin 61–90
J. Hopke J. Donath S. Blechert W. Boland (1994) ArticleTitleHerbivore-induced volatiles: The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid FEBS Lett. 352 146–150 Occurrence Handle10.1016/0014-5793(94)00948-1 Occurrence Handle7925964
R. Karban I. T. Baldwin (1997) Induced Responses to Herbivory University of Chicago Press Chicago, IL
R. Karban A. A. Agrawal M. Mangel (1997) ArticleTitleThe benefits of induced defences against herbivores Ecology 78 1351–1355
A. Kessler I. T. Baldwin (2001) ArticleTitleDefensive function of herbivore-induced plant volatile emissions in nature Science 291 2141–2144 Occurrence Handle10.1126/science.291.5511.2141 Occurrence Handle11251117
A. Kessler I. T. Baldwin (2002) ArticleTitlePlant responses to insect herbivory: the emerging molecular analysis Annu. Rev. Plant Biol. 53 299–328 Occurrence Handle10.1146/annurev.arplant.53.100301.135207 Occurrence Handle12221978
S. T. Kim S. Yu K. S. Cho S. G. Kim J. C. Hong H.-D. Han H.-D. Bae M. H. Nam K. Y. Kang (2003) ArticleTitleProteomic analysis of differentially expressed proteins induced by rice blast fungus and elicitor in suspension-cultured rice cells Proteomics 3 2366–2378
P. J. Landolt J. H. Tumlinson D. H. Alborn (1999) ArticleTitleAttraction of Colorado potato beetle (Coleoptera: Chrysomelidae) to damaged and chemically induced potato plants Environ. Entomol. 28 973–978
F. Liu Y. Lou J. Cheng (2002) ArticleTitleVolatiles-mediated intra- and interspecific interactions of the rice brown planthopper, Nilaparvata lugens, and the white-backed planthopper, Sogatella furcifera Chin. J. Rice Sci. 16 162–166
Y. Lou I. T. Baldwin (2003) ArticleTitleManduca sexta recognition and resistance among allopolyploid Nicotiana host plants Proc. Natl. Acad. Sci. USA 100 14581–14586 Occurrence Handle10.1073/pnas.2135348100 Occurrence Handle14530394
Y. Lou I. T. Baldwin (2004) ArticleTitleNitrogen supply influences herbivore-induced direct and indirect defenses and transcriptional responses in Nicotiana attenuata Plant Physiol. 135 496–506 Occurrence Handle10.1104/pp.104.040360 Occurrence Handle15133153
Y. Lou J. Cheng (1994) ArticleTitleThe kairomone from Nilaparvata lugens (Stål) and its relation to rice varieties Acta Phytophylacica Sin. 21 327–332
Y. Lou J. Cheng (1996) ArticleTitleThe behavioral responses of Anagrus nilaparvatae Pang et Wang to the volatile of rice varieties Entomol. J. East China 5 60–64
Y. Lou J. Cheng (2003) ArticleTitleRole of rice volatiles in the foraging behaviour of the predator Cyrtorhinus lividipennis Reuter for the rice brown planthopper Nilaparvata lugens (Stål) Biocontrol 48 73–86 Occurrence Handle10.1023/A:1021291427256
Lou, Y., Cheng, J., and Du, M. 1999. Role of rice volatiles in the host selection behavior of the parasitoid, Anagrus nilaparvatae Pang et Wang: isolation and identification of volatile rice synomone, p. 608, in G. Zhou (ed.). First Annual Meeting of Science Society of China: Science and Technology Progress and Society and Economy Development Beyond 2000. Science and Technology Press of China, Beijing, China.
