Abstract
The administration of phage therapy for aquaculture disease has been anticipated by the researchers over a decade as an effective and an alternative control mechanism, though the application of phages as a disease control agent in aquaculture projects various beneficial aspects, critical limitations, and negative influence on production. This present scenario made a pressure to review the possible disclosure of phage therapy with its critical boundaries and limiting influences towards the disease control management of aquaculture (fish, shrimps, lobsters, bivalve mollusks, etc.). The phage therapy has proven its efficacy as a biocontrol agent towards aquaculture disease, although the sustainability of the phage therapy needs further investigation on the following: commercial application, formulation of bacteriophage for layman usage, and development of protocol for various diseases with consistent results. The marginal space existing between the inventors and the end user must be fulfilled by the awareness program and the government policies. The administration of the phage therapy could be effective for long-term safety and negatively influence the development of multidrug-resistant bacteria pathogens in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The government authorities have permitted the antibiotics towards the common bacterial infections of the fish, like tail and fin rot, epizootic skin erosion, ulcerative syndrome, and gill damage for-off. It seems to be an economically viable and sensible remedy than the critical financial defeat caused by the bacterial infections; hence, it has its own limitation due to multidrug resistance (MDR) of bacteria, microbial changeover, and accumulation of residual chemicals, in the environment, and general public (Park et al. 2000; Perreten 2005; Oliveira et al. 2012). Noteworthy, this current problem has been effectively initiated to resolve by using the bacteriophages, since it is being a natural, low cost, viable, and an alternative biomedicine for antibiotics and the chemicals used as an antibacterial substance, to control the dispersal of multidrug-resistant bacteria in the aquatic fish cultivation (Nakai and Park 2002; Oliveira et al. 2012; Subharthi 2015).
Bacterial viruses are actually viruses that may possibly survive in the bacterial host through infections and gradually consume the entire host known as bacteriophages or phages. Phages are as similar as virus in obligate parasitic feature; hence, it does not have any basic or specific metabolic function to survive its life rather it depends the metabolic support of the host (Al-Sum and Al-Dhabi 2014). As the nature omnipresent, bacteriophages are generally occurring in soil, fresh and marine environment, and in particular mammal intestinal tract. In the period of 1919–1920, scientist Félix d’Herelle, who was the discoverer of phages, has first introduced the phage therapy. In this method, the bacterial infectious diseases were treated with phages (Citorik et al. 2014). This phage therapy has become the booming solution to protect the fish population from the pathogens and effectively applied in the real cultivation (Rao and Lalitha 2015) The effect of bacteriophage therapy and its potential application in aquaculture practices has been documented by Oliveira et al. (2012). The crucial achievement of this technique using phage is more environmental friendly, and it has an uninterrupted host phage mechanism. This review has extensively highlighted the research finding of the phages and their potential mechanism against vast bacterial pathogens of aquaculture (Richards 2014).
Efficacy of phage therapy
The efficacy of the phage therapy is comparably high than the chemotherapy: (1) the reports are evidenced with effective mechanisms of phages towards MDR pathogenic bacteria because it induces bacteriolysis through a completely different mechanism from antibiotics (Matsuzaki et al. 2003); (2) the phage has high host-specific nature towards the bacterial pathogens, and the replacement of microbes could not be possible with this; (3) the immediate counter action like a mutation of phages could take place against the phage-resistant mutant bacteria; (4) in the economical point of view, the expenditure required for the phage therapy is considerably very less than the antibiotic dosage; and (5) still no reports stated on the side effects of the phage and phage products particularly lysin against eukaryotic cells (Hagens and Offerhaus 2014; Ly-Chatain 2014).
Mechanism of phage therapy
The phage mechanisms involved in the pathogenic bacteriolysis is well defined with following steps and is primarily started with the phage infection, which can be initiated by the adsorption of phages on the particular receptors of proteins or sugars (peptidoglycan (PG), teichoic acids, oligosaccharides, lipopolysaccharide (LPS), capsule, flagellum, type IV fimbriae, and sex pilus) of the pathogens cell wall. In general, the phages have the potential to attract the exact targeted bacterial pathogens in a species level or even in strain level, in very few studies reported with cross bacterial pathogens at species or genus level and named as polyvalent phages (Ross et al. 2016). The phage therapy finally achieved to destroy the complete bacterial infections caused by various sources of the aquaculture with the notable advantage of not harming natural flora of the host (Loc-Carrillo and Abedon 2011). In the phage life cycle, the insertion of phage genetic material into the pathogen cytoplasm is being the most important mechanism. The cell wall lysis of the pathogen particularly mediated by the phage depolymerases enzyme which includes a peptidoglycan hydrolases, endosialidases, endorhamnosidases, alginate lyases, and hyaluronate lyases (Drulis-Kawa et al. 2015) Then, the phage pre genes have been expressed and the related proteins (holing, endolysin, and spanins) are synthesized which would act as a main part in the control/destroy mechanism of the targeted bacterial pathogen system. The protein holin mean to make a hole in the cell wall of the pathogenic bacteria and give a way to the lysin enter into the peptidioglycan layer; it was known as a peptidoglycan hydrolase which could effectively degrade the peptidoglycans. As the same as a phage, the enzyme lysin has also been used as an alternative agent in the therapy. At the end of the phage life cycle, the new progeny phages released from the bacterial host and which could be mainly mediated by the protein endolysin (Young et al. 2000). The enzymes spanins (i-spanin and o-spanin) specially needed for the phages those who infect through disrupt the outer cell wall of Gram-negative pathogens (Kongari et al. 2018). As a subsequent process, the new progeny of the phage potentially infect the nearby pathogen. The basic mechanism of the phage to kill the bacteria pathogen has been effectively applied as a therapy against both Gram-positive and Gram-negative pathogens. Hence, it would be possible when all the infectious steps stated in the phage life cycle should entirely finish. Lack of studies on the mechanism of bacteriophages infecting aquaculture pathogens further limits the development of phage therapy as an alternative to antibiotics.
Active role of receptors and resistance to phages
In the phage infectious cycle, adsorption of the phage on the bacterial cell wall receptors has been considered as one of the vital stage and it could be possible by the exploitation of bacterial surface proteins (as receptors), host parts like flagella, capsules, fimbriae, and lipopolysaccharide (LPS) (Drulis-Kawa et al. 2015). In addition to this, the phages has the potential enzymes, which significantly works on the cell wall puncture mechanism for the effective entry to the bacterium. The well-known phage reported was PK1A with endosialidase secretion in its tail and deconstruct the polysialc acid capsule of E. coli K1 (Pelkonen et al. 1992; Skurnik and Strauch 2006). The Escherichia coli PK1A2 bacteriophage was also reported for effective mechanism (Lehti et al. 2017). Literature showed the role of microtubules in cargo trafficking which has been demonstrated in eukaryotic viruses. Particularly Pseudomonas phage capsid assembles on the membrane to move along tubulin-like protein, PhuZ on the phage nucleus facilitates DNA packaging (Chaikeeratisak et al. 2019). This study demonstrated that “a transport and distribution mechanism in which capsids attached to the sides of filaments are trafficked to the nucleus by PhuZ polymerization at the poles indicates the phage cytoskeleton evolved cargo-trafficking capabilities in bacteria”.
The bacteria might have been anti-phage resistance at various situations: one when a mutation occurs and another one reported was loss/modification of receptors (Labrie et al. 2010). Even the bacterial host system expresses anti-phage mechanism to phage due to the action of lysogeny, in some circumstances the Escherichia coli trigger restriction-modification system which can destroy the genetic materials of the T4 phage (Manning and Kuehn 2011). The genetic mutation of the phage in the bacterial system may interrupt the reproduction and assembly mechanism of the phage. Though this condition has not been reported as worse because the resistance of the bacteria could change or decrease the physical strength of it, and in case the receptor represents the virulence factor used by the phage, then it could be declined by the phage significantly (Levin and Bull 2004; Azam and Tanji 2019).
Basics of phage therapy
The expectation of phage therapy has been limited to control certain number of infectious bacteria in the aquaculture animals, the rest can be taken care by the defense mechanism of the animals (Levin and Bull 2004). The mechanism of phage multiplication in targeted bacteria was well defined, though due to the unpredictable factors, it is still in unsuccessful stage. To meet the success end point in the phage therapy set of repeatable, multidimensional and quantitative efforts have to be taken in particularly in vivo state.
The phage therapy should come in practice once it has done in vivo state with complete revealing of the mechanism because both bacterial infectious mechanisms as the same way the phage controlling mechanisms are intricate. The formulation of phage might fulfill the complete protection from bacterial and other infectious contaminants. The healthy population of phages must be a main fraction of the commercial formulation that should be comply with product certification. The phage formulation should be specified with their respective receptor. The probability of occurring incomplete receptors/altered receptors and impulsive phage-resistant mutants would be possible with the bacterial population of 106–108. The predictable mutation in the receptor with virulence nature of the bacteria could end up with the bacteria with avirulent bacteria that promote the immune system to eradicate with no trouble. The successful technology with animal model testing should be making them efficient with phage unique character.
Aquaculture vs phage therapy
The phage therapy has been reported for its significant application in the fields of medicine, both human and veterinary, agricultural, and food sectors. The phage has extended its application towards aquaculture, especially to vast aquaculture organisms (Table 1) (Oliveira et al. 2012).
The vibrio species are normal ubiquitous bacteria of the aquatic system responsible for most of the bacterial outbreaks recorded across the countries (Kiran et al. 2016). The phage therapy against fish bacterial control was first reached in the application stage at 1981 and was demonstrated first in Japanese eel Anguilla japonica (Wu et al. 1981; Wu and Chao 1982; Oliveira et al. 2012). Further, the continuation of research in the field of control mechanism towards pathogens with field trials in fish aquaculture experimented at 1977. The phage therapy with positive note on protective mechanism was reported successfully in yellow tail (Seriola quinqueradiata) against the pathogenic effect of L. garvieae infections (Nakai et al. 1999) when there was no remedy for the drug-resistant strains. Those phages with virulent effect against L. garvieae were denoted as PLgY and known as the family of Siphoviridae (Park et al. 1997). In accordance with the sensitivity to phages, the pathogenic strains L. garvieae of fish and their ecosystem were classified into various groups (Park et al. 1998).
The phage verified to have significant effects on cultured ayu fish (Plecoglossus altivelis) against the bacterial pathogen of P. plecoglossicida at the time the licensed chemotherapeutic agents were not permitted for aquaculture application. The key points were investigated from the research reports of phage therapy as follows: (1) the food prepared with the infused phage has given optimistic results against experimental infection, and it positively correlated with massive phage activity at in vivo state (Park et al. 2000; Park and Nakai 2003); (2) in addition, application of phage suspension in open water system has effectively controlled the further spreading over of the pathogen for secondary (Oliveira et al. 2012; Almeida et al. 2019); (3) noteworthy, the research extensively reported no evidence for the presence of phage-resistant organisms and nullifying compounds released by the immune system of the both healthy as well as infected fish (Oliveira et al. 2012; Rao and Lalitha 2015); (4) it is evidently proved with the application of phage leads with very less percentile of infectious and fish mortality rate (Park and Nakai 2003).
The research findings of the model studies are a benchmark for biocontrol mechanism that could be effectively implemented for numerous fish cultivation models. Since, it serves up a significant and sustainable biocontrol therapy against various uncontrollable infectious diseases in aquaculture industry (Nakai and Park 2002). The combined mechanism with bacteriophage has given proven results in the fish farms. Hence, it is highly suitable for prophylactic use and not recommend for to treat furunculosis caused by Aeromonas salmonicida and in Atlantic salmon (Imbeault et al. 2006; Verner-Jeffreys et al. 2007).
The vibrio species are normal ubiquitous bacteria of the aquatic system responsible for most of the bacterial outbreaks recorded across the countries (Austin and Zhang 2006; Kiran et al. 2016). The use of lytic phages on the control of V. harveyi infection in Penaeusmonodon larvae was demonstrated by Karunasagar et al. (2007). In this report, the bacteriophages isolated from oyster and P. monodon hatchery water were testedagainst V. harveyi in vitro and the results showed lysis 55–70% V. harveyi tested. The effective phages belonged to Siphoviridae family. Significant findings of this studyinclude effective elimination of V. harveyi cells from the biofilm formed on high density polyethylene (HDPE) surface, the tanks used in the P. monodon hatchery.Another report by Vinod et al (2006), showed a double stranded DNA phage belong to Siphoviridae was isolated from farm water P. monodon ponds. The lytic phageeffectively controlled luminous vibrios in microcosm experiments.
Based on the reports from the aquaculture industry, the success of phagetherapy depends on delivery methods which ensure delivery of phages in the infected site and a titre required to eliminate the pathogenic bacteria (Kalatzis et al. 2018). Most of the successful phage therapy reports showed injection of diseased fish, however, this could not be a reliable method for treatment of a population. Application ofphage therapy to contain a well-known zoonotic pathogen Vibrio parahaemolyticus was demonstrated by Jun et al. (2014). The commercialization of phage therapy inaquaculture production systems requires economic and consistent phage production methods. The production method that could reduce the chances of emerging resistant bacteriophages need to be preferred include cellstat and a two-stage self-cycling process (García et al. 2019).
Disease control management in shrimps
Antibiotics
The use of antibiotics in shrimp aquaculture pose potential threat include residual antibiotics in the food systems (Selvin and Lipton 2003, 2004) and possible emergence of resistantstrains. In aquaculture industry 36 types of antibiotics were used in seven major aquaculture production countries (Rico et al. 2012). In marine invertebrates, especially in penaeid shrimp (economically important shrimp), a serious disease was caused by Vibrio harveyi (Karunasagar et al. 2004; Austin and Zhang 2006). Antibiotics are frequently used in some of hatcheries to control the diseases; it sometimes leads failure to control the luminous bacteria. The growth of antibiotic-resistant V. harveyi increased in larval tanks causing serious mortality in P. monodon larvae (Lavilla-Pitogo et al. 1990; Karunasagar et al. 1994). Due to these effects, some of the shrimp farmers used to apply diverse group of antibiotics on a daily basis as a precautionary measure (Holmstrom et al. 2003) Antibiotics are used as both prophylactic and reactive mode oftreatments. Among the antibiotics used in the aquaculture, oxytetracycline and chloramphenicol were most prevalent in many production countries (Selvin and Lipton 2003, 2004). Considering the impacts of antibiotics, the use of chloramphenicol was banned in aquaculture (Rico et al. 2012).
Bacteriophage therapy
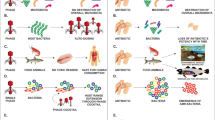
The use of bacteriophage based treatments obviously known as ‘phagetherapy’ become a green alternative to the use of antibiotics in aquaculture. The effect of phage therapy can be improved with a combination of lytic phages and/orphages with antimicrobial substances, which ultimately prevents the emergence of phage resistance (Karunasagar et al. 2005; Shivu et al. 2007; Rao and Lalitha 2015). This report also showed the possible threat of lysogenic phages might transform the virulent gent into the non-virulent strains. Almeida et al. (2019) demonstrated the effect of phage therapy in the control of Flavobacterium columnare infection in rainbow trout Oncorhynchus mykiss cultured in recirculating aquaculture systems (RAS). This study demonstrated the persistence of exposed phage for three weeks in a RAS, evidenced the potential application of phage therapy in RAS. Further this reportrevealed the highest concentration of phage in biofilm layers over plastic surfaces indicating the possibility of developing immobilization techniques for sustained releaseof phase in RAS. Several literatures have reported the usage of the bacteriophages for the biocontrol of luminous vibriosis with complete characterization of bacteriophages (Fig. 1). The consistent results needed to certify the effective application of bacteriophage to control luminous vibriosis in shrimps, and it is necessary to extend the application to other organisms to which this disease is associated.
Disease control management in lobsters
Bacteriophage therapy is one of the alternative techniques that potentially control and remove the pathogenic Vibrio spp. in the larval culture of the tropical rock lobster, Panulirus ornatus (Payne 2007). In spiny lobster, has been identified with diseases caused by V. harveyi (Vinod et al. 2006). Among eight bacteriophages investigated for lytic activity against V. harveyi, six come under Siphoviridae and two from Myoviridae, among them one from the Siphoviridae family had noticed in clear and no apparent transducing property. Crothers-Stomps et al. (2010) demonstrated the use of bacteriophage on the control of V. harveyi strains infectingphyllosoma larvae of tropical rock lobster Panulirus ornatus. The bacteriophage strains showed effectively controlled vibrio strains such as V. rotiferianus, V. harveyi, V. campbellii, and V. parahaemolyticus belong to the family Siphoviridae and Myoviridae. Significant outcome of this study by Crothers-Stomps et al. (2010) include demonstration of phage induced bacteriocin production.
Disease control management in bivalve mollusks
The bacteriophage treatment has been used as a control mechanism towards the infection of Vibrio splendidus in a cultured larvae stage of the Pacific oyster, Crassostrea gigas (Sugumar et al. 1998; Park and Nakai 2003), and often used for bacterial infections of molluscan aquaculture (Berthe 2005). The pathogens V. harveyi and Vibrio alginolyticus have been found to be a main disease causing agent in pearl oysters, hence, the application of bacteriophages as an antimicrobial strategy to overcome this disease was not yet to be proved. In present scenario, the available research reports are very less on biocontrol of microbial disease with bacteriophages for any other bivalve species.
Enzybiotics
The diverse group of bacteriophage products (lysins and bacteriophage tail-like bacteriocins) has been isolated in purified form, and the alternative therapy results revealed its anti-infective nature (Skurnik and Strauch 2006; Oliveira et al. 2018). The enzyme lysin has efficiently worked on Gram-positive bacteria and effectively destroys the peptidoglycan layer from outside of the cell wall. It has the potential to degrade the cell walls by the bacteriolytic effects on non-growing bacteria within a few seconds after applying the lysins (Drulis-Kawa et al. 2015). On the other hand, cell wall-degrading antibiotics like penicillin and cephalosporin requires a channel to reach within the cell and inhibit peptidoglycan synthesis of bacteria in division phase only. The instantaneous administration of two lysins that have different peptidoglycan cutting sites has been reported to possess a synergistic effect. The bacteriophage lysin was also been reported to be fairly specific for bacterial species, eliminating the specific bacteria without disturbing the natural habitats. However, the lysins studied so far are inactive against Gram-negative cell wall, since lysin does not have the ability to enter the outer membrane of Gram-negative bacteria (Parisien et al. 2008).
Bacteriophage tail-like bacteriocins are one of the groups of bacteriophage products with high molecular weight fragments of bacteriophages, synthesized by a number of Enterobacteriaceae and other Gram-negative bacteria (Bradley 1967; Daw and Falkiner 1996). These special groups of bacteriocins have been reported to eliminate the target bacterial cell by pore formation in the cell wall and lead to a rapid loss of ions. Phage-specific lysins and phage tail-like bacteriocins isolated and analyzed by many researchers as an alternative potential therapy than to use whole bacteriophage (Inal 2003).
Phage therapy vs hindrance
The following challenges should be addressed in the phage therapy: (1) development of phage resistance, (2) risk of anaphylaxis reactions, (3) capture and transfer of bacterial toxin gene, and (4) utility against intracellular pathogens.
Concern to the phage resistant, it has been reported and proved that the development of mutants occurred at laboratory investigations which holds the resistant to bacteriophages. Hence, to implement the bacteriophage therapy, the above state one should be a core concern, and also act as a limiting factor to the positive approach of the bacteriophage therapy on multidrug resistance pathogens (Tang et al. 2019). The resistance mechanism of bacteria to bacteriophages could induce by a variety of means; for example, it happens through a change in the phage-receptors molecules in Gram-negative bacteria or via CRISPR-Cas systems (Ormala and Jalasvuori 2013). This problem should be addressed by re-isolation of a new phage from the environment. Since the phage and bacterial host exist together in the same environment, a phage species might attack mutated/resistant bacteria. The bacteriophage being a more significant finding than the discovery of many chemical antibiotics since it needs prolonged time to develop a new antibiotic. Though, the bacteriophage resistance has not been considered as a bigger challenge than the drug-resistant mechanism (Haq et al. 2012).
The administration of high dosage of bacteriophage as a treatment may induce an extreme response such as anaphylaxis, sometimes they reflect the opposite side effects, though it has not been reported yet (Sulakvelidze et al. 2001). To the above stated issue, the bacteriophage therapy has exhibited the potential recovery of endotoxin when causesing the death of the bacteria especially in Gram-negative bacteria. This may negatively impact the efficacy of the bacteriophage therapy as same as the exploitation of antibiotics (Wittebole et al. 2014). The warning has been given by the researchers that the excess amount of endotoxin released either by the phage therapy or antibiotics could cause fever, septic shock, and finally ends up with death (Nobrega et al. 2015).
The capability of the phage to carry the genetic material by horizontal gene transfer was an important factor that being a challenge to practice the bacteriophage therapy. In addition, in the time of deletion of the bacteriophage DNA from the host chromosome, the host DNA may also assimilate with the bacteriophage DNA. Thus, lysogenic bacteriophages could initiate the horizontal transmission of bacterial genes from one bacterium to another bacterium to improve the bacterial virulence or genes for antibiotic resistance. The CTX ɸ prophage infection has been considered as a classical example to improve the virulence factor of V. cholerae (Meaden and Koskella 2013). The few other bacteriophages also possess the ability to hold the genetic materials by means of horizontal gene transfer. Due to this, the bacteriophage has been routed to generate toxins (enterotoxins and exfoliating toxins) of bacteria (Mohammed-Ali and Jamalludeen 2015). In addition to the above stated, the toxins CTX cholera toxin, botulinum toxin, Shiga toxin, and diphtheria toxin were also being reported as a toxin of bacteriophage. To overcome this issue and develop the successful bacteriophage therapy, the bacteriophages are anticipated to refuse the host DNA (Wittebole et al. 2014). The complete understanding of bacteriophage genome sequence paws the way to defeat the barriers of phage therapy. Lytic phages since do not assimilate into the host’s DNA and do not influence the host’s virulence factor, and the features made them an ideal candidate for therapeutic use.
The bacteriophage therapy has not been reported as potential one to kill the intracellular pathogens particularly Salmonella species. Because of the lack of ability of the bacteriophage to reach the intracellular pathogens, especially eukaryotic cell, they are still being a challenge to the researchers to completely destroy this intracellular pathogen through the phage therapy. Although the bacteriophages are not a direct pathogens of eukaryotic cells, the human immune system may identify bacteriophage as foreign antigens and respond by producing bacteriophage-neutralizing antibodies (Sulakvelidze et al. 2001).
Conclusions and future prospective
The production of different species of fish, crustaceans, and mollusks made the aquaculture industry as one of the significant elements of Indian economy. The disease control management in aquaculture has been updated constantly by the researchers to overcome the next-generation pathogenic diseases. Though, somewhere the bacterial infectious disease leaves the economic lags. The administration of antibiotics has existed as the only route to control the pathogenic effect and be a protective mechanism of aquaculture farming so far. Though the intelligence of the pathogenic microbes has been upgraded and introduced, the novel character of multidrug resistant (MDR) against almost all antibiotics.
