Abstract
This study aimed to evaluate the protective effects of quercetin on the biochemical parameters, immunity, and growth performance in malathion-exposed common carp, Cyprinus carpio. The methods six experimental groups, including the control group, fish exposed to concentrations of 1.04 and 2.08 mg/l malathion, fish supplemented with quercetin (200 mg/kg diet), and fish treated with quercetin + malathion for 21 days, were considered for the experiment. After the feeding period, in results the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GST) were significantly decreased in the hepatocyte, while malondialdehyde (MDA) content increased in response to malathion. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities and glucose, cortisol, and urea levels significantly increased after exposure to malathion. Exposure of fish to malathion-induced decreases in protease, lysozyme, and alternative complement (ACH50) activities and total immunoglobulin (total Ig) in the mucosa. Changes in other parameters were different depending on malathion concentrations. The supplementation of fish with quercetin had no ameliorating effect on the malathion-related alternations of mucosal lysozyme and protease activities. However, quercetin ameliorated the depressing effects of malathion on biochemical and immunological parameters. Changes in the growth performance and hematological parameters indicated the toxic effect of malathion. In conclusion, quercetin could efficiently reduce the toxic effects of malathion on the biochemical, immune, and hematological parameters of the common carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of aquatic environments is known as one of the most important environmental problems in the world. Today, a variety of pollutants such as pesticides, heavy metals, oils and their derivatives, and hydrocarbons threaten the life of organisms in both freshwater and seawater (Khabbazi et al. 2015; Heidary et al. 2016). Organophosphate insecticides are used in Iran to control pests and thus increase crop yield (Chorehi et al. 2013; Hedayati et al. 2015; Shahbazi Naserabad et al. 2015; Bekele et al. 2021). Malathion (C10H19O6PS2) is one of the most widely used organophosphate pesticides in the world (Bharti and Rasool 2021). Growing use of malathion in urban and agricultural environments has increased the release of this insecticide into aquatic ecosystems mainly through surface runoff, farmland drainage, or direct application (Bharti and Rasool 2021; Rebechi et al. 2021). Following penetration and leakage of pesticides into surface waters, they can enter fish’s body through the food chain and absorption via the skin and gills, causing oxidative stress, genotoxicity, and behavioral and physiological changes (Ince et al. 2017; Ullah et al. 2018; Guo et al. 2021).
Physiological and behavioral disorders have been observed in experiments testing the effects of malathion on aquatic organisms (Shahbazi Naserabad et al. 2015; Alavinia et al. 2019). Bharti and Rasool (2021) detected that spotted snakehead, Channa punctatus (Bloch), shows biochemical changes and histopathological lesions after exposure to malathion. Histopathological damages and changes in the expression of oncogenes were also found in tambaqui, Colossoma macropomum, after exposure to malathion (de Souza et al. 2020). Malathion caused hematotoxicity and also disruptions in biochemical homeostasis in catfish (Heteropneustes fossilis) and rohu (Labeo rohita, Hamilton) (Karmakar et al., 2013; Ullah et al. 2018). Endocrine disruptive toxicity was observed in male zebrafish, Danio rerio, and catfish, Clarias batrachus (Linn.), following exposure to malathion (Lal et al. 2013; Guo et al. 2021). The genotoxicity and neurotoxicity potential of malathion was shown in the rainbow trout, Oncorhynchus mykiss (Alavinia et al. 2019).
Due to the increasing growth of industries and following increases in environmental pollutants, exposure of fish to xenobiotics seems to be an inevitable event (Heidary et al. 2016; Ballesteros et al. 2017; Burgos-Aceves et al. 2019; Ibor et al. 2019). Therefore, researchers are looking for practical ways to reduce the toxicity of pollutants in aquatic animals.
Today, use of dietary supplements to improve the immune system against environmental stressors, especially diseases and toxins in fish and other vertebrates, has widely increased (Fallahpour et al. 2014; Solomon et al., 2018; Ghafarifarsani et al. 2021b; Hedayati et al. 2021; Rashidian et al. 2022). One of these ways is use of natural and synthetic antioxidants to improve the detoxifying and antioxidant defense system in organisms (Solomon et al., 2018; Hajirezaee et al., 2020; Zeng et al. 2021).
Quercetin is a plant flavonoid, which is absorbed in gut cells as glycosides and enters the intestinal lumen after being hydrolyzed to glycine (Parhi et al. 2020). Intestinal microflora plays a vital role in the hydrolysis and absorption of quercetin in the intestine (Liu et al. 2014; Jia et al. 2019; Zhang et al. 2020).
As a natural antioxidant, quercetin has a significant effect on the activity of antioxidant enzymes and cellular glutathione levels and inhibition of lipid peroxidation (Pês et al. 2018; Jia et al. 2019). Quercetin can also affect cellular signalling pathways such as nuclear factor, erythroid 2-like 2 (NRF2), mitogen-activated protein kinase (MAPK), and AMP-activated protein kinase (AMPK) (Tang, et al. 2020). Quercetin has anti-inflammatory properties and reduces inflammation by inhibiting the secretion of cytokines, reducing the production of cyclooxygenase-2 (COX-2) and lipoxygenase (LOX), and maintaining the stability of mast cells (Chaturvedi et al. 2020). Also, it is recognized that interleukin (IL-1, IL-6, IL-10) and tumor necrosis factor (TNF-α) are affected by quercetin (Karimi et al. 2021). Quercetin can inhibit fibrosis in cholestasis liver injury rats by downregulating the expression of TGF-β1 and α-SMA and preventing oxidative stress (Xiao et al. 2020). Quercetin has neuroprotective effects and anti-cancer activity (Tang et al. 2020). The protective effects of quercetin on the prevention of oxidative stress in oxytetracycline-treated fish have been reported (Pês et al. 2018). Moreover, the impact of quercetin on growth performance, antioxidant capability, and innate immunity of fish has been studied by Jia et al. (2019) and Bhattacharjee et al. (2020). Common carp, Cyprinus carpio, is one of the most important farmed fish species in the world (Monsef et al. 2016; Saffar Shargh et al. 2017; Abilov et al. 2021). The nutritional management of the common carp has been the focus of many studies to improve growth, immunity, and fish welfare (Mohammadi et al. 2020; Al-Shawi et al., 2021; Ghafarifarsani et al., 2021a; Hedayati et al. 2021; Raissy et al. 2022; Rudiansyah et al. 2022).
This study aimed to evaluate the protective effects of dietary quercetin toxicity induced by malathion in C. carpio. The results of this study may help find a natural solution to moderate the effects of insecticides on fish.
Materials and methods
Fish
The common carp, Cyprinus carpio, with an average weight of 15.35 ± 0.08 g, were purchased from a local fish farm in Rasht, Iran, and transferred to a private farm. Specimens were acclimatized with lab conditions for 2 weeks in aerated freshwater (temperature: 24 ± 2 °C; dissolved oxygen: 6–7 mg/l; pH: 7.4 ± 0.2; photoperiod: 13 h light and 11 h dark). During the acclimatization period, fish were fed two times daily with formulated diet from Faradaneh, Co., Shahrekord, Iran (38–41% crude protein, 4–8% crude lipid, 3–6% crude fiber, 1–1.5% digestible phosphorus, 5–11% moisture, 7–11% ash).
Quercetin
Quercetin (> 95% purity, Sigma Chemical Co., USA). Adjusted concentrations of quercetin were mixed with commercial diet to achieve 200 mg/kg diet. In this regard, the basal diet was first powdered by a mill, the quercetin at an adjusted level added, and then water added to form a paste. Then, the paste was pelleted (diameter: 3 ± 0.3 mm) using an industrial meat grinder and kept at room temperature for 24 h to dry. To adjust the pellet size with fish mouth, the pellets were crushed after drying. Finally, the feeds were stored in the refrigerator at 4 °C (Ghafarifarsani et al. 2022a, b a, b). The dietary levels of quercetin were selected according to the levels used in other studies (Park et al. 2010; Zhai and Liu 2013; Pês et al. 2016; Jia et al. 2019; Xu et al. 2019).
Malathion
Malathion (57% EC) was bought from a store. Stock solution of malathion was mixed with distilled water to achieve 1.04 mg/l (25% of LC50 96 h) and 2.08 mg/l (50% of LC50 96 h). LC50 96 h was calculated for fish in a previous test (Table 1). The exposure concentrations were selected according to the concentrations used by other studies (Topal et al. 2014; Hamed 2015; Ghafari Farsani et al. 2016; Poorbagher et al. 2018; Khoei 2021).
Experimental design
Specimens were distributed randomly into six groups in three replicates with a stocking rate of 20 fish per replicate (a total of 360 fish). The fish were fed with 200 mg/kg quercetin for 21 days and were simultaneously exposed to different concentrations of malathion.
Experimental groups included T1: control group fed with commercial diet only, T2: fish exposed to 1.04 mg/l malathion, T3: fish exposed to 2.08 mg/l malathion, T4: fish fed supplemented diet with 200 mg/kg quercetin, T5: fish fed supplemented diet with 200 mg/kg quercetin and exposed to 1.04 mg/l malathion, and T6: fish fed supplemented diet with 200 mg/kg quercetin and exposed to 2.08 mg/l malathion.
All fish fasted for 24 h before sampling. After anesthetizing the fish (10 fish/tank) with clove powder (150 mg/l), blood was collected from the venous stem vein using a 2.5-cc syringe impregnated with anticoagulant. Then, the blood samples were aliquot into two parts for hematology and blood biochemical analysis. A portion of the blood was centrifuged at 4000 rpm for 15 min at 4 °C to obtain plasma. Plasma was collected and stored at − 70 °C until biochemical analysis.
Growth performance
After blood sampling, all fish per tank (n = 20) were captured and weighted individually and growth performance was measured based on the following formula (Fallahpour et al. 2014; Hajiradkouchak et al. 2019):
Hematological parameters
The blood was immediately used to count the number of erythrocytes (RBC) and leukocytes (WBC) by means of a hemocytometer slide at a magnification of 400 × (Sohn and Henry 1969). Hematocrit (Hct) was estimated by the microhematocrit method (Blaxhall and Daisley 1973), and hemoglobin (Hb) content was measured by using the cyanohemoglobin method (Rawling et al. 2012). Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) as red blood cell indices were calculated based on the following formula (Blaxhall and Daisley 1973):
Biochemical parameters
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and acid phosphatase (ACP) activities were measured using biochemical reagents obtained from Pars Azmun Co, Iran. Total protein, albumin, glucose, cholesterol, triglyceride, urea, and creatinine levels were estimated using diagnostic biochemical kits obtained from Pars Azmun Co, Iran. Measured using an automatic biochemical analyzer (Roche Hitachi 911 Chemistry Analyzer, Tokyo, Japan). Cortisol levels were detected using a commercial ELISA kit (Zellbio®, Berlin, Germany).
Immunological parameters
The myeloperoxidase activity (MPO) in serum was assayed following the method presented by Zheng et al. (2018). The total immunoglobulin (total Ig) levels were assayed using polyethylene glycol (Banaee et al. 2019a). Alternative complement (ACH50) activity was assessed using sheep red blood cells and vernal buffer (Ahmadi et al. 2014). Lysozyme activity was measured using lyophilized Micrococcus luteus (sigma).
Oxidative stress biomarkers
Superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST), and catalase (CAT) activities and malondialdehyde (MDA) content were determined using a commercial kit (Zellbio®, Berlin, Germany).
Statistical analyses
Data analysis was done by SPSS statistical software. All data were analyzed by one-way analysis of variance (ANOVA), after performing the data normality test by Kolmogorov–Smirnov test. When differences were found among treatments, Tukey’s test was used to compare means. Differences were considered at a significant level of p < 0.05.
Results
Growth parameters
The final weight (FW) and WG (g) significantly decreased in all malathion-exposed fish compared to control (Table 2, p < 0.05). In contrast, these parameters significantly increased in fish supplemented only with quercetin (T4) compared to control (T1) (Table 2, p < 0.05). WG% showed significant decreases in malathion-exposed fish (Table 2, p < 0.05), while there were no significant differences between only quercetin-supplemented fish and control (Table 2, p > 0.05). The values of FCR significantly increased in malathion-exposed fish compared to control (Table 2, p < 0.05). However, quercetin in the diet somewhat moderated the malathion-induced increases in FCR, so that the FCR values in fish supplemented only with quercetin and those of 1.04 mg/l malathion + quercetin (T5) were similar to control (Table 2, p < 0.05). Except for only quercetin-supplemented fish, the SGR values significantly decreased in all groups compared to control (Table 2, p < 0.05). The SGR values were significantly higher in the treatment only quercetin than those in control (Table 2, p < 0.05).
Hematology
The RBC and WBC concentrations significantly increased in fish supplemented only with quercetin and those with 2.08 mg/l malathion + quercetin (T6) compared to control (Table 3, p < 0.05). The values of Hct showed significant increases in fish supplemented only with quercetin compared to control (Table 3, p < 0.05). Hct significantly decreased in the treatment 1.04 mg/l malathion compared to control (Table 3, p < 0.05). There were no significant differences in MCHC and MCH values between the experimental groups (Table 3, p > 0.05). However, the values of MCV showed significant decreases in the treatment 1.04 mg/l malathion compared to control (Table 3, p < 0.05). There were no significant differences in MCV values between the other groups and control (Table 3, p > 0.05).
Biochemical compounds in serum
The levels of total protein and albumin were significantly lower in malathion-exposed fish and treatments malathion + quercetin compared to control (Table 4, p < 0.05). Fish supplemented only with quercetin and those with 1.04 mg/l malathion + quercetin showed no significant differences with control (Table 4, p > 0.05). There were no significant differences in globulin levels between experimental groups (Table 4, p > 0.05). The levels of triglyceride significantly decreased in the treatment 2.08 mg/l malathion and fish supplemented only with quercetin (Table 4, p < 0.05). There were no significant differences in triglyceride levels between other experimental groups with control (Table 4, p > 0.05). The levels of glucose (Table 4, p < 0.05) and cortisol (Table 4, p < 0.05) significantly increased in malathion treatments and 2.08 mg/l malathion + quercetin compared to control. The glucose (Table 4, p < 0.05) and cortisol (Table 4, p < 0.05) concentrations significantly decreased in fish supplemented only with quercetin compared to control. The concentrations of creatinine significantly increased in all malathion-exposed fish compared to control (Table 4, p < 0.05). The urea levels significantly elevated in almost all malathion-exposed fish compared to control (Table 4, p < 0.05). However, urea levels significantly decreased in fish supplemented only with quercetin (Table 4, p < 0.05).
Hepatic metabolic enzymes
The activity of ALT, ALP, and AST significantly decreased in fish supplemented only with quercetin compared to control and other groups (Table 5, p < 0.05). The activity of these enzymes in malathion-exposed fish was significantly higher than that in control (Table 5, p < 0.05). The treatments malathion + quercetin showed no significant differences with control fish (Table 5, p > 0.05).
Serum immune parameters
The activity of ACP and lysozyme and total Ig levels significantly decreased in response to malathion compared to control (Table 6, p < 0.05). The lysozyme levels showed significant increases in fish supplemented only with quercetin compared to control (Table 6, p < 0.05). There were no significant differences between control and fish of malathion + quercetin (Table 6, p > 0.05). The activity of ACH50 significantly decreased in response to 2.08 mg/l malathion compared to control (Table 6, p < 0.05). The ACH50 activity in only quercetin-supplemented fish and fish of 1.04 mg/l malathion + quercetin showed significant elevations compared to control (Table 6, p < 0.05). The activity of MPO significantly decreased in response to malathion compared to control (Table 6, p < 0.05). The lysozyme levels showed significant decreases in fish exposed to 1.04 mg/l malathion and the treatment 1.04 mg/l malathion + quercetin (Table 6, p < 0.05). The maximum MPO activity was observed in fish supplemented only with quercetin (Table 6, p < 0.05).
Mucosal immune parameters
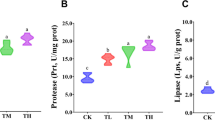
The activity of protease (Fig. 1A) and lysozyme (Fig. 1C) and total Ig levels (Fig. 1B) significantly decreased in response to malathion compared to control (p < 0.05). These parameters significantly increased in fish supplemented only with quercetin compared to control. There were no significant differences between control and fish of the treatment 1.04 mg/l malathion + quercetin (Fig. 1, p > 0.05). In the treatment 2.08 mg/l malathion + quercetin, protease (Fig. 1A) and lysozyme (Fig. 1C) activities and total Ig levels (Fig. 1B) decreased compared to control (p < 0.05).
The effects of quercetin on skin mucus parameters (protease activities, total immunoglobulin (total Ig), lysozyme activity, and alternative complement activity (ACH50)) in malathion-exposed common carp (C. carpio) after 21 days. Low dose, fish exposed to concentrations of 1.04 mg/l malathion; High dose, 2.08 mg/l malathion; QR, fish supplemented with quercetin (200 mg/kg diet); Low-QR, fish treated with quercetin + malathion (1.04 mg/l); High-QR, quercetin (200 mg/kg diet) + malathion (2.08 mg/l) for 21 days. Data are presented as mean ± SE. Different letters (a–d) in the same row indicate significant differences (p < 0.05)
The activity of ACH50 significantly decreased in response to 2.08 mg/l malathion compared to control (Fig. 1D, p < 0.05), while it showed a significant increase in fish supplemented only quercetin and in the treatment 1.04 mg/l malathion + quercetin compared to control (Fig. 1D, p < 0.05). There were no significant differences between control and the treatment 2.08 mg/l malathion + quercetin (Fig. 1D, p > 0.05).
Antioxidant enzymes
CAT activity in malathion and 2.08 malathion + quercetin treatments showed a significant decrease compared to control (Table 7, p < 0.05). In fish fed a quercetin-containing diet, CAT activity was significantly increased compared to the control group (Table 7, p < 0.05). SOD activity showed a significant decrease in malathion and malathion + quercetin treatments compared to control (Table 7, p < 0.05). While the activity of this enzyme in the treatment of quercetin alone was higher than the control (Table 7, p < 0.05). The activity of GPx in 2.08 malathion treatment showed a significant decrease compared to the control (Table 7, p < 0.05), while the activity of this enzyme in fish fed only with quercetin showed a significant increase compared to the control (Table 7, p < 0.05). The other experimental groups did not show a significant difference with the control (Table 7, p > 0.05). GST enzyme activity showed a significant increase in quercetin alone treatment compared to control (Table 7, p < 0.05). Other groups did not show significant differences in the activity of this enzyme compared to control (Table 7, p < 0.05). MDA levels in fish supplemented only with quercetin showed a significant decrease compared to control (Table 7, p < 0.05). Except for 1.04 malathion + quercetin, the other groups showed higher levels of MDA than control (Table 7, p < 0.05).
Discussion
The growth performance results are shown in Table 2. Exposure of fish to malathion caused a decrease in the SGR value, as reported in other fish exposed to insecticides (Mahmoud et al. 2021). The highest FW and WG values were obtained following quercetin administration, whereas the lowest value was observed in the fish exposed to malathion alone and in combination with quercetin. The increase in FW in quercetin-supplemented fish may be related to appetite stimulation and improvement in the efficiency of the gastrointestinal tract in absorbing nutrients (Najafabadi et al. 2018). In agreement with our results, specific growth rate and condition factor of Nile tilapia, Oreochromis niloticus, improved in fish supplemented with quercetin (Zhai and Liu 2013). However, no significant differences were observed in feed conversion rate and survival rate between quercetin-supplemented fish and non-supplemented individuals (Zhai and Liu 2013). In the study of Kim et al. (2013), no improvement was observed in olive flounder, Paralichthys olivaceus, supplemented with quercetin. In blunt snout bream, Megalobrama amblycephala, the supplementation of fish with diet containing high levels of quercetin could improve the growth indices (Jia et al. 2019). Similarly, in grass carp, Ctenopharyngodon idella, the WG increased in fish supplemented with 0.4 g/kg quercetin, which was accompanied by a significant decrease in feed conversion ratio (Xu et al. 2019). Ghafarifarsani et al. (2022a) observed higher final weight, weight gain, specific growth rate, survival rate, and lower feed conversion ratio in common carp supplemented with quercetin.
Gholamhosseini et al. (2020) observed an increase in the growth performance parameters in the Caspian kutum, Rutilus frisii kutum, after feeding with Mentha longifolia supplement. Also, the weight gain and SGR were significantly improved in Siberian sturgeon, Acipenser baerii, fed Lemon verbena, Aloysia citrodora, extract (Adel et al. 2021). Improvement in the growth performance and survival rate was also reported in rainbow trout, Oncorhynchus mykiss, fed 2% of Artemisia dracunculus (Gholamhosseini et al. 2021). Xu et al. (2020) recorded an increase in SGR and a decrease in FCR in Japanese seabass, Lateolabrax japonicus, fed mixture of erula sinkiangensis, Medicago falcata L., and Allium sativum. In this study, the highest value of FCR was observed in fish exposed to a high dose of malathion. The FCR value of fish exposed to a low dose of malathion combined with quercetin was significantly higher than the control groups. In addition, fish SR decreased significantly after exposure to malathion. Although feeding fish with a quercetin supplement significantly affected the SR of fish exposed to malathion, the SR of these fish was still considerably lower than the control group. These results clearly show that dietary quercetin has no effect in improving SR and FCR efficiency in malathion-exposed fish.
In the present study, malathion and quercetin affected the hematological indices of C. carpio. Hematological indices are known as indicators of fish health (Abarghoei et al. 2015; Heidary et al. 2016; Monsef Rad et al. 2016). In this study, the highest values of Hct, RBC, and WBC were observed in the fish fed a quercetin supplement. Quercetin administration may stimulate hematopoiesis in the hematopoietic tissues of fish (Dutta et al. 2021). Similar results were also reported for plant-based supplements containing quercetin. For example, the dietary Allium sativum and Aloe vera increased RBC, Hct, Hb, MCV, MCH, MCHC, WBC, lymphocytes, monocytes, and granulocytes in African catfish, Clarias gariepinus (Gabriel, et al. 2021). Yousefi et al. (2021) obtained similar results in the common carp fed dietary marjoram. The supplementation of fish with quercetin returned the Hct level to the same levels as in the control and only quercetin-supplemented fish. In contrast, exposing fish to a high dose of malathion led to a significant decrease in Hct and MCV value than the control group. The decrease in hematocrit may be due to a decrease in hemoglobin concentration in the erythrocytes of only malathion-exposed fish (Abarghoei et al. 2015). At the same time, oral administration of quercetin was able to increase the percentage of Hct in fish exposed to malathion. Amaeze et al. (2020) showed significant changes in hematological parameters in African catfish exposed to pesticides. In common carp, although WBC count increased in response to lindane, RBC count, Hb, and Hct levels decreased after exposure (Saravanan et al. 2011).
The results of blood biochemical parameters are presented in Table 4. Exposure of fish to a low dose of malathion resulted in a significant reduction in total protein, which may be due to the impaired protein biosynthesis in the liver and amino acid absorption in the intestine of the exposed fish. Albumin levels decreased significantly in the fish exposed to malathion alone and in combination with quercetin, which may be attributed to a reduction in total protein biosynthesis in the liver. However, dietary administration of quercetin increased albumin levels significantly. Elevated albumin levels may indicate the role of albumin in the transportation of quercetin in the blood (Alsaif et al. 2020).
Fish exposed to a low dose of malathion showed an increase in triglyceride levels, which may be due to the increased rate of breakdown of fat stored in adipose tissue (Banaee et al. 2019b). In contrast, there was a significant decrease in the triglyceride levels in the fish fed quercetin supplement. Similar results were observed in O. niloticus, where the triglyceride levels in serum decreased in response to quercetin supplementation (Zhai and Liu 2013).
Quercetin may reduce triglyceride levels by inhibiting enzymes involved in triglyceride biosynthesis (Kuipers et al. 2018). In addition, quercetin may reduce blood triglyceride levels by inhibiting fat adipogenesis (Jia, et al. 2019).
Exposing fish to a low dose of malathion displayed an increase in cholesterol levels compared to the control group. Malathion may increase cholesterol by impairing the biliary excretion of cholesterol and reducing the synthesis of high-density lipoprotein (Haque et al. 1987; Abdel-Daim et al. 2020). Quercetin may restore cholesterol levels to normal levels in fish exposed to malathion by affecting cholesterol-carrying lipoproteins’ biosynthesis in the blood (Jia et al. 2019). Jia et al. (2019) observed reduced levels of triglyceride and cholesterol in fish supplemented with quercetin. Quercetin can decrease blood cholesterol levels by reducing cholesterol absorption in the intestine and affecting lipoproteins’ biosynthesis (Jia, et al. 2019).
Glucose, cortisol, and urea levels significantly increased after exposure to malathion. The secretion of cortisol and subsequent elevations in glucose can be a sign of stress in the exposed fish to meet the energetic needs of fish for coping with stressful conditions (Banaee et al. 2020). The administration of fish with quercetin significantly decreased glucose, cortisol, and urea levels. This result may indicate the ameliorating effect of quercetin on stress induced by malathion.
In this study, the levels of urea and creatinine increased in response to malathion, which may be attributed to disruptions in renal nephron function. In contrast, the dietary quercetin ameliorated such disruptions, as we observed decreases in urea and creatinine levels in the quercetin-supplemented fish.
The activities of liver enzymes in serum are presented in Table 5. The activities of ALP, AST, and ALT increased significantly in the serum of fish exposed to malathion, which may be attributed to the damages to hepatocytes (Banaee et al. 2019b). Similarly, significant increases were observed in AST, ALT, and ALP activities in exposed catfish (Heteropneustes fossilis) to fertilizer industry effluent (Singh and Pandey 2021). In contrast, the lowest activities of AST, ALT, and ALP were observed in the fish fed quercetin supplement. Quercetin may increase the oxidative capacity of cells and thus reduce the risk of lipid peroxidation. The regulation of AST and ALT activities in quercetin-treated fish may be related to its free radical scavenging properties (Zhang, et al. 2020). Improving the stability of cell membranes can prevent AST and ALP leakage and modulate their activity in plasma (Jia et al., 2019).
Changes in the immunological parameters in the serum of fish were presented in Table 6. Lysozyme activity, ACP activity, and total immunoglobulin were significantly decreased in malathion-exposed fish. The origin of serum and mucosal lysozyme is related to neutrophils and macrophages. Therefore, a decrease in lysozyme activity may indicate a decline in the number of neutrophils and macrophages. Raibeemol and Chitra (2020) reported decreased lysozyme activity in the serum of orange chromide, Pseudetroplus maculatus, exposed to chlorpyrifos. Similarly, the lysozyme activity decreased in rainbow trout after exposure to diazinon (Ahmadi et al. 2014). In rainbow trout, exposure to diazinon significantly reduced Ig levels (Ahmadi et al. 2014). Decreased lysozyme and ACP activities and total immunoglobulin levels may indicate the immunosuppressing effects of malathion, which may makes fish susceptible to diseases. Results of the present study showed increases in serum lysozyme and ACP activities and Ig levels following supplementation with quercetin. Dietary administration of quercetin could return lysozyme activity to the normal range. In addition, an increase in lysozyme activity may be due to the improved immune system of fish fed quercetin. Therefore, quercetin can act as an immune stimulant. In agreement with our results, lysozyme activity significantly increased in olive flounder fed diet containing spirulina + quercetin (Shin et al. 2010; Kim et al. 2013). In blunt snout bream, Megalobrama amblycephala, the supplementation of fish with high levels of quercetin could enhance immunity parameters (Jia et al. 2019). In zebrafish, results indicated that the immune indices including ACP, MPO, lysozyme activities, and complement activity and IgM increase in response to 1 μg/l quercetin in the diet.
Similar results were reported by Hoseini et al. (2021), where significant increases in lysozyme activity were observed in common carp fed diets containing Russian olive, Elaeagnus angustifolia, extract. A significant increase in serum lysozyme activity was also reported in O. niloticus in fish supplemented with pineapple peel powder (Doan et al., 2021). A significant decrease in ACP activity was observed in the zebrafish exposed to fluoride (Cao et al. 2020). In contrast, Zhang et al. (2019) found that exposure of Chinese mitten crab, Eriocheir sinensis, to deltamethrin increased ACP activity. An increase in immune parameters, including ACP and lysozyme activities and total Ig levels in the plasma and mucus of common carp, fed dietary marjoram, Origanum majorana, extract, was also reported by (Yousefi et al., 2021).
MPO activity is an indicator of the antibacterial response of neutrophils. Neutrophils contain MFO in their granular cytoplasm, which can kill bacteria by producing H2O2 and other free radicals. Therefore, alternations in the activity of MPO can be an indicator of the bactericidal power of neutrophils. Borgia et al. (2018) found that exposure of common carp to electroplating industrial effluent decreases MPO activity. In this study, the activity of MPO in the fish exposed to a high concentration of malathion was significantly lower than in the control group, which may be due to the depressing effects of malathion on the bactericidal activity of neutrophils. The highest activity of MPO was detected in the fish fed quercetin, indicating enhancing effects of quercetin on the malathion-related reductions in the activity of neutrophils.
The oxidative biomarkers in the hepatocytes are presented in Table 7. Significant decreases were observed in the CAT, SOD, GPx, and GST activities in the hepatocyte of the malathion-exposed fish. A significant reduction in the CAT activity may be due to the inhibition of its activity by metabolites of malathion. CAT is an oxidoreductase that plays a vital role in decomposing hydrogen peroxide into water and oxygen (Ullah et al. 2018). A decrease in the CAT activity may increase H2O2 levels in the hepatocyte of fish exposed to malathion. Increased H2O2 could increase peroxidation of bio-macromolecules and the occurrence of oxidative damage (Rezaei Shadegan and Banaee 2018). GST can play an essential role in conjugating the reduced form of glutathione (GSH) to xenobiotic through transferring glutathione (Banaee et al. 2013; Awasthi et al. 2019).
GPx also plays an active role in the breakdown of hydrogen peroxide using glutathione (Ullah et al. 2018; Shirazi and Khakdan, 2021). The dietary administration of quercetin caused a significant increase in CAT, SOD, GST, and GPx activity. Although dietary quercetin could not adjust the SOD activity of exposed fish, it returned the CAT and GPx activity to normal values in olive flounder; the supplementation of fish with quercetin significantly enhanced the hepatic antioxidant capacity (Kim et al. 2013). In blunt snout bream, the supplementation of fish with diet containing high levels of quercetin could improve the hepatic antioxidant capacity (Jia et al. 2019). Similarly, in grass carp, Ctenopharyngodon idella, the alkaline phosphatase and superoxide dismutase activity increased in fish supplemented with 0.4 g/kg quercetin (Xu et al. 2019). Ghafarifarsani et al. (2022b) reported increases in the activity of liver antioxidant enzymes in common carps fed quercetin. In zebrafish, quercetin significantly elevated the activity of hepatic antioxidant enzymes including SOD, GPx, CAT, and T-AOC (Wang et al., 2020). In contrast, in olive flounder, the H2O2 concentration and activity of SOD and CAT decreased in response to quercetin, suggesting the direct scavenging role of quercetin in reducing the oxidative stress–related production of reactive oxygen species (ROS) (Shin et al., 2010). Similarly, in silver carp, quercetin decreased the activity of SOD, GPx, and GST in the muscle of fish exposed to oxytetracycline, which was attributed to the direct scavenging role of quercetin in reducing the ROS (Pês et al., 2018). Dietary administration of beluga sturgeon, Huso huso, increased CAT and SOD activity in serum (Safari et al. 2020).
The MDA content in the hepatocyte was increased after exposure to malathion, which could be due to the oxidative stress induced by the insecticide. The lowest level of MDA was detected in the fish treated only with quercetin. However, quercetin ameliorated the increased levels of MDA in fish exposed to low concentration of malathion. Therefore, these results may show the ameliorating effect of quercetin supplement on the oxidative stress induced by malathion. However, such an effect seems to depend on the exposure concentration of malathion. Similarly, the levels of MDA significantly decreased in zebrafish, following feeding with diet containing 1 and 10 μg/l quercetin (Wang et al., 2020).
Khalil et al. (2020) found that plant flavonoids could reduce the impact of pesticides by modulating the expression of antioxidant genes. The antioxidant properties of quercetin can play an essential role in regulating antioxidant enzymes and inhibiting lipid peroxidation (Jia, et al. 2019). The protective effect of quercetin on the regulation of CAT, SOD, GST, and GPx activities has been confirmed in oxytetracycline-treated silver catfish, Rhamdia quelen, by Pês et al. (2018).
Exposure of fish to malathion led to a decrease in the protease activity in the mucosa. However, the protease activity in the fish fed quercetin was significantly higher than in the control group. In addition, dietary administration of quercetin had no significant effect on the return of protease activity. Mucosal proteases play an essential role in the breakdown of proteins involved in inflammatory, coagulation, apoptotic, and tissue regeneration processes. Therefore, inhibition of protease activity could cause interference in cellular inflammatory reactions. Furthermore, a decrease in the protease activity may lead to disturbance in the activation of the zymogen, an inactive precursor involved in the breakdown of proteins.
The total Ig levels in the mucosa were significantly decreased after exposure to malathion. Decreased total Ig indicates a reduction in immunity in response to malathion. A significant increase in the total Ig was observed in fish fed with quercetin supplement, indicating the enhancing effects of quercetin on the immunity of malathion-exposed fish.
The exposure of fish to malathion decreased mucosal lysozyme activity. The lowest activity of lysozyme and ACH50 was detected in the fish exposed to low concentrations of malathion. A decrease in the lysozyme and ACH50 activity may reduce mucosal defenses against bacterial infiltration (Yang et al. 2021). The lysozyme and ACH50 activity in the mucosa of fish fed quercetin were significantly higher than in the control group, which may be related to the immunostimulatory properties of flavonoids. However, lysozyme and ACH50 activity could not be returned to the normal ranges after exposure to malathion in quercetin-supplemented fish. Doan et al. (2021) showed that oral administration of pineapple peel powder increased mucosal ACH50 in O. niloticus. Similar results were observed in the Nile tilapia fed with fishwort, Houttuynia cordata, powder (Srichaiyo et al. 2020).
Conclusion
This study showed that exposure of fish to malathion could cause significant changes in growth indices, biochemical and antioxidant parameters, and humoral and mucosal immunity of fish. Oral administration of quercetin had antioxidant and immunostimulatory properties for fish. The protective effect of quercetin on the regulation of some biochemical, immunological, and hematological parameters could be confirmed. However, oral administration of quercetin could not return changes in some other parameters to normal levels in fish exposed to malathion. In conclusion, the results show that quercetin administration can improve the toxic effects of malathion and prevent oxidative damage, immunosuppression, and biochemical disorders in fish.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abarghoei, S., Hedayati, S. A., Ghafari Farsani, H., & Gerami, M. H. 2015. Hematological responses of Goldfish (Carassius auratus) to different acute concentrations of Silver Sulfate as a toxicant. Pollution, 1(3), 247-256.
Abdel-Daim, M M, A I Abushouk, S G Bungău, M Bin-Jumah, A F El-Kott, A A Shati, L Aleya, and S Alkahtani. 2020. ''Protective effects of thymoquinone and diallyl sulphide against malathion-induced toxicity in rats. ''Environmental Science and Pollution Research 27(10):10228-35.
Abilov, B., Isbekov, K., Assylbekova, S., Bulavina, N., Kulmanova, G., S. K, K., Nikolova, L. 2021. Evaluation of Production and Economic Performance of Farmed Carp Using Small Lake-Commercial Fish Farms System in Southeastern Kazakhstan. Archives of Razi Institute, 76(4), 1143-1154. https://doi.org/10.22092/ari.2021.355785.1722
Adel, M, M A O Dawood, A Gholamhosseini, F Sakhaie, and M Banaee. 2021. "Effect of the extract of lemon verbena (Aloysia citrodora) on the growth performance, digestive enzyme activities, and immune-related genes in Siberian sturgeon (Acipenser baerii)." Aquaculture 541: 736797. https://doi.org/10.1016/j.aquaculture.2021.736797.
Ahmadi, K, A R Mirvaghefei, M Banaee, and A R Vosoghei. 2014. "Effects of long-term diazinon exposure on some immunological and haematological parameters in rainbow trout Oncorhynchus mykiss (Walbaum, 1792)." Toxicology and Environmental Health Sciences 6: 1–7. https://doi.org/10.1007/s13530-014-0181-1.
Alavinia, S J, A Mirvaghefi, H Farahmand, G Rafiee, S J Alavinia, N Shiry, and S Moodi. 2019. "DNA damage, acetylcholinesterase activity, and hematological responses in rainbow trout exposed to the organophosphate malathion." Ecotoxicology and Environmental Safety 182: 109311, https://doi.org/10.1016/j.ecoenv.2019.05.081.
Alsaif, N A, T A Wani, A H Bakheit, and S Zargar. 2020. "Multi-spectroscopic investigation, molecular docking and molecular dynamic simulation of competitive interactions between flavonoids (quercetin and rutin) and sorafenib for binding to human serum albumin." International Journal of Biological Macromolecules 165(B): 2451-2461. https://doi.org/10.1016/j.ijbiomac.2020.10.098.
Al-Shawi, S. G., Al-Younis, Z. K., Yousif, A. Y., Shichiyakh, R. A., Zekiy, A. O., & Naserabad, S. S. 2021. Dietary silymarin, Silybum marianum extract ameliorates cadmium chloride toxicity in common carp, Cyprinus carpio. Annals of Animal Science, 20210065–20210065.
Amaeze, N H, B O Komolafe, B F Salako, K K Akagha, T M D Briggs, O O Olatinwo, and M A Femi. 2020. "Comparative assessment of the acute toxicity, haematological and genotoxic effects of ten commonly used pesticides on the African Catfish, Clarias gariepinus Burchell 1822." Heliyon 6(8): e04768. https://doi.org/10.1016/j.heliyon.2020.e04768.
Awasthi, Y, A Ratn, R Prasad, M Kumar, A Trivedi, J P Shukla, and S P Trivedi. 2019. "A protective study of curcumin associated with Cr6+ induced oxidative stress, genetic damage, transcription of genes related to apoptosis and histopathology of fish, Channa punctatus (Bloch, 1793)." Environmental Toxicology and Pharmacology 71: 103209. https://doi.org/10.1016/j.etap.2019.103209.
Ballesteros M L, N G Rivetti, D O Morillo, L Bertrand, M V Amé, and M A Bistoni. 2017. "Multi-biomarker responses in fish (Jenynsia multidentata) to assess the impact of pollution in rivers with mixtures of environmental contaminants." Science of The Total Environment 595: 711-722. https://doi.org/10.1016/j.scitotenv.2017.03.203" https://doi.org/10.1016/j.scitotenv.2017.03.203 .
Banaee, M, A Sureda, A R Mirvaghefi, and K Ahmadi. 2013. "Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon." Fish Physiology and Biochemistry 39(3): 489-501. https://doi.org/10.1007/s10695-012-9714-1.
Banaee, M, S Soltanian, A Sureda, A Gholamhosseini, B N Haghi, M Akhlaghi, and A Derikvandy. 2019a. "Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (cyprinus carpio)." Chemosphere 236: 124335. https://doi.org/10.1016/j.chemosphere.2019.07.066.
Banaee, M, S Tahery, B Nematdoost Haghi, S Shahafve, and M Vaziriyan. 2019b. "Blood biochemical changes in common carp (Cyprinus carpio) upon co-exposure to titanium dioxide nanoparticles and paraquat." Iranian Journal of Fisheries Sciences 18(2): 242-255. https://doi.org/10.22092/ijfs.2019.118174.
Banaee, M, M Akhlaghi, S Soltanian, A Sureda, A Gholamhosseini, and M Rakhshaninejad. 2020. "Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish pontastacus leptodactylus." Ecotoxicology 29(9): 1500-1515. https://doi.org/10.1007/s10646-020-02233-0.
Bekele, T G, H Zhao, and Q Wang. 2021. "Tissue distribution and bioaccumulation of organophosphate esters in wild marine fish from Laizhou Bay, North China: Implications of human exposure via fish consumption." Journal of Hazardous Materials 401: 123410. https://doi.org/10.1016/j.jhazmat.2020.123410.
Bharti, S, and F Rasool. 2021. "Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish." Toxicology Reports 8: 443-455. https://doi.org/10.1016/j.toxrep.2021.02.018.
Bhattacharjee, P, A Borah, and S Das. 2020. "Quercetin-induced amelioration of deltamethrin stress in freshwater teleost, Channa punctata: Multiple biomarker analysis." Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 227: 108626. https://doi.org/10.1016/j.cbpc.2019.108626
Blaxhall, P. C., & Daisley, K. W. (1973). Routine haematological methods for use with fish blood. Journal of fish biology 5(6): 771-781.
Borgia, V J F, A J Thatheyus, A G Murugesan, S C P Alexander, and I Geetha. 2018. "Effects of effluent from electoplating industry on the immune response in the freshwater fish, Cyprinus carpio." Fish & Shellfish Immunology 79: 86-92. https://doi.org/10.1016/j.fsi.2018.05.010.
Burgos-Aceves, M A, L Lionetti, and C Faggio. 2019. "Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish." Science of The Total Environment 670: 1170-1183. https://doi.org/10.1016/j.scitotenv.2019.03.275.
Cao, J, C Feng, L Xie, L Li, J Chen, S Yun, W Guo, T Wang, Y Wu, R Meng, and G Wang. 2020. "Sesamin attenuates histological alterations, oxidative stress and expressions of immune-related genes in liver of zebrafish (Danio rerio) exposed to fluoride." Fish & Shellfish Immunology 106: 715-723. https://doi.org/10.1016/j.fsi.2020.08.039.
Chaturvedi, S, M Y Malik, M Rashid, S Singh, V Tiwari, P Gupta, S Shukla, S Singh, and M Wahajuddin. 2020. "Mechanistic exploration of quercetin against metronidazole induced neurotoxicity in rats: Possible role of nitric oxide isoforms and inflammatory cytokines." NeuroToxicology 79: 1-10. https://doi.org/10.1016/j.neuro.2020.03.002.
Chorehi, M. M., Ghaffari, H., Hossaini, S. A., Niazie, E. H. N., Vajargah, M. F., & Hedayati, A. (2013). Acute toxicity of Diazinon to the Caspian vimba, Vimba vimba persa (Cypriniformes: Cyprinidae). International Journal of Aquatic Biology, 1(6), 254-257.
de Souza, S S, R N Machado, J C da Costa, D F Campos, G S da Silva, and V M de Almeida-Val. 2020. "Severe damages caused by Malathion exposure in Colossoma macropomum."Ecotoxicology and Environmental Safety 205:111340. https://doi.org/10.1016/j.ecoenv.2020.111340.
Doan, H V, S H Hoseinifar, R Harikrishnan, T Khamlor, M Punyatong, W Tapingkae, M Yousefi, J Palma, and E El-Haroun. 2021. "Impacts of pineapple peel powder on growth performance, innate immunity, disease resistance, and relative immune gene expression of Nile tilapia, Oreochromis niloticus." Fish & Shellfish Immunology 114: 311-319. https://doi.org/10.1016/j.fsi.2021.04.002.
Dutta, A, A Dahiya, and S Verma. 2021."Quercetin-3-rutinoside protects against gamma radiation inflicted hematopoietic dysfunction by regulating oxidative, inflammatory, and apoptotic mediators in mouse spleen and bone marrow."Free Radical Research 27:1-6. https://doi.org/10.1080/10715762.2021.1914334
Fallahpour, Fahimeh, Mahdi Banaee, and Narges Javadzade. "Effects of Dietary Marshmallow (Althaea Officinalis L.) Extract on Growth Performance and Body Composition of Common Carp (Cyprinus Carpio)." Int. J. Adv. Biol. Biomed. Res. 2, no. 8 (2014): 2453-60.
Gabriel, N N, M R Wilhelm, H M Habte-Tsion, P Chimwamurombe, and E Omoregie. 2021. "The effects of dietary garlic (Allium sativum) and Aloe vera crude extract mixtures supplementation on growth performance, feed utilization, hematological parameters, whole body composition, and survival at low pH in African catfish (Clarias gariepinus)." Scientific African 11: e00671. https://doi.org/10.1016/j.sciaf.2020.e00671.
Ghafari Farsani, H., Poorbagher, H., & Farahmand, H. 2016. Effects of malathion on DNA breakage in the liver and gill of rainbow trout (Oncorhynchus Mykiss) using weighted averaging. Journal of Fisheries, 69(1), 89-99.
Ghafarifarsani, H., Hoseinifar, S. H., Adorian, T. J., Ferrigolo, F. R. G., Raissy, M., & Van Doan, H. 2021a. The effects of combined inclusion of Malvae sylvestris, Origanum vulgare, and Allium hirtifolium boiss for common carp (Cyprinus carpio) diet: Growth performance, antioxidant defense, and immunological parameters. Fish & Shellfish Immunology, 119, 670-677.
Ghafarifarsani, H., Kachuei, R., & Imani, A. 2021b. Dietary supplementation of garden thyme essential oil ameliorated the deteriorative effects of aflatoxin B1 on growth performance and intestinal inflammatory status of rainbow trout (Oncorhynchus mykiss). Aquaculture, 531, 735928.
Ghafarifarsani, H., Hoseinifar, S. H., Javahery, S., & Van Doan, H. 2022b. Effects of dietary vitamin C, thyme essential oil, and quercetin on the immunological and antioxidant status of common carp (Cyprinus carpio). Aquaculture, 553, 738053.
Ghafarifarsani, H., Hoseinifar, S. H., Javahery, S., Yazici, M., & Van Doan, H. 2022a. Growth performance, biochemical parameters, and digestive enzymes in common carp (Cyprinus carpio) fed experimental diets supplemented with vitamin C, thyme essential oil, and quercetin. Italian Journal of Animal Science, 21(1), 291-302.
Gholamhosseini, A, M Adel, M A O Dawood, and M Banaee. 2020. "The potential benefits of mentha longifolia on growth performance and innate immunity parameters in caspian kutum (rutilus frisii kutum)." Aquaculture Research 51(12): 5212-5227.
Gholamhosseini, A, S Hosseinzadeh, S Soltanian, M Banaee, A Sureda, M Rakhshaninejad, A A Heidari, and H Anbazpour. 2021. "Effect of dietary supplements of artemisia dracunculus extract on the haemato-immunological and biochemical response, and growth performance of the rainbow trout (oncorhynchus mykiss)." Aquaculture Research 52(5), 2097-2109. https://doi.org/10.1111/are.15062.
Guo, D, W Liu, T Yao, M Ma, Q Wang, J Qiu, and Y Qian. 2021. "Combined endocrine disruptive toxicity of malathion and cypermethrin to gene transcription and hormones of the HPG axis of male zebrafish (Danio rerio)." Chemosphere 267: 128864. https://doi.org/10.1016/j.chemosphere.2020.128864
Hajiradkouchak, E., Patimar, R., Harsij, M., Ghorbani, R. 2019. Age determination, growth indices and reproduction biology of Prussian carp, Carassius gibelio (Bloch, 1782) from four reservoirs in Golestan Province, Southeast Caspian Sea. Caspian Journal of Environmental Sciences, 17(4), 337-351. https://doi.org/10.22124/cjes.2019.3807
Hajirezaee, S., Mohammadi, G., & Naserabad, S. S. 2020. The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2-NPs). Aquaculture, 518, 734734.
Hamed, H.S. 2015. Impact of a short-term malathion exposure of Nile tilapia, (Oreochromis niloticus): The protective role of selenium. Int J Environ Monit Anal, 3(5-1), pp.30-7.
Haque, N, S J Rizvi, and MB Khan. 1987. "Malathion induced alterations in the lipid profile and the rate of lipid peroxidation in rat brain and spinal cord."Pharmacology & toxicology 61(1):12-5. https://doi.org/10.1111/j.1600-0773.1987.tb01764.x.
Hedayati, S. A. A., Ghafari Farsani, H., Shahbazi Naserabad, S., & Gerami, M. H. (2015). Acute toxicity and behavioral changes associated with diazinon in Rutilus rutilus caspicus and Hypophthalmicthys molitrix. Iranian Journal of Toxicology, 9(30), 1354-1359.
Hedayati, S. A., Sheikh Veisi, R., Hosseini Shekarabi, S. P., Shahbazi Naserabad, S., Bagheri, D., & Ghafarifarsani, H. (2021). Effect of Dietary Lactobacillus casei on Physiometabolic Responses and Liver Histopathology in Common Carp (Cyprinus carpio) After Exposure to Iron Oxide Nanoparticles. Biological Trace Element Research, 1–9.
Heidary, S, A Hajimoradloo, A Bani, M Aghamaali, and R Ghorbani. "Changes in biochemical and physiological responses of common carp, Cyprinus carpio L. after long-term exposure to Pb (II)." Caspian Journal of Environmental Sciences 14(4): 311-320.
Heidary, S., Hajimoradloo, A., Bani, A., Aghamaali, M., Ghorbani, R. 2016. Changes in biochemical and physiological responses of common carp, Cyprinus carpio L. after long-term exposure to Pb (II). Caspian Journal of Environmental Sciences, 14(4), 311-320.
Hoseini, S M, A Taheri Mirghaed, Y Iri, S H Hoseinifar, H D Doan, and M Reverter. 2021. "Effects of dietary Russian olive, Elaeagnus angustifolia, leaf extract on growth, hematological, immunological, and antioxidant parameters in common carp, Cyprinus carpio." Aquaculture 536: 736461. https://doi.org/10.1016/j.aquaculture.2021.736461.
Ibor, O R, A O Adeogun, F Regoli, and A Arukwe. 2019. "Xenobiotic biotransformation, oxidative stress and obesogenic molecular biomarker responses in Tilapia guineensis from Eleyele Lake, Nigeria." Ecotoxicology and Environmental Safety 169: 255-265. https://doi.org/10.1016/j.ecoenv.2018.11.021
Ince, S, D Arslan-Acaroz, H H Demirel, N Varol, H A Ozyurek, F Zemheri, and I Kucukkurt. 2017. "Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats." Biomedicine & Pharmacotherapy 96: 263-268. https://doi.org/10.1016/j.biopha.2017.09.141
Jia E, Yan Y, Zhou M, Li X, Jiang G, Liu W, Zhang D. 2019. Combined effects of dietary quercetin and resveratrol on growth performance, antioxidant capability and innate immunity of blunt snout bream (Megalobrama amblycephala). Animal Feed Science and Technology. 256. https://doi.org/10.1016/j.anifeedsci.2019.114268
Karimi, A, F Naeini, V A Azar, M Hasanzadeh, H R Niazkar, and H Tutunchi. 2021. "A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis." Phytomedicine 86: 153567. https://doi.org/10.1016/j.phymed.2021.153567.
Karmakar, S, K Patra, S Jana, D P Mandal, and S Bhattacharjee. 2013. "Exposure to environmentally relevant concentrations of malathion induces significant cellular, biochemical and histological alterations in Labeo rohita."Pesticide biochemistry and physiology126:49-57. https://doi.org/10.1016/j.pestbp.2015.07.006.
Khabbazi, M., Harsij, M., Hedayati, S. A. A., Gholipoor, H., Gerami, M. H., & Ghafari Farsani, H. (2015). Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchus mykiss and their potential toxicity. Nanomedicine Journal, 2(1), 67-73.
Khalil, S R, Y A Elhakim, A H A El-fattah, M R Farag, N E A El-Hameed, and A E EL-Murr. 2020. "Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): Stress and immune encoding gene expression." Fish & Shellfish Immunology 100: 208-218. https://doi.org/10.1016/j.fsi.2020.03.009.
Khoei, A. J. (2021). Evaluation of potential immunotoxic effects of iron oxide nanoparticles (IONPs) on antioxidant capacity, immune responses and tissue bioaccumulation in common carp (Cyprinus carpio). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 244, 109005.
Kim, SS, S Rahimnejad, K W Kim, B J Lee, and K J Lee. 2013. "Effects of dietary supplementation of Spirulina and Quercetin on growth, innate immune responses, disease resistance against Edwardsiella tarda, and dietary antioxidant capacity in the juvenile olive flounder Paralichthys olivaceus."Fisheries and aquatic sciences 16(1):7-14.
Kuipers, E N, A D Dam, N M Held, I M Mol, R H Houtkooper, P C Rensen, and M R Boon. 2018. "Quercetin lowers plasma triglycerides accompanied by white adipose tissue browning in diet-induced obese mice."International journal of molecular sciences 19(6): 1786.
Lal, B, M K Sarang, and P Kumar. 2013. "Malathion exposure induces the endocrine disruption and growth retardation in the catfish, Clarias batrachus (Linn.)."General and comparative endocrinology 181:139-45. https://doi.org/10.1016/j.ygcen.2012.11.004.
Liu, H N, Y Liu, L L Hu, Y L Suo, L Zhang, F Jin, X A Feng, N Teng, and Y Li. 2014. "Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens." Poultry Science 93(2): 347-353. https://doi.org/10.3382/ps.2013-03225".
Mahmoud, H K, F M Reda, M Alagawany, and M R Farag. 2021. "The stress of abamectin toxicity reduced water quality, growth performance, immunity and antioxidant capacity of Oreochromis niloticus fish: Modulatory role of Simmondsia chinensis extract as a dietary supplement." Aquaculture 534: 736247. https://doi.org/10.1016/j.aquaculture.2020.736247.
Mohammadi, G., Rashidian, G., Hoseinifar, S. H., Naserabad, S. S., & Van Doan, H. 2020. Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish & Shellfish Immunology, 99, 267-273.
Monsef Rad, S., Paighambari, S., Haji Moradlou, A., Mashayekhi, F., Qorbani, R. (2016). Evaluation of electronarcosis and clove oil for short-term anesthesia in common carp, Cyprinus carpio L. 1758. Caspian Journal of Environmental Sciences, 14(4), 229-310.
Najafabadi, R E, N Kazemipour, A Esmaeili, S Beheshti, and S Nazifi. 2018. "Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain."BMC Pharmacology and Toxicology 19(1):1-2. https://doi.org/10.1186/s40360-018-0249-7.
Parhi, B, D Bharatiya, and S K Swain. 2020. "Application of quercetin flavonoid based hybrid nanocomposites: A review." Saudi Pharmaceutical Journal 28(12): 1719-1732. https://doi.org/10.1016/j.jsps.2020.10.017
Park MS, Shin HS, Lee J, Kil GS, Choi CY. 2010. Influence of quercetin on the physiological response to cadmium stress in olive flounder, Paralichthys olivaceus: Effects on hematological and biochemical parameters. Mol. Cell. Toxicol. 6:151–159. https://doi.org/10.1007/s13273-010-0022-5
Pês TS, Saccol EMH, Ourique GM, Londero ÉP, Gressler LT, Golombieski JI, Glanzner WG, Llesuy SF, Gonçalves PBD, Neto JR, Baldisserotto B, Pavanato MA. 2016. Quercetin in the diet of silver catfish: Effects on antioxidant status, blood parameters and pituitary hormone expression. Aquaculture 458, 100–106. https://doi.org/10.1016/j.aquaculture.2016.02.020
Pês, T S, E M H Saccol, E P Londero, C A Bressan, G M Ourique, T M Rizzetti, O D Prestes, R Zanella, B Baldisserotto, and M A Pavanato. 2018. "Protective effect of quercetin against oxidative stress induced by oxytetracycline in muscle of silver catfish." Aquaculture 484: 120-125. https://doi.org/10.1016/j.aquaculture.2017.10.043.
Poorbagher, H., Ghaffari Farsani, H. and Farahmand, H. 2018. A method to quantify genotoxicity of malathion in rainbow trout using the weighted averaging. Toxicology mechanisms and methods, 28(8), 607-614.
Raibeemol, K A, and K C Chitra. 2020. "Induction of immunological, hormonal and histological alterations after sublethal exposure of chlorpyrifos in the freshwater fish, Pseudetroplus maculatus (Bloch, 1795)." Fish & Shellfish Immunology 102: 1-12. https://doi.org/10.1016/j.fsi.2020.04.005.
Raissy, M., Ghafarifarsani, H., Hoseinifar, S. H., El-Haroun, E. R., Naserabad, S. S., & Van Doan, H. 2022. The effect of dietary combined herbs extracts (oak acorn, coriander, and common mallow) on growth, digestive enzymes, antioxidant and immune response, and resistance against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Aquaculture, 546, 737287.
Rashidian, G., Mahboub, H. H., Hoseinifar, S. H., Ghafarifarsani, H., Zare, M., Punyatong, M., & Van Doan, H. 2022. Allium hirtifolium protects Cyprinus carpio against the detrimental responses mediated by foodborne zinc oxide nanoparticle. Aquaculture, 738252.
Rawling, M D, D L Merrifield, D L Snellgrove, H Kühlwein, A Adams, and S J Davies. 2012. "Haemato-immunological and growth response of mirror carp (Cyprinus carpio) fed a tropical earthworm meal in experimental diets." Fish & Shellfish Immunology 32(6): 1002-1007.
Rebechi, D, A M Palacio-Cortés, V S Richardi, T Beltrão, M Vicentini, