Abstract
Indian aquaculture industry was growing steadily and showed a sixfold growth in production over the last two decades. However, the overall development of aquaculture in India did not reach the levels as projected due to frequent disease outbreaks and related issues. Shrimp and fish are predominantly affected by viruses including white spot syndrome virus, monodon baculovirus, hepatopancreatic parvovirus, viral nervous necrosis, white tail disease, and hypodermal and hematopoietic necrosis baculovirus that cause outbreaks across the countries. Owing to these viral pathogens, the production of aquaculture fishes and crustaceans has dramatically been dropped. There are no specific measures to control these viral infections since it causes mortality at all life stages of cultured aquatic organisms. Early detection of the diseases may be beneficial to prevent the spreading and mass mortality. In India, the Central Institute of Brackish Water Aquaculture has developed a non-invasive diagnostic tool for early and precise detection of monodon baculovirus infection in Indian tiger shrimp Penaeus monodon using SYBR Green-based real-time polymerase chain reaction (PCR) technique. More than twenty-five cell lines from freshwater, brackish water, and marine fish have been developed, characterized, and stored in C. Abdul Hakeem College for research and viral diagnosis. This review reveals the distinctive tools that are being used in aquaculture for the detection of pathogens and preventive measures. Advanced molecular methods such as nested PCR and SYBR Green-based real-time PCR are found to be sensitive and effective for the quantitation. Simple and inexpensive methods such as microscopic evaluation and histopathological analysis detects the progression of the disease. These assays can be used as a diagnostic method for emerging diseases in addition to avoid forthcoming of another.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indian aquaculture has demonstrated a sixfold growth in production over the last two decades, with freshwater aquaculture contributing the major share. The carp culture in freshwater and shrimp culture in brackish water form the major activity in Indian aquaculture systems. In contrast, the development of brackish water aquaculture has been confined to a very few species, although India offers immense potential for the development of mariculture. Cultivable aquatic organisms in India are Penaeus monodon (tiger shrimp), Penaeus vannamei (white shrimp), Macrobrachium rosenbergii (freshwater prawn), Lates calcarifer (sea bass fish), and Indian major carps and Catfish (Jana and Jana 2003). Global aquaculture production in 2011 was 63 million tonnes (inland 44 and marine 19 million tonnes), and in India, it was about 4.6 million tonnes. In India, fish production was 9.1 million tonnes (i.e., through capture 4.5 and culture 4.6). Export value in freshwater (Indian major carps) and brackish water culture (shrimp) was Rs. 165970 millions. The Department of Biotechnology demonstrated a semi-intensive culture and developed a technology to achieve the production level of 10 t/ha per annum in two crops in shrimp and more than 18–20 t/ha in fish production per annum through polyculture with the introduction of high-yielding hybrids, quality feed and breed, and better health management. This has been a paradigm shift in fish and shrimp production in the country.
In aquatic health management, technology is being developed for screening healthy population of brooders based on molecular markers, maturation in captivity, completion of maturation in indoor facility wherever feasible, and breeding and spawning in controlled systems. Most of the shrimp farmers did not have awareness and/or knowledge of antibiotics use in aquaculture industry and was a critical issue in the management of diseases. Antibiotics are being a choice of therapeutics for even viral outbreaks with secondary bacterial infections (Selvin et al. 2009). In China, the farmers are aware of the problems associated with antibiotic use in aquaculture and they have been practicing water quality management using probiotics and disinfectants (Li et al. 2016). However, the effect of such practices on control/prevention of disease outbreaks was not documented. Novel concept on raceway technology using aerobic microbial flocculent technology is being propagated in designing, construction, and operation of raceways to raise crops of various kinds including shrimp, scampi, and freshwater ornamental fishes for future and to ensure the bio-security of used water for practicing sustainable aquaculture.
In India, the loss of shrimp due to white spot syndrome virus (WSSV) has been estimated about US$ 1.50 million per year (Central Institute of Brackish Water Aquaculture, CIBA report 2008). Application of various technologies especially biotechnological tools has made an impact in reducing disease risk and advance technologies are contributing to the future enhancement of aquaculture production. Improved nutrition, use of probiotics, improved disease resistance strain, water quality, seed and feed, use of immunostimulants, rapid detection of pathogens, and the use of affordable vaccines have all assisted in health control measures in aquaculture (Selvin et al. 2009). The rapid diagnostic method is helpful in the detection of pathogens in different situations such as in clinically infected animals, in subclinically infected animals, or in the environment. There is an urgent need to develop a simple, rapid, cost-effective, sensitive detection tool for the diagnosis of various pathogens through innovation methods and addressing fish health management issues and bio-safety aspects in aquaculture.