Several research attempts have been initiated to target the multidrug-resistant pathogens through the development of new antibiotics or novel compounds/drugs against MDR pathogens. The government bodies have rolling announcement to submit proposals to discover the viable and new drugs with industrial collaboration. In addition to the above, it is also essential to create awareness programs about the recent research and inventions of aqua farming among the aquaculture producers. Natural compounds from the resource like flora and fauna of terrestrial and marine habitats have been focused on the implementation of new drugs. It is noteworthy that the bacteriophage therapy has a unique approach towards infectious disease control management in aquaculture farming and does not comparably hazardous as antibiotics. The bacteriophage therapy has been tried well in many aquaculture farming for the past 38 years especially fish (Japanese eel, yellow tail, aya), shrimps, lobsters (rock lobster), and bivalve mollusks. The promising phage therapy seems to be an economical, eco-friendly, biologically safe than the regular chemotherapy.
The main hindrance of the phage therapy was noticed recently as the phage-resistant mechanism developed by the bacterial defense system. Though it was evidently proved, the strategy of co-evaluation between the bacteriophage and pathogenic bacteria would preserve the phage therapy for certain promising extant. The phage therapy on the phage-resistant bacteria impacts the structural change which is responsible for the virulence mechanism and limits the virulence though it does not completely destroy the bacteria. To extend to this, the above discussed apertures are still needed to be fulfilled only through the impending research areas like application of bacteriophage against intracellular pathogens and in all taxonomic groups of aquaculture (fish, shrimp, etc.) disease control management, etc. with effective cocktail formulation of bacteriophage for commercial application as an anti-pathogenic agent.
References
Al-Sum AB, Al-Dhabi NA (2014) Isolation of bacteriophage from Mentha species in Riyadh, Saudi Arabia. J Pure Appl Micro 8(2):945–949
Almeida GMF, Mäkelä K, Laanto E, Pulkkinen J, Vielma J, Sundberg L-R (2019) The Fate of Bacteriophages in Recirculating Aquaculture Systems (RAS)—Towards Developing Phage Therapy for RAS. Antibiotics 8 (4):192
Austin B, Zhang XH (2006) Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 43(2):119–124
Azam AH, Tanji Y (2019) Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl Micro Biotech 103:2121–2131
Berthe FCJ (ed) (2005) Diseases in mollusc hatcheries and their paradox in health management, Fish Health Section. Asian Fisheries Society, Manila
Bradley DE (1967) Ultrastructure of bacteriophage and bacteriocins. Bact Rev 31:230–314
Chaikeeratisak V, Khanna K, Nguyen KT, Sugie J., Egan ME, Erb ML, Vavilina A, Nonejuie P, Nieweglowska E, Pogliano K, Agard DA, Villa E, Pogliano J (2019) Viral capsid trafficking along treadmilling tubulin filaments in Bacteria. Cell 177: 1771–1780
Chen L, Yuan S, Liu Q, Mai G, Yang J, Deng D, Zhang B, Liu C, Ma Y (2018) In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front Microbiol 9:1476. https://doi.org/10.3389/fmicb.2018.01476
Citorik RJ, Mimee M, Lu TK (2014) Bacteriophage-based synthetic biology for the study of infectious diseases. Curr Opi Micro 19:59–69
Crothers-Stomps C, Hoj L, Bourne DG, Hall MR, Owens L (2010) Isolation of lytic bacteriophage against Vibrio harveyi. J Appl Microbiol 108(5): 1744–1750, 2010
Daw MA, Falkiner FR (1996) Bacteriocins: nature, function and structure. Micron 27:467–479
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B (2015) Bacteriophages and phage-derived proteins – application approaches. Curr Med Chem 22(14):1757–1773
García R, Latz S, Romero J, Higuera G, García K and Bastías R (2019) Bacteriophage Production Models: An Overview. Front. Microbiol. 10: 1187
Hagens S, Offerhaus ML (2014) Bacteriophages – new weapons for food safety. Food Technol 62:46–54
Haq IU, Chaudhry WN, Akhtar MN, Andleeb S, Qadri I (2012) Bacteriophages and their implications on future biotechnology: a review. Virol J 9(9):1–8
Holmstrom K, Graslund S, Wahlstrom A, Poungshompoo S, Bengtsson BE, Kautsky N (2003) Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int J Food Sci Technol 38(3):255–266
Imbeault S, Parent S, Lagace M, Uhland CF, Blais JF (2006) Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed Brook Trout. J Aquat Anim Health 18(3):203–214
Inal JM (2003) Phage therapy: a reappraisal of bacteriophages as antibiotics. Arch Immunol Ther Exp 51:237–244
Jun JW, Shin TH, Kim JH, Shin SP, Han JE, Heo GJ, De Zoysa M, Shin GW, Chai JY, Park SC. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple-antibiotic-resistant O3: K6 pandemic clinical strain. J Infect Dis. 2014;210:72–8
Jun J, Giri SS, Kim HJ, Yun SK, Chi C, Chai JY, Lee BC, Park SC (2016) Bacteriophage application to control the contaminated water with Shigella. Sci Rep 6:22636. https://doi.org/10.1038/srep22636
Jun JW, Han JE, Giri SS, Tang KFJ, Zhou X, Aranguren LF, Kim HJ, Yun S, Chi C, Kim SG, Park SC (2018) Phage application for the protection from acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei. Ind J Micro 58:14–117
Kalatzis P, Castillo D, Katharios P, Middelboe M (2018) Bacteriophage Interactions with Marine Pathogenic Vibrios: Implications for Phage Therapy. Antibiotics 7 (1):15
Karunasagar I, Pai R, Malathi GR, Karunasagar I (1994) Mass mortality of Penaeus monodon larvae due to antibiotic resistant Vibrio harveyi infection. Aquaculture 128(3–4):203–209
Karunasagar I, Vinod MG, Kennedy B, Vijay A (2005) Biocontrol of bacterial pathogens in aquaculture with emphasis on phage therapy, In: Walker PJ, Lester RG, Bondad-Reantaso MG (ed) Diseases in Asian aquaculture V. Fish Health section, Asian Fisheries Society, Manila, pp 535–542
Karunasagar I, Shivu MM, Girisha SK, Krohne G (2007) Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 268(1–4):288–292
Kiran GS, Priyadharshini S, Dobson A, Gnanamani E, Selvin J (2016). Degradation intermediates of polyhydroxy butyrate inhibits phenotypic expression of virulence factors and biofilm formation in luminescent Vibrio sp. PUGSK8. NPJ Biofilms and Microbiomes 2: 16002.
Kongari R, Rajaure M, Cahill J, Rasche E, Mijalis E, Berry J, Young R (2018) Phage spanins: diversity, topological dynamics and gene convergence. BMC Bioinformatics 15 19(1): 326
Laanto E, Bamford JKH, Ravantti JJ, Sundberg LR (2015) The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front Microbiol 6: 829. https://doi.org/10.3389/fmicb.2015.00829
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327
Lavilla-Pitogo CR, Baticados MCL, Cruz-Lacierda ER, .de la Pena LD (1990) Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture 91(1–2): 1–13
Lehti TA, Pajunen MI, Skog MS, Finne J (2017) Internalization of a polysialic acid-binding Escherichia coli bacteriophage into eukaryotic neuroblastoma cells. Nat Commun 8(1915):1–12
Levin BR, Bull JJ (2004) Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol 2:166–173
Loc-Carrillo C, Abedon ST (2011) Pros and cons of phage therapy. Bacteriophage 1(2):111–114
Ly-Chatain MH (2014) The factors affecting effectiveness of treatment in phages therapy. Front Microbiol 5(51):1–7
Manning AJ, Kuehn MJ (2011) Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol 11:258
Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman MD, Wakiguchi H, Sugihara S, Sugiura T, Koda S, Muraoka A, Imai S (2003) Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J Infect Dis 187:613–624
Meaden S, Koskella B (2013) Exploring the risks of phage application in the environment. Front Microbiol 4:358
Mohammed-Ali MN, Jamalludeen NM (2015) Isolation and characterization of bacteriophage against methicillin resistant Staphylococcus aureus. J Med Microb Diagn 5: 213
Nakai T, Park SC (2002) Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol 153:13–18
Nakai T, Sugimoto R, Park KH, Matsuoka S, Mori K, Nishioka T, Maruyama K (1999) Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis Aquat Org 37:33–41
Nobrega FL, Costa AR, Kluskens LD, Azeredo J (2015) Revisiting phage therapy: new applications for old resources. Trends Microbiol 23:185–191
Oliveira J, Castilho F, Cunha A, Pereira MJ (2012) Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquacult Int 20:879–910
Oliveira H, Sao-Jose C, Azeredo J (2018) Phage derived peptidoglycan degrading enzymes: challenges and future prospects for in vivo therapy. Viruses 10(6):292
Ormala AM, Jalasvuori M (2013) Phage therapy: should bacterial resistance to phages be a concern, even in the long run? Bacteriophage 3:e24219
Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ (2008) Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J Appl Micro 104:1–13
Park SC, Nakai T (2003) Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis Aquat Org 53:33–39
Park KH, Matsuoka S, Nakai T, Muroga K (1997) A virulent bacteriophage of Lactococcus garvieae (formerly Enterococcus seriolicida) isolated from yellowtail Seriola quinqueradiata. Dis Aquat Org 29(2):145–149
Park KH, Kato H, Nakai T, Muroga K (1998) Phage typing of Lactococcus garvieae (formerly Enterococcus seriolicida) a pathogen of cultured yellowtail. Fish Sci 64:62–64
Park SC, Shimamura I, Fukunaga M , Mori KI, Nakai (2000) Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol 66:1416–1422
Payne M (2007) Towards successful aquaculture of the tropical rock lobster, Panulirus ornatus: the microbiology of larval rearing, PhD Thesis, University of Queensland
Pelkonen S, Aalto J, Finne J (1992) Differential activities of bacteriophage depolymerase on bacterial polysaccharide: binding is essential but degradation is inhibitory in phage infection of K1-defective Escherichia coli. J Bacteriol 174:7757–7761
Pereira C, Silva YJ, Santos AL, Cunha Â, Gomes NCM, Almeida A (2011) Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure. Marine Drugs 9 (11):2236–2255
Pereira C, Moreirinha C, Teles L, Rocha RJM, Calado R, Romalde JL, Nunes ML (2017) Adelaide Almeida, Application of phage therapy during bivalve depuration improves Escherichia coli decontamination. Food Microbiol 61: 102–112
Perreten V (2005) Resistance in the food chain and in bacteria from animals: relevance to human infections. In: White DG, Alekshun MN, McDermott PF (eds) Frontiers in antimicrobial resistance. American Society for Microbiology, Washington, DC, p 575
Rao BM, Lalitha KV (2015) Bacteriophages for aquaculture: Are they beneficial or inimical. Aquaculture 437:146-154
Richards GP (2014) Bacteriophage remediation of bacterial pathogens in aquaculture: a review of the technology. Bacteriophage 4(4): e975540 1-12
Rico A, Satapornvanit K, Haque MM, Min J, Nguyen PT, Telfer TC, van den Brink PJ, (2012) Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Reviews in Aquaculture 4 (2):75-93
Selvin J, Lipton AP (2003). Leaching and residual kinetics of chloramphenicol incorporated medicated feed treated to juvenile black tiger shrimp Penaeus monodon Fabricious. Fish Technol 40: 13-17
Selvin J, Lipton AP (2004) Leaching and residual kinetics of oxytetracycline incorporated medicated feed treated to juvenile black tiger shrimp Penaeus monodon Fabricious. Fish Technol 41: 93-100
Ross A, Ward S, Hyman P (2016) More is better: selecting for broad host range bacteriophages. Front Microbiol 7(1352):1–6
Shivu MM, Rajeeva BC, Girisha SK, Karunasagar I, Krohne G, Karunasagar I (2007) Molecular characterization of Vibrio harveyi bacteriophages isolated from aquaculture environments along the coast of India. Environ Microbiol 9(2): 322–331, 2007
Skurnik M, Strauch E (2006) Phage therapy: facts and fiction. Int J Med Microbiol 296:5–14
Stomps CC, Hoj L, Bourne DG, Hall MR, Owens L (2010) Isolation of lytic bacteriophage against Vibrio harveyi. J Appl Micro 108:1744–1750
Subharthi P (2015) Phage therapy an alternate disease control in aquaculture: a review on recent advancements. IOSR J Agric Vet Sci 8(9):68–81
Sugumar G, Nakai T, Hirata Y, Matsubara D, Muroga K (1998) Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Dis Aquat Org 33:111–118
Sulakvelidze A, Alavidze Z, Morris Jr JG (2001) Bacteriophage therapy. Antimicrob. Agents Chemother 45: 649–659
Tang SS, Biswas SK, Tan WS, Saha AK, Leo BF (2019) Efficacy and potential of phage therapy against multidrug resistant Shigella spp. Peer J 7:e6225
Verner-Jeffreys DW, Algoet M, Pond MJ, Virdee HK, Bagwell NJ, Roberts EG (2007) Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 270(1–4):475–484
Vinod MG, Shivu MM, Umesha KR, Rajeeva BC, Krohne G, Karunasagar I, Karunasagar I (2006) Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 255:117–124. https://doi.org/10.1016/j.aquaculture.2005.12.003
Wang Y, Bartn M, Elliott L, Li X, Abraham S, Dae MO, Munr J (2017) Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 473:251–258
Wittebole X, De Roock S, Opal SM (2014) A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 5:226–235
Wu JL, Chao WJ (1982) Isolation and application of a new bacteriophage, ET-1, which infect Edwardsiella tarda, the pathogen of edwardsiellosis, Rep Fish Dis Res IV (8): 8–17
Wu JL, Lin HM, Jan L, HSU YL, Chang LH (1981) Biological control of fish bacterial pathogen, Aeromonas hydrophila, by bacteriophage AH1. Fish Pathol 15:271–276
Young R, Wang IN, Roof WD (2000) Phages will out strategies of host cell lysis. Trends Microbiol 8:120–128
Funding
The University Grants Commission, New Delhi, provided research grant and support to SS and PR. GSK received research grant from DBT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by anyof the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ninawe, A.S., Sivasankari, S., Ramasamy, P. et al. Bacteriophages for aquaculture disease control. Aquacult Int 28, 1925–1938 (2020). https://doi.org/10.1007/s10499-020-00567-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00567-4