Abstract
White spot syndrome virus (WSSV) occurs worldwide and causes high mortality and considerable economic damage to the shrimp farming industry. Considering the global environmental, the economic and sociological importance of shrimp farming, and the constraints of high intensity cultivation, development of novel control measures against the outbreak of WSSV become inevitable. In this study, we have explored the protective efficacy of DNA vaccination and tissue distribution of the recombinant plasmid in immunized Litopenaeus vannamei. The VP28 gene was cloned in the eukaryotic expression vector pVAX1, and the construct vector was named as lpv28. The protective effect of lpv28 against WSSV was evaluated in L. vannamei by injecting lpv28 construct and later challenging with WSSV. Expression of these proteins from the recombinant plasmids was confirmed in vitro by RT-PCR and Western blot analysis. The result of vaccination trials showed that a survival rate in shrimp vaccinated with lpv28 was 52.5% at most compared to control groups (100% mortality). The immunological parameters analyzed in the vaccinated and control groups showed that the vaccinated groups owned a high level of lysozyme, alkaline phosphatase, and total superoxide dismutase when compared to the control group. Furthermore, protein expression analysis indicated that VP28 can be detected in gill, muscle and head soft tissue of the shrimps in the immunized group after 14th day injection. Thus, the result indicated that DNA vaccination strategy has a potential utility against WSSV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White spot syndrome virus (WSSV) has been one of the most serious viral disease for the shrimp culture industry broken out in China and Southeast Asia since 1990s. At present, it caused large economic loss, which therefore attracts much attention of many research teams throughout the world (Flegel 1997; Lightner 1996). WSSV did not only attack different kinds of shrimps, but also other aquatic crustacean such as crab, crayfishes, lobster, etc. (Sahul Hameed et al. 2001, 2003; Syed Musthaq et al. 2006a). Based on the existing biological evidence, the virus contains a 305-kb double-stranded circular DNA and has been approved recently to be a representative of a new genus, Whispovirus, by the International Committee on Taxonomy of Viruses (ICTV) (Mayo 2002).
To prevent and cure WSSV, traditional control strategies such as change of environmental conditions, stocking of specific pathogen-free shrimp post-larvae and increase in disease resistance by oral immunostimulants are used to control WSSV infection (Lo et al. 1996; Chang et al. 1998). Recently, the development of vaccines against WSSV would be considerable, because shrimps, which are invertebrates, lack specific immunity, while possess effective and quick immunoreaction mechanism to identify foreign matter, including cellular immunity and humoral immunity. And some studies show that vaccination against WSSV is feasible using either inactivated pathogens, recombinant proteins or DNA (Witteveldt et al. 2004; Bright Singh et al. 2005; Jha et al. 2006; Vaseeharan et al. 2006; Rout et al. 2005; Rajesh Kumar et al. 2008). The protective responses of shrimp can be inducible (Loker et al. 2004; Kurtz and Franz 2003).
The experiment aims at successfully construct DNA vaccine, which will be injected into the shrimps. Through comparison with other non-immunized shrimps (control groups), we aimed at demonstrating the efficacy of DNA vaccine and analyze how it will affect the immune parameters of lysozyme, T-SOD, AKP in the shrimps.
Materials and methods
The experimental animals
The shrimps used in this experiment were obtained from QingDao Laoshan Shrimp Aquacultural Grounds. The shrimps were kept in the tanks of 50 cm long, 30 cm high, 30 cm wide, with air-lift biological filters, and they were fed with artificial pellet feed. They were kept in these tanks for 7 days for acclimatization prior to the experiments. Then, five shrimps were picked out randomly from those shrimps for PCR examination to exclude viral infection, because only healthy individuals were used.
Virus preparation
The virus used in this study was isolated from infected crayfish Procambarus clarkii from China and stored in Yellow Sea Fisheries Research Institute, Qingdao, China. Infected tissue was homogenized in TNE buffer (50 mM Tris/HCl, 400 mM NaCl, 5 mM EDTA, pH 8.5) and then centrifuged at 3,500g for 5 min at 4°C. The supernatant was then recentrifuged at 15,000g for 30 min at 4°C, and sediment was resuspended in TN buffer (20 mM Tris/HCl, 400 mM NaCl, pH 7.4). The number of copies of the virus was calculated (Zhou et al. 2007).
DNA purification
Viral DNA was isolated from purified virions by treatment with proteinase K (0.2 mg/ml) and Sarkosyl (1%) at 65°C for 2 h, followed by phenol and chloroform extraction and dialysis against TE. The purity and concentration of the DNA were determined by agarose gel electrophoresis.
Construction and preparation of DNA vaccine
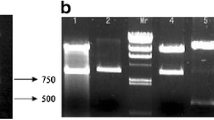
VP28 gene was amplified by the polymerase chain reaction (PCR) using WSSV genome DNA as template and specific primers (VP28-S 5′CGCGGATTCTATGGATCTTTCTTTCTTTCAC; VP28-A 5′CGCGAATTCTTACTCGGTCTCAGTG). Then, amplified VP28 gene was cloned into the eukaryotic expression vector pVAX1. This vector carries the human cytomegalovirus (CMV) immediate early promoter, the bovine growth hormone (BGH) polyadenylation signal, the kanamycin resistance gene and the pUC origin. The forward primer contained a BamHI restriction site, the reverse primer contained a EcoRI restriction site. Then, the PCR product was cloned into pVAX1 and transformed into Escherichia coli Top 10 cells (Tiangen.com). The recombinant clone was selected by kanamycin resistance, and monoclone was cultured in 10 ml of LB liquid culture medium, shaked a night at 37°C; then, plasmid DNA was extracted by Extraction Kit (BioFlux, China). The recombinant clone was digested using the restriction enzymes BamHI and EcoRI and analyzed by electrophoresis on 0.7% agar gel. The recombinant was named as lpv28.
Preparation of anti-VP28 polyclonal antibody
Recombinant protein VP28 used to produce antiserum was expressed and purified as previously described (Liu et al. 2009). Purified VP28 (20 μg) was mixed with an equal volume of Freund’s complete adjuvant (Sigma) for the first injection. Subsequent injections were conducted using 20 μg of antigen mixed with an equal volume of Freund’s incomplete adjuvant (Sigma). Four days after the last injection, mice were exsanguinated, and antisera were collected.
Immunization and challenge
Shrimps were divided into six big groups (three big groups for first trial, three big groups for last trial); each big group divided into two small groups (lpv28 group, PBS group, pVAX1group, 20 shrimps/group), then shrimps of each group were injected with lpv28, PBS and pVAX1, respectively (20 μg/shrimp). On the 7th day after immunization, the shrimps in the first three big groups were challenged by intramuscular injection with WSSV (102–103 copies/μl, muscle injection), and shrimps were observed every day. On the 14th day after immunization, the shrimps in the last three big groups were challenged with WSSV (102–103 copies/μl, muscle injection), and shrimps were observed every day. The dead shrimps were collected, and the DNA was extracted from gill to identify whether the shrimps were infected with WSSV.
Analysis of immunological parameters
For immunological parameters analysis, 40 shrimps were injected with lpv28 and PBS, respectively (20 μg/shrimp, 20 shrimps/group). Hemolymph from five shrimps at various stages of immunization was collected in anticoagulant solution (3.8% sodium citrate) at an anticoagulant hemolymph/ratio of 1:2. The supernatant was collected by centrifugation at 800g for 10 min. The supernatant was stored and used for analyzing immunological index.
Lysozyme analysis
Lysozyme was analyzed according to the method of Grinde et al. (1988). Samples were incubated with Micrococcus lysodeikticus (0.2 mg/ml), pH 6.2, at 25°C, and the change in absorbance was recorded at 520 nm over 3 min. The specific activity (IU/mg protein) for lysozyme was determined.
Total SOD analysis
Superoxide dismutase activity was evaluated by measuring the inhibition of pyrogallol autoxidation (Jiang and Mu 1999), and one unit of the SOD activity was defined as the amount of enzyme that caused 50% inhibition of pyrogallol autoxidation.
ALP analysis
Alkaline phosphatase (ALP) activities were examined by using disodium phenyl phosphate as a substrate (Jiang and Mu 1999). One unit of ALP activity was defined as the appearance of 1 mg hydroxybenzene after reaction with the substrate for 60 or 15 min at 37°C.
PCR, RT-PCR and western blot analysis of lpv28 expression in vivo
For PCR, RT-PCR, and protein expression analysis, 20 μg of lpv28 was injected to 30 shrimps, and six shrimps were used to isolate DNA and RNA from various tissues on 3rd, 7th, 10th, 14th day post-immunization. On the 3rd, 7th, 10th, 14th day after immunization, DNA was extracted from gill, muscle, head soft tissue of the shrimp and analyzed by PCR using special primers. Total RNA was extracted from frozen gills using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions and digested with RNase-free DNaseI (TaKaRa) before carrying out the RT-PCR. Reverse transcription of total RNA was done according to kit’s recommendations (Tiagen.com). In addition, the expression of the VP28 gene of WSSV at the transcriptional level was analyzed by RT-PCR. Finally, Western blotting method was used to analyze protein expression of immunization shrimp from the gill, muscle, head soft tissue. Briefly, shrimp muscle was solubilized in lysis buffer (50 mM Tris–HCl, 100 mM NaCl, 1 mM PMSF, 1% Triton X-100, pH 7.5) at 4°C. After centrifugation at 10,000×g, extraction muscle protein was subjected to 12% SDS–PAGE analysis according to the method of Laemmli (Laemmli 1970) and then transferred onto nitrocellulose membranes using a semidry electroblot apparatus (Bio-Rad). The membrane was immersed in blocking buffer (3% BSA, 20 mM Tris, 0.9% NaCl, pH 7.2) at 4°C overnight, followed by incubation with polyclonal mouse anti-VP28 (1:500) and mouse antibody (1:1000)(HRP-labeling) for 1 h, respectively. Then the membrane was washed twice with 10 ml of PBST for 5 min with gentle agitation. Subsequently, detection was performed with a DAB (4-chloro-1-naphthol, Sigma) solution (1 mg/ml).
Statistical analysis
All data obtained from the experiments were analyzed using one-way ANOVA (P < 0.05 as significant level).
Result
Construction of DNA vaccine
VP28 gene fragment was acquired by PCR amplification and cloned into pVAX1 vector. The recombinant VP28-pVAX1 was confirmed by restriction digestion (Fig. 1) and DNA sequencing. The VP28 can be seen clearly (Fig. 1), it means that the DNA vaccine was constructed successfully.
Immunological assays
The lysozyme activity of shrimp treated with lpv28 was significantly (P < 0.01) higher than the negative control groups (PBS) on 7th, 10th day post-immunization (Fig. 2). After 10 days post-immunization, the lysozyme activity of shrimp decreased gradually but it did not reach levels similar to control groups. The level of AKP activity observed in the treatment shrimp on 14th day was higher than that observed in the control group. The level of AKP did not change significantly in the treatment shrimp on 3th, 7th, and 10th day compared to the negative control groups at the same days (Fig. 3). Significantly increasing levels of T-SOD in the shrimp treated on 3rd and 7th day was observed compared to the negative control groups (PBS). Then, T-SOD activity reached levels similar in both the groups (Fig. 4).
In vivo expression of lpv28 by PCR, RT-PCR and western blot analysis
The distribution of VP28 was determined in different tissues of immunized shrimp by PCR, and results showed that the expression of VP28 protein in gut, muscle, and head tissue on day 3rd, 7th, 10th, and 14th day post-immunization (Fig. 5a–d). In vivo transcriptional analysis indicated that the presence of VP28 in muscle on 3rd, 7th, 10th, and 14th post-immunization (Fig. 6). No band was detected in muscle tissue of control group (PBS and pVAX1) by PCR and RT-PCR analysis. For further confirming this, Western blotting analysis was performed. VP28 protein can be detected in muscle tissue on day 7th and 14th post-immunized shrimp. No protein band showed in the control shrimp (Fig. 7).
Tissue distribution of the lpv28 DNA in the immunized shrimp at different time by PCR analysis with VP28 primers on day 3, (a) day 7, (b)day 10 (c) and day 14 (d) after vaccination. Lane M 2,000 bp Marker; Lane 1 control group inject with PBS into the shrimp; Lane 2 control group inject with pVAX1 into the shrimp; Lane 3 DNA template from gills; Lane 4 DNA template from muscle; Lane 5 DNA template from head soft tissue
In vivo transcriptional analysis of the DNA vaccine construct in shrimp muscle. Lane M 2,000 bp marker; Lane 1 control group inject with PBS 7 days after immunization; Lane 2 control group inject with pVAX1 7 days after immunization; Lane 3 RT-PCR product from immunized shrimp muscle 3 days after immunization; Lane 4 RT-PCR product from immunized shrimp muscle 7 days after immunization; Lane 5 RT-PCR product from immunized shrimp muscle 10 days after immunization; Lane 6 RT-PCR product from immunized shrimp muscle 14 days after immunization
Challenge and immune experiment
The groups of shrimp immunized with lpv28, pVAX1, and PBS were challenged with WSSV on the 7th and 14th day post-immunization. The survival rate of group treated with lpv28 on the 7th day post-immunization reached 52.5%, whereas 100% mortality was observed in control groups (pVAX1 group and PBS group) (Fig. 8) on 5th day post-challenge. The calculated survival percentage was statistically significant when compared to control groups of shrimp treated with pVAX1 and PBS (P < 0.01) on 5th day post-challenge. Only 20% survival rate was observed in the groups treated with lpv28 on the 14th day post-immunization, whereas 100% mortality was observed in control groups (pVAX1 group and PBS group) on 5th day post-challenge (Fig. 9). A significant retarding cumulative mortality was observed in groups immunized with lpv28. Mortality occurred significantly later in the groups immunized with lpv28.
Discussion
Shrimps belong to the invertebrates, which possess non-specific immune system and differ from fish, which possess specific immune systems (Kimbrell and Beutler 2001). However, recent research showed that the host response to pathogen infection in other invertebrate species revealed that the invertebrate immune system is capable of priming and immune memory (Kurtz and Franz 2003).
The result of this experiment indicated that DNA vaccine-lpv28 can be effectively expressed in the body of shrimps and was able to protect L. vannamei against WSSV infection as supported by survival rates. The VP28 gene of WSSV was selected because it plays a role in the initial period when the disease breaks out (Van Hulten et al. 2001). And the other important reason for selecting VP28 gene for DNA vaccine construction is its significant protective effect in shrimp against WSSV (Van Hulten et al. 2001; Syed Musthaq et al. 2006b). In this experiment, it has been demonstrated that lpv28 has been expressed in different tissues of the L. vannamei such as the gill, muscle, and the head soft tissues, etc. But the intensity of expression declines with the increase in days after immunization, because survival rate decreases to 20% compared to 52.5% obtained by challenging shrimps 7 days after immunization. Much data indicated that the protection against WSSV may require continuos immunogenic protein in shrimp tissues. DNA constructs may help for persistent expression of immunogenic proteins in shrimp tissue and be more suitable for long-term protection instead of protein vaccines in the case of shrimp. Previous study indicated that shrimp intramuscularly injected with a DNA construct showed resistance to the WSSV for about 30 days (Rajesh Kumar et al. 2008). Rout (Rout et al. 2007) also reported that intramuscularly DNA vaccinated shrimp developed resistance against this virus for about 7 weeks.
In crustacean cells, phosphatase is the most important element of lysosomal enzymes, which perform the double function of digestion and defense (Jiang and Mu 1999). The lysozyme activity of the groups immunized with lpv28 is stronger than that of the control group. On the 10th day of post-immunization, the activity of LZM reached the highest level and then it begins to decline. The LZM activity changed with the efficiency of protection. This suggested that LZM probably plays an extremely important role in fighting against WSSV.
Superoxide dismutase is one of the main antioxidant defense pathways that accelerates the conversion of O2 − to H2O2 and O2 and thereby prevents the direct toxic ejects of the reactive oxygen species (Fridovich 1989). Equally, T-SOD data indicated that the amount of T-SOD in shrimps was higher than that of the compared group after being injected lpv28. In the immunized group, after being injected the vaccine, the quantity of T-SOD increases dramatically and then dropped gradually with the days going on. The higher content of T-SOD in shrimp corresponds to the higher protection rate against WSSV.
Concerning AKP, the activity almost remained the same during the first 13 days after being injected lpv28, but on the 14th day post-immunization, it was much more active than that of other days in immunized shrimps compared to control group. This phenomenon indicates that lpv28 is able to induce the variation in AKP in shrimp; however, it is just a simple change of enzyme activity, which was ineffective in fighting against WSSV, because the quantity of AKP on the 7th day after immunization was obviously less than that of the 14th day. While in terms of the protecting ability, the survival rate of shrimps challenged 7 days after immunization was indeed higher than that of after 14 days. Future research will be addressed at studying how to express DNA vaccine more effectively and persistently.
References
Bright Singh IS, Manjusha M, Somnath Pai S, Rosamma Philip (2005) Fenneropenaeus indicus is protected from white spot disease by oral administration of inactivated white spot syndrome virus. Dis Aquat Org 66:265–270
Chang PS, Chen HC, Wang YC (1998) Detection of white spot syndrome associated baculovirus (WSBV) in experimentally infected wild shrimp, crabs and lobsters by in situ hybridization. Aquaculture 164:233–242
Flegel TW (1997) Major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World J Microbiol Biotechnol 13:433–442
Fridovich I (1989) Superoxide dismutases an adaptation to aparamagnetic gas. J Biol Chem 264:7761–7764
Grinde B, Lie O, Poppe T, Salte R (1988) Species and individual variation in lysozyme activity in fish of interest in aquaculture. Aquaculture 68:299–304
Jha RK, Xu ZR, Bai SJ, Sun JY, Li WF, Shen J (2006) Protection of Procambarus clarkii against white spot syndrome virus using vaccine expressed in Pichia pastoris. Fish Shellfish Immunol 22:295–307
Jiang XL, Mu HJ (1999) Immune mechanisms of penaeid shrimp. In: Guan HS (ed) Studies on the immunology, cell culture and disease of marine animals. Shandong Science and technology Press, Jinan, China, pp 5–21 (in Chinese)
Kimbrell DA, Beutler B (2001) The evolution and genetics of innate immunity. Nat Rev Gen 2:256–267
Kurtz J, Franz K (2003) Innate defence: evidence for memory in invertebrate immunity. Nature 425:37–38
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lightner DV (1996) A handbook of pathology and diagnostic procedures for diseases of penaeid shrimp. World Aquaculture Society, Baton Rouge, LA, USA, p 304
Liu QH, Ma CY, Chen WB, Zhang XL, Dong SL, Huang J (2009) White spot syndrome virus VP37 interacts with VP28 and VP26. Dis Aquat Org 85:23–30
Lo CF, Ho CH, Peng SE, Chen CH, Hsu HC, Chiu YL (1996) White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis Aquat Org 27:215–225
Loker ES, Adema CM, Zhang SM, Kepler TB (2004) Invertebrate immune systems-not homogeneous, not simple, not well understood. Immunol Rev 198:10–24
Mayo MA (2002) A summary of taxonomic changes recently approved by ICTV. Arch Virol 147:1655–1656
Rajesh Kumar S, Ishaq Ahamed VP, Sarathi M, Nazeer Basha A, Sahul Hameed AS (2008) Immunological responses of Penaeus monodon to DNA vaccine and its efficacy to protect shrimp against white spot syndrome virus (WSSV). Fish Shellfish Immunol 244:467–478
Rout N, Citarasu T, Ravindran R, Murugan V (2005) Transcriptional and translational expression profile of a white spot syndrome viral (WSSV) gene in different organs of infected shrimp. Aquaculture 245:31–38
Rout N, Kumar S, Jaganmohan S, Murugan V (2007) DNA vaccines encoding viral envelope proteins confer protective immunity against WSSV in black tiger shrimp. Vaccine 25:2778–2786
Sahul Hameed AS, Yoganandhan K, Sathish S, Murugan V, Rasheed M, Jayaraman K (2001) Experimental pathogenicity of white spot syndrome virus (WSSV) in two freshwater crabs (Partelphusa hydrodomous and P. pulvinata). Aquaculture 201:179–186
Sahul Hameed AS, Balasubramanian G, Syed Musthaq S, Yoganandhan K (2003) Experimental infection of twenty species of Indian marine crabs with white spot syndrome virus (WSSV). Dis Aquat Org 57:157–161
Syed Musthaq S, Sudhakaran R, Balasubramanian G, Sahul Hameed AS (2006a) Experimental transmission and tissue tropism of white spot syndrome virus (WSSV) in two species of lobsters, Panulirus homarus and Panulirus ornatus. J Invertebr Pathol 93:75–80
Syed Musthaq S, Yoganandhan K, Sudhakaran R, Rajesh Kumar S, Sahul Hameed AS (2006b) Neutralization of white spot syndrome virus of shrimp by antiserum raised against recombinant VP28. Aquaculture 253:98–104
Van Hulten MCW, Witteveldt J, Snippe M, Vlak JM (2001) White spot syndrome virus envelope protein VP28 is involved in the systemic infection of shrimp. Virology 285:228–233
Vaseeharan B, Prem Anand T, Murugan T, Chen JC (2006) Shrimp vaccination trials with the VP292 protein of white spot syndrome virus. Lett Appl Microbiol 43:137–142
Witteveldt J, Cifuentes C, Vlak JM, van Hulten MCW (2004) Protection of Penaeus monodon against white spot syndrome virus by oral vaccination. J Virol 78:2057–2061
Zhou Q, Qi YP, Yang F (2007) Application of spectrophotometry to evaluate the concentration of purified white spot syndrome virus. J Virol Meth 146:288–292
Acknowledgment
This study was supported by the High Technology Development Program of China (863) (Grant: 2006AA100312), the special project for shrimp (Grant: 200803012), the National Basic Research Program (973) of China (Grant: 2006CB101801), and the National Science Foundation of China (Grant: 30871942)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Liu, Qh., Hou, L. et al. Effect of VP28 DNA vaccine on white spot syndrome virus in Litopenaeus vannamei . Aquacult Int 18, 1035–1044 (2010). https://doi.org/10.1007/s10499-010-9321-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-010-9321-z