The Department of Biotechnology (DBT), Ministry of Science and Technology of India, is addressing various aquatic health issues through establishment of diagnostic laboratories to screen the shrimp brooders and seeds for viral pathogens using molecular tools such as polymerase chain reaction (PCR), real-time PCR (RT-PCR), and immunodiagnostics through the projects supported at various aquaculture institutes in the country. Support is also provided to the programmes such as production specific pathogen free (SPF) brooders and seeds using molecular tools including RNA interference (RNAi) technology and developing brood stock banks to supply high-quality brooders to hatchery operators. Fish cell lines and shrimp primary cell culture, the outcome of DBT funded projects, are being used for propagation of viruses for production of whole-virus vaccines. There is a need of National Referral Facility to take care of aquatic animal health by providing diagnostic facility and control and preventive measures for all bacterial and viral diseases encountered in Indian aquaculture system. Detection of viral diseases is done by two strategies: DNA based and protein based. DNA-based detection is carried out by RT-PCR which is sensitive and indicative of different stages of infection. Protein-based methods such as Western blot, dot blot, and enzyme-linked immunosorbent assay (ELISA) are specific, simple, and rapid for the detection. Nested PCR is used for the detection of Enterocytozoon hepatopenaei (EHP) with specific primers in every step. PCR-based diagnostic methods are being developed for recent outbreak of EHP in shrimp aquaculture (Rajendran et al. 2016). In this review, we report advancements in diagnosis and control measures based on reports and research works progressed in India.
Common diseases of aquaculture
The diseases caused by infectious agents’ particularly viral and bacterial pathogens pose a major threat to a thriving aquaculture industry and result in severe economic losses worldwide. According to reports, global losses due to shrimp disease are more than US $3000 million. The loss of shrimp production due to WSSV and yellow head virus (YHV) has been estimated about US$1 billion per year in Asian countries (Adams et al. 2008). The viral pathogens encountered in Indian aquaculture were WSSV, hepatopancreatic parvovirus (HPV), hypodermal and hematopoietic necrosis baculovirus (IHHNV) and monodon-type baculovirus (MBV), infectious myonecrosis virus (IMNV) in shrimps, and Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) in prawns and fish nodavirus in sea bass (Hameed et al. 1998, 2001, 2003, 2004; Musthaq et al. 2006). The bacterial pathogens reported in Indian aquaculture were Vibrio harveyi, V. anguillarum, Aeromonas hydrophila, A. caviae, and Edwardsiella tarda (Selvin et al. 2009). A detail strategy is highlighted in this paper to address various tools for the diagnosis of existing and emerging viral and bacterial diseases of shrimp, prawn, and fish.
White spot syndrome virus (WSSV) of shrimp
WSSV of shrimp was isolated for the first time in India by Hameed et al. (1998). Research on morphology, clinical signs, pathogenicity, tissue tropism, effect on host, cause of death due to WSSV, host range, protein- and DNA-based diagnostic methods, recombinant and DNA vaccines, antiviral plant compounds against WSSV, developing in vitro model for WSSV, and application of RNAi techniques to control WSSV are still undergoing. WSSV has been named in different names by different workers. Durand et al. (1996) named WSSV as hypodermal and hematopoietic necrosis baculovirus (HHNBV); Wang et al. (1995) named as Penaeus monodon non-occluded baculovirus (PmNOBIII); Inouye et al. (1994) as rod-shaped nuclear virus (RV-PJ); Whereas Venegas et al. (2000) called as penaeid rod-shaped DNA virus. Wongteerasupaya et al. (1995) and Hameed et al. (1998) named systemic ectodermal and mesodermal baculovirus (SEMBV); Chou et al. (1995) and Lightner (1996) named as white spot baculovirus, but now it has been named in single name as white spot syndrome virus (WSSV). This WSSV has been assigned to new Genus Whispovirus and new family Nimaviridae.
The SDS-PAGE analysis of purified WSSV revealed the presence of three prominent protein bands with molecular weight of 27 (VP28), 22 (VP24) and 18 (VP19) (Hameed et al. 1998). Development polyclonal antibodies against VP28 helped for the detection of WSSV by immunological methods (Western blot, dot blot, and ELISA).
As stated by Hameed et al. (1998), WSSV is highly pathogenic to cultured penaeid shrimp and causing 100 % mortality within 3–10 days, when the viruses were administered by immersion method, oral route, or intramuscular injection. Experimental data revealed that the cells of stomach, gills, and integument are possible sites for viral entry into the cells.
Tissue tropism of WSSV
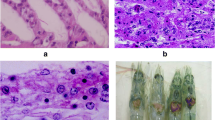
Tissue tropism of WSSV has been studied by histology (Fig. 1), Western blot (Fig. 2), PCR (Fig. 3), and bioassay (Hameed et al. 1998, 2004; Yoganandhan et al. 2003). These studies revealed that the WSSV targets cells of mesodermal and ectodermal organs including gills, heart, hemocytes, antennal gland, lymphoid organs, muscle, stomach, and intestine. Yoganandhan et al. (2003) examined the biochemical and hematological changes provoked by white spot syndrome virus (WSSV) in hemolymph, hepatopancreas, and muscle of shrimp. The changes include the following:
-
1.
Significant reductions in total hemocyte counts (THC)
-
2.
Hemolymph from WSSV-infected shrimp failed to clot
-
3.
Reduction in oxygen consumption and ammonia excretion in WSSV-infected shrimp
-
4.
Dysfunction of respiratory system and causing hypoxia in tissue
-
5.
Reduction in oxygen affinity of hemocyanin
-
6.
Infected animals may die due to anoxia.
The results of their study indicated the failure of vital functions such as clotting of hemolymph defense mechanism, exchange of respiratory gas, and excretion in WSSV-infected shrimp.
Demonstration of WSSV-VP28 protein by Western blot using antiserum of r-VP28 in different organs of WSSV-infected Penaeus monodon. M marker, lane 1 positive control, 2 negative control, 3 eye stalk, 4 gill tissue, 5 heart, 6 stomach, 7 intestine, 8 pleopods, 9 head muscle, 10 abdominal muscle, 11 tail muscle, 12 hepatopancreas
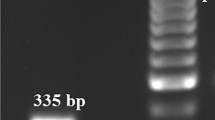
a PCR detection of WSSV-DNA in the experimentally infected shrimp after 12 h.p.i. of WSSV. M marker, 1 positive control, 2 negative control (normal shrimp), 3 eyestalk, 4 heart, 5 gill, 6 head soft tissue, 7 appendages, 8 hemolymph, 9 connective tissue, 10 stomach, 11 hepatopancreas. b Multiplex PCR for simultaneous DNA and RNA viruses of shrimp and prawn
Host range of WSSV
WSSV has been found to be highly pathogenic to penaeid shrimp and has a wide host range that includes crabs, lobster, crayfish, non-decapod crustacean, copepods, and arthropods. More than 100 species of arthropods have been reported as host or carriers of the WSSV either from culture facilities, the wild, or experimental infection. (Hameed et al. 1998, 2000, 2001, 2003, Musthaq et al. 2006). Detection methods used in above-mentioned studies were PCR, in situ hybridization, transmission electron microscopy (TEM), and indirect immunofluorescence assay.
Pathogenicity of WSSV in freshwater crabs
Two species of freshwater crabs (Paratelphusa hydrodomous and P. pulvinata) are found to be highly susceptible to WSSV. This indicated that WSSV maintains its infectivity in freshwater also. This was confirmed by histology, PCR, Western blot, and bioassay test in shrimp (Hameed et al. 2001). Overall, available diagnostic methods for WSSV were histology; DNA-based diagnostic methods such as PCR (single step and nested PCR) and in situ hybridization; protein-based diagnostic methods such as ELISA and Western blot.
Control measures for WSSV
Any practical method to eradicate or inactivate WSSV in the culture systems would be enormous practical benefits for the shrimp farmers and hatchery operators as there are no drugs to treat WSSV-infected shrimp or even vaccination is not possible because shrimp has simple defense mechanism. In C. Abdul Hakeem College, various strategies to control or prevent WSSV infection in shrimp has been experimented. The strategies being followed to contain WSSV outbreaks include,
-
1.
Screening various previously reported antiviral plants against WSSV
-
2.
Use of various recombinant viral proteins as immunostimulants to protect shrimp.
-
3.
Application of RNAi technology to prevent WSSV infection in shrimp by silencing the expression of VP28 gene in WSSV.
-
4.
Use of antibodies raised against VP28 of WSSV as immunotherapy.
-
5.
VP28 gene of WSSV was used for producing recombinant VP28 for making immunostimulant, antibody production for immunotherapy, and dsRNA for gene silencing.
-
6.
Recombinant VP28 protein-induced resistance against WSSV
-
7.
Recombinant VP28 DNA vaccine against WSSV (Li et al. 2010)
In a study conducted on acquired resistance against WSSV via recombinant VP28 protein, purified r-VP28 protein (4 µg per gm of body weight) administered intramuscularly following WSSV challenge showed 0 % PCG and 45.8 % VG at 21 d.p.i. While administration via oral route as coated feed (commercial pellets weighing approximately 0.02 g were coated with approximately 108 inactivated bacteria per pellet) for 10 days and challenged orally with WSSV 10 days after vaccination showed 0 % PCG and 59.4 % VG at 21 d.p.i. The protection conferred by these recombinant protein vaccines has been found to be short lived. Hence, long-term antigen expression through genetic immunization could be a useful strategy to adopt against viral diseases in shrimp.
Application of RNAi technology to control WSSV
In 1998, Andrew Fire and Craig Mello described a new technology that was based on the silencing of specific genes by double-stranded RNA (dsRNA), a technology they called RNA interference (RNAi). RNAi is a phenomenon in which the introduction of double-stranded RNA (dsRNA) into a diverse range of organisms and cell types causes degradation of the complementary mRNA, that is, knock down the expression of genes. The idea is that RNAi makes the gene not to work, or silencing or knock down the expression, it is called post-transcriptional gene silencing. Several recent studies have reported that siRNAs (Westenberg et al. 2005; Xu et al. 2007) or dsRNA synthesized by in vitro methods (Kim et al. 2007; Robalino et al. 2004, 2005) serve as potential therapeutic agents for treating white spot syndrome disease. These approaches are costly and laborious for the synthesis of large amounts of siRNA or dsRNA by in vitro methods. In a study conducted by Sarathi et al. (2008), targeted five major WSSV structural genes VP28, VP26, VP24, VP19, and VP15 to produce corresponding dsRNA because these proteins expressed by these genes are abundantly present in the WSSV and play a vital role during the infection. The VP28 gene is very important because it is involved in WSSV attachment and penetration into shrimp cells, so it was targeted for silencing by bacterially synthesized VP28-dsRNA. This study was the first attempt to use the bacterially synthesized dsRNA encoding genes of WSSV to protect the shrimp against WSSV.
Efficacy of dsRNA against various genes of WSSV
The antiviral activity of bacterially expressed dsRNA against WSSV was tested by intramuscular injection. The shrimp were injected with bacterially expressed dsRNA specific to various genes of WSSV 24 h before WSSV challenge. The cumulative mortalities during the 30 days following the challenge with WSSV in different groups intramuscularly injected with dsRNA corresponding to VP28, VP26, VP24, VP19, VP15, and control dsRNA were 0,100, 37, 66, 37, and 100 %, respectively. Shrimp injected with VP26 dsRNA and control dsRNA showed no survival as observed in positive control group, but mortality of VP26 dsRNA-injected shrimp was significantly delayed when compared to the positive control group.
Infectious hypodermal and hematopoietic necrosis virus
Based on histopathological observations in India (Fig. 4), IHHNV was first reported by Felix and Devaraj in the year 1993. Later Karunasagar et al. (2009) reported the occurrence of IHHNV using molecular methods and it is associated with slow growth. Nested PCR is carried out to identify the viral infection and the PCR-positive IHHNV samples were further sectioned and stained for histological analysis. The phylogenetic analysis obtained from the PCR product of IHHNV-positive samples indicates that the sequences are similar to southeast Asian IHHNV isolates signifies that the entry of virus to India from southeast Asia.
Monodon baculo virus
MBV affects all life stages but severe in post-larval and adult stage. First reported in 1995 by Alavandi (Shrimp Diseases, Their Prevention and Control, CIBA Bulletin-3, 1995). Initially diagnosed by staining with 0.1 % malachite green and multiple spherical intranuclear occlusion bodies were observed under phase contrast microscope (Fig. 5). The Central Institute of Brackish Water Aquaculture (CIBA) has developed SYBR Green-tagged real-time PCR technique for early and precise diagnosis of MBV (Kumar et al. 2014). The assay was non-invasive detects early infection stage of MBV using fecal matter of infected shrimp. RT-PCR was carried out with SYBR Green master mix (PE Applied Biosystems), and amplification and detection were done simultaneously (Fig. 6). Also the results were compared with MBV by conventional PCR. The sensitivity is 10–80 folds higher than the conventional PCR. Also SYBR Green dye will bind more with larger amplicon and give off fluorescence which is higher than the smaller amplicon (Mouillesseaux et al. 2003; Dhar et al. 2001). SYBR Green RT-PCR is an effective assay to detect other types of viral infections such as IHHNV, HPV, and WSSV (Richards et al. 2004; Kumar et al. 2014; Yadav et al. 2015).
Viral nervous necrosis
Viral nervous necrosis (VNN) is a fish viral disease, and the causative agent is the nodavirus, which belong to the Genus Betanoda virus and Family Nodaviridae. It is non-enveloped virus, spherical and 25 nm in diameter. It is reported that this virus infects more than 50 fish species. Stages affected were larvae, juveniles, market size, and adult fish. The clinical signs include darkening of body, lethargic, and spiraling (whirling) movement. The targeted organs are brain, spinal cord, and retina, and rarely affect broodstock gonads. They cause extensive vacuolation in the target organs. Immunization with vaccines may effective in controlling the disease. In India, the occurrence of betanodavirus has been reported for the first time by Azad et al. (2005). In their study, the confirmation was done by histopathology and RT-PCR. This virus has been isolated for the first time in India using sea bass cell lines in our laboratory (Parameswaran et al. 2008). The fish nodavirus has been replicated in sea bass cell lines in large scale and developed three types of vaccines under Indo-Norwegian program on fish vaccine development, which includes whole nodavirus vaccine, recombinant capsid protein vaccine, and DNA vaccine using capsid gene.
Fish cell lines: development, characterization, and applications
Totally, twenty-five cell lines from Indian marine, freshwater, and brackish water fish were developed and all the cell lines have been characterized. All these cell lines are being applied in isolation of fish viruses, using as an in vitro assay system for toxicological studies and gene expression studies. The cell lines developed from fish have been used to isolate fish nodavirus (Parameswaran et al. 2008; Fig. 8) and used for propagation of viruses for developing viral vaccines (Babu et al. 2013). Research has been initiated to develop cell lines from economically important fish Lates calcarifer and that would be India’s first marine fish cell line from Asian sea bass (Hameed et al. 2006). Shrimp aquaculture industry was continuously suffered with intermittent viral outbreaks. The early diagnosis and antiviral drug screening require stable shrimp cell lines. Ma et al. (2015) reported research progress on penaeid shrimp cell line development including media and cultivation methods.
Production of whole nodavirus vaccine using the cell lines
Fish cell lines developed from sea bass, grouper, and Etroplus suratensis were tested for their susceptibility to nodavirus. Viral replication has been confirmed by CPE, RT-PCR, ELISA, TCID50, and real-time PCR. Among the eight cell lines, cell lines of sea bass and grouper were found to be highly susceptible and more suitable for nodaviral propagation in large scale. The cell lines developed from sea bass, grouper, and pearl spot were found to be highly susceptible to nodavirus and the infected cell lines showed clear CPE of multiple vacuolations specific to nodavirus.
Confirmation of nodaviral infection in infected cell lines with prominent CPE by RT-PCR using different primer sets including 175 bp (Nishizawa et al. 1994), 294 bp (Thiery et al. 1999), 426 bp (Nishizawa et al. 1994), 540 bp (CAHC, Melvisharam), and 1017 bp (Huang et al. 2001; modified with restriction).
Vaccine against fish nodavirus
For the production of whole nodavirus vaccine, the virus propagated in SISS cell line was harvested and inactivated by heating at 60 °C in water bath for 1 h as per the protocol followed by Frerichs et al. (2000). The inactivation of virus was confirmed by inoculating the inactivated samples which failed to produce CPE in SISS cell line. The inactivated whole nodavirus was used in vaccination trial in sea bass fingerlings. Relative percent survival (RPS) in fish vaccinated by oral route, immersion method, and intramuscular method was 89.8, 69.7, and 81.3 %, respectively. Asian sea bass was immunized with purified r-FNCP42 and was challenged with nodavirus by intramuscular injection. The vaccinated sea bass was protected from nodaviral infection, and 76 % of RPS was recorded. The processes involved in the preparation of DNA vaccine against fish nodavirus was depicted in Fig. 9, and the in vitro gene expression and transcription analysis of pFNVE38 DNA in SISK cell line by immunohistochemistry and RT-PCR are shown in Fig. 10.
Distribution of pFNVE38 (DNA vaccine) in different organs of sea bass was analyzed by PCR and analysis of capsid gene expression in different organs of sea bass injected with pFNVE38 intramuscularly (Vimal et al. 2014). Analysis of clearance of DNA vaccine in fish tissues at different time intervals showed positive results. With this, RPS of DNA vaccine had been estimated to about 77.3 % in vaccinated fish which in turn indicates its efficacy.
Conclusion
Despite the application of numerous strategies, the problems associated with aquaculture infection still remain unabated. Fitness and virulence studies on different genotypes of etiological agents (viruses) suggest that all the strains are equally virulent, but the one with smaller genomic size is the fittest. Studies also have shown that mixed genotype infection of viruses correlates with lower disease outbreaks. Good management practices including different molecular diagnostic tools help to prevent the occurrence of infection in aquaculture culture systems. In addition to these measures, the best way to eradicate viral diseases is to use PCR-screened SPF broodstock for rearing. The rapid early diagnostics and innovative control measures would decrease the incidences of viral outbreaks in aquaculture. The new approaches including RNAi, genome engineering, and synthetic biology would possibly revamp aquaculture industry free from viral outbreaks. Further research needs to be carried out to deploy effective strategies to overcome the spread of diseases by developing different vaccines based on various viral proteins and also to develop a cost-effective and practical mode for administering it.
References
Adams A, Aoki T, Berthe CJ, Grisez L, Karunasagar I (2008) Recent technological advancements on aquatic animal health and their contributions toward reducing disease risks - a review. In: Bondad-Reantaso MG, Mohan CV, Crumlish Margaret, Subasinghe RP (eds) Diseases in Asian Aquaculture VI. Fish Health Section. Asian Fisheries Society, Manila, pp 71–88
Azad IS, Shekhar MS, Thirunavukkarasu AR, Poornima M, Kailasam M, Rajan JJS, Ali SA, Abraham Mathew, Ravichandran P (2005) Nodavirus infection causes mass mortalities in hatchery produced larvae of Asian seabass, Lates calcarifer, Bloch: first report from India. Dis Aquat Org 63:113–118
Babu SV, Abdul Majeed S, Nambi KSN, Taju G, Madan N, Sundar Raj N et al (2013) Comparison of betanodavirus replication efficiency in ten Indian fish cell lines. Arch Virol 158:1367–1375
Chou HY, Huang CY, Wang CH, Chiang HC, Lo CF (1995) Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis Aquat Org 23:165–173