Y. Lou J. Cheng X. Ping F. Tang S. Ru M. Du (2002) ArticleTitleMechanisms on host discrimination between two hosts Nilaparvata lugens and Sogatella furcifera by the egg parasitoid Anagrus nilaparvatae Acta Entomol. Sin. 45 770–776
L. Mattiacci M. Dicke M. A. Posthumus (1995) ArticleTitleBeta-glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps Proc. Natl. Acad. Sci. USA 92 2036–2040 Occurrence Handle11607516
T. Meiners M. Hilker (2000) ArticleTitleInduction of plant synomones by oviposition of a phytophagous insect J. Chem. Ecol. 26 221–232 Occurrence Handle10.1023/A:1005453830961
N. Obara M. Hasegawa O. Kodama (2002) ArticleTitleInduced volatiles in elicitor-treated rice blast fungus-inoculated rice leaves Biosci. Biotechnol. Biochem. 66 2549–2559 Occurrence Handle10.1271/bbb.66.2549 Occurrence Handle12596847
R. Ozawa K. Shiojiri M. W. Sabelis G.-I. Arimura T. Nishioka J. Takabayashi (2004) ArticleTitleCorn plants treated with jasmonic acid attract more specialist parasitoids, thereby increasing parasitization of the common armyworm J. Chem. Ecol. 30 1797–1808 Occurrence Handle10.1023/B:JOEC.0000042402.04012.c7 Occurrence Handle15586675
R. Rakwal S. Komatsu (2000) ArticleTitleRole of jasmonate in the rice (Oryza sativa L.) self-defense mechanism using proteome analysis Electrophoresis 21 2492–2500 Occurrence Handle10.1002/1522-2683(20000701)21:12<2492::AID-ELPS2492>3.0.CO;2-2 Occurrence Handle10939463
R. Rakwal G. K. Agrawal N.-S. Jwa (2001) ArticleTitleCharacterization of a rice (Oryza sativa L.) Bowman-Birk proteinase inhibitor: tightly light regulated induction in response to cut, jasmonic acid, ethylene and protein phosphatase 2A inhibitors Gene 263 189–198 Occurrence Handle10.1016/S0378-1119(00)00573-4 Occurrence Handle11223257
E. Rubia-Sanchez Y. Suzuki K. Miyamoto T. Watanabe (1999) ArticleTitleThe potential for compensation of the effects of the brown planthopper Nilaparvata lugens (Stål) (Homoptera: Delphacidae) feeding on rice Crop Prot. 18 39–45 Occurrence Handle10.1016/S0261-2194(98)00087-8
M. Sabelis A. Janssen A. Pallini M. Venzon J. Bruin B. Drukker P. Scutareanu (1999) Behavioral responses of predatory and herbivorous arthropods to induced plant volatiles: from evolutionary ecology to agricultural applications A. A. Agrawal S. Tuzun E. Bent (Eds) Induced Plant Defenses Against Pathogens and Herbivores APS Press Saint Paul, MN 269–296
E. A. Schmelz H. T. Alborn J. H. Tumlinson (2001) ArticleTitleThe influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays Planta 214 171–179 Occurrence Handle11800380
E. A. Schmelz H. T. Alborn E. Banchio J. H. Tumlinson (2003) ArticleTitleQuantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory Planta 216 665–673 Occurrence Handle12569409
Y. Suzuki K. Sogawa Y. Seino (1996) ArticleTitleOvicidal reaction of rice plants against the whitebacked planthopper, Sogatella furcifera Horváth (Homoptera: Delphacidae) Appl. Entomol. Zool. 31 111–118
B. Sznajder J. A. Harvey (2003) ArticleTitleSecond and third trophic level effects of differences in plant species reflect dietary specialisation of herbivores and their endoparasitoids Entomol. Exp. Appl. 109 73–82 Occurrence Handle10.1046/j.1570-7458.2003.00096.x
S. Tamogamia R. Rakwalb O. Kodamaa (1997) ArticleTitlePhytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid FEBS Lett. 412 61–64 Occurrence Handle10.1016/S0014-5793(97)00743-6 Occurrence Handle9257690
J. S. Thaler (1999) ArticleTitleJasmonate-inducible plant defences cause increased parasitism of herbivores Nature 399 686–688 Occurrence Handle10.1038/21420
T. C. J. Turlings F. L. Wäckers (2004) Recruitment of predators and parasitoids by herbivore-damaged plants R. T. Cardé J. Millar (Eds) Advances in Insect Chemical Ecology Cambridge University Press Cambridge 21–75
T. C. J. Turlings U. B. Lengwiler M. L. Bernasconi D. Wechsler (1998) ArticleTitleTiming of induced volatile emissions in maize seedlings Planta 207 146–152 Occurrence Handle10.1007/s004250050466
L. E. M. Vet M. Dicke (1992) ArticleTitleEcology of infochemicals use by natural enemies in a tritrophic context Annu. Rev. Entomol. 37 141–172 Occurrence Handle10.1146/annurev.en.37.010192.001041
Wang, X., Du, M., Lou, Y., and Cheng, J. 2005. Jasmonates signaling pathway is not involved in the production of Nilaparvata lugens-induced rice volatiles. J. Zhejiang Uni. (Agri. and Life Sci.) (in press).
T. Watanabe H. Kitagawa (2000) ArticleTitlePhotosynthesis and translocation of assimilates in rice plants following phloem feeding by the planthopper Nilaparvata lugens (Homoptera: Delphacidae) J. Econ. Entomol. 93 1192–1198 Occurrence Handle10985030
M. Yamasaki A. Yoshimura H. Yasui (2003) ArticleTitleGenetic basis of ovicidal response to whitebacked planthopper (Sogatella furcifera Horváth) in rice (Oryza sativa L.) Mol. Breed. 12 133–143 Occurrence Handle10.1023/A:1026018821472
Acknowledgments
We thank Sandrine Gouinguené, Maria Elena Hoballah, Thomas Degen, Zhou Guoxin, and Wang Xia for their invaluable assistance with laboratory work and the Ministry of Science and Technology of China (973) (G2000016208) and the National Natural Science Foundation of China (30270233) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, YG., Du, MH., Turlings, T.C.J. et al. Exogenous Application of Jasmonic Acid Induces Volatile Emissions in Rice and Enhances Parasitism of Nilaparvata lugens Eggs by theParasitoid Anagrus nilaparvatae. J Chem Ecol 31, 1985–2002 (2005). https://doi.org/10.1007/s10886-005-6072-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-6072-9