Dhar AK, Roux MR, Klimpel KR (2001) Detection and quantification of infectious hematopoietic and haematopoietic necrosis virus (IHHNV) and white spot virus (WSV) of shrimp by real time quantitative PCR using SYBR Green chemistry. J Clin Microbiol 39:2835–2845
Durand J, Parrado EA, Massey DS (1996) Migradollars and development: a reconsideration of the Mexican case. Int Migr Rev 30:423–444
Frerichs GN, Tweedie A, Starkey WG, Richards RH (2000) Temperature, pH and electrolyte sensitivity, and heat, UV and disinfectant inactivation of sea bass (Dicentrarchus labrax) neuropathy nodavirus. Aquaculture 185:13–24
Hameed ASS, Anil Kumar M, Stephen Raj ML, Kunthala J (1998) Studies on the pathogenicity of systemic ectodermal and mesodermal baculovirus and its detection in shrimp by immunological methods. Aquaculture 160:31–45
Hameed ASS, Xavier Charles M, Anilkumar M (2000) Tolerance of Macrobrachium rosenbergii to white spot syndrome virus. Aquaculture 183:207–213
Hameed ASS, Yoganandhan K, Sathish S, Murugan V, Rasheed M, Jayaraman K (2001) Experimental pathogenicity of white spot syndrome virus (WSSV) in two freshwater crabs (Partelphusa hydrodomous and P. pulvinata). Aquaculture 201:179–186
Hameed ASS, Balasubramanian G, Syed Musthaq S, Yoganandhan K (2003) Experimental infection of twenty species of Indian marine crabs with white spot syndrome virus (WSSV). Dis Aquat Org 57:157–161
Hameed ASS, Yoganandhan K, Sri Widada J, Bonami JR (2004) Studies on the occurrence of Macrobrachium rosenbergii nodavirus and extra small virus-like particles associated with white tail disease of M. rosenbergii in India by RT-PCR detection. Aquaculture 238:127–133
Hameed ASS, Parameswaran V, Shukla R, Singh ISB, Thirunavukkarasu AR, Bhonde RR (2006) Establishment and characterisation of India’s first marine fish cell line (SISK) from the kidney of sea bass (Lates calcarifer). Aquaculture 257:92–103
Huang B, Tan C, Chang SF, Munday B, Mathew JA, Ngoh GH, Kwang J (2001) Detection of nodavirus in barramundi, Lates calcarifer (Bloch), using recombinant coat protein-based ELISA and RT-PCR. J Fish Dis 24:135–141
Inouye K, Miwa S, Oseko N, Nakano H, Kimura T, Momoyama K, Hiraoka M (1994) Mass mortalities of cultured kuruma shrimp Penaeus japonicus in Japan in 1993: electron microscopic evidence of the causative virus. Fish Pathol 29:149–158
Jana BB, Jana S (2003) The Potential and Sustainability of Aquaculture in India. J Appl Aquacult 13:3–4
Karunasagar I, Rai P, Pradeep B, Karunasagar I (2009) Detection of viruses in Penaeus monodon from India showing signs of slow growth syndrome. Aquaculture 289:231–235
Kim CS, Kosuke Z, Nam YK, Kim SK, Kim KH (2007) Protection of shrimp (Penaeus chinensis) against white spot syndrome virus (WSSV) challenge by double stranded RNA. Fish Shellfish Immunol 23:242–246
Kumar RD, Sanjuktha M, Rajan JJS, Ananda Bharathi R, Santiago TC, Alavandi SV, Poornima M (2014) Development of SYBR Green based real time PCR assay for detection of monodon baculovirus in Penaeus monodon. J Virol Methods 205:81–86
Li X, Liu QH, Hou L, Huang J (2010) Effect of VP28 DNA vaccine on white spot syndrome virus in Litopenaeus vannamei. Aquacul Int 18:1035–1044
Li K, Liu L, Clausen JH, Lu M, Dalsgaard A (2016) Management measures to control diseases reported by tilapia (Oreochromis spp.) and whiteleg shrimp (Litopenaeus vannamei) farmers in guangdong, china. Aquaculture 457:91–99
Lightner DV (1996) White spot syndrome baculovirus complex (WSBV). In: Lightner DV (ed) A handbook of pathology and diagnostic procedures for diseases of penaeid shrimp Sect 3, viruses/WSBV. World Aquaculture Society, Baton Rouge, pp 1–8
Ma J, Zeng L, Lu Y (2015) Penaeid shrimp cell culture and its applications. Rev Aquacult 1–11
Mouillesseaux KP, Klimpel KR, Dhar AK (2003) Improvement in the specificity and sensitivity of detection for the Taura syndrome virus and yellow head virus of penaeid shrimp by increasing the amplicon size in SYBR Green real-time PCR. J Virol Methods 111:121–127
Musthaq S, Yoganandhan K, Sudhakaran R, Kumar S, Sahul Hameed AS (2006) Neutralization of white spot syndrome virus of shrimp by antiserum raised against recombinant VP28. Aquaculture 253:98–104
Nishizawa T, Mori K, Nakai T, Furusawa I, Muroga K (1994) Polymerase chain reaction (PCR) amplification of RNA of stripped jack nervous necrosis virus (SJNNV). Dis Aquat Org 18:103–107
Parameswaran V, Kumar SR, Ishaq AVP, Hameed ASS (2008) A fish nodavirus associated with mass mortality in hatchery-reared Asian Sea bass, Lates calcarifer. Aquaculture 275:366–369
Rajendran KV, Shivam SP, Praveena E, Rajan JJS, Kumar TS, Avunje S et al (2016) Emergence of Enterocytozoon hepatopenaei (EHP) in farmed Penaeus (Litopenaeus) vannamei in India. Aquaculture 454:272–280
Richards GP, Watson MA, Kingsley DH (2004) A SYBR Green real-time RT-PCR method to detect and quantitate Norwalk virus in stools. J Virol Methods 116:63–70
Robalino J, Browdy CL, Prior S, Metz A, Parnell P, Gross P, Warr G (2004) Induction of antiviral immunity by double-stranded RNA in a marine invertebrate. J Virol 78:10442–10448
Robalino J, Bartlett T, Shepard E, Prior S, Jaramillo G, Scura E, Chapman RW, Gross PS, Browdy CL, Warr GW (2005) Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response? J Virol 79:13561–13571
Sarathi M, Simon MC, Ishaq Ahmed VP, Rajesh Kumar S, Sahul Hameed AS (2008) Silencing VP28 gene of white spot syndrome virus of shrimp by bacterially expressed dsRNA. Mar Biotechnol 10:198–206
Selvin J, Ninawe AS, Lipton AP (2009) Shrimp disease management: prospective approaches. ANE Publishers, New Delhi, p 281
Thiery R, Arnauld C, Delsert C (1999) Two isolates of sea bass, Dicentrarchus labrax L., nervous necrosis virus with distinct genomes. J Fish Dis 22:201–207
Venegas CA, Nonaka L, Mushiake K, Nishizawa T, Muroga K (2000) Quasi-immune response of Penaeus japonicus to penaeid rod-shaped DNA virus (PRDV). Dis Aquat Org 42:83–89
Vimal S, Farook MA, Madan N, Majeed AS, Nambi KSN, Taju G, Raj SN, Venu S, Subburaj R, Thirunavukkarasu AR, Hameed ASS (2014) Development, distribution and expression of a DNA vaccine against nodavirus in Asian Seabass, Lates calcarifier (Bloch, 1790). Aquac Res 47:1209–1220
Wang C, Lo C, Leu J, Chou C, Yeh P, Chou H, Tung M, Chang C, Su M, Kou G (1995) Purification and genomic analysis of baculovirus associated with white spot syndrome (WSBV) of Penaeus monodon. Dis Aquat Org 23:239–242
Westenberg M, Heinhuis B, Zuidema D, Vlak JM (2005) siRNA injection induces sequence-independent protection in Penaeus monodon against white spot syndrome virus. Virus Res 114:133–139
Wongteerasupaya C, Vickers JE, Sriurairatana S, Nash GL, Akarajamorn A, Boonsaeng V, Panyim S, Tassanakajon A, Withyachumnamkul B, Flegel TW (1995) A non-occluded systemic baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn Penaeus monodon. Dis Aquat Org 21:69–77
Xu J, Han F, Zhang X (2007) Silencing shrimp white spot syndrome virus (WSSV) genes by siRNA. Antiviral Res 73:126–131
Yadav R, Paria Anutosh, Smruti Mankame M, Makesh Aparna Chaudhari, Rajendran KV (2015) Development of SYBR Green and TaqMan quantitative real-time PCR assays for hepatopancreatic parvovirus (HPV) infecting Penaeus monodon in India. Mol Cell Probes 29:442–448
Yoganandhan K, Thirupathi S, Sahul Hameed AS (2003) Biochemical physiological and hematological changes in white spot syndrome virus-infected shrimp, Penaeus indicus. Aquaculture 221:1–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ninawe, A.S., Hameed, A.S.S. & Selvin, J. Advancements in diagnosis and control measures of viral pathogens in aquaculture: an Indian perspective. Aquacult Int 25, 251–264 (2017). https://doi.org/10.1007/s10499-016-0026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0026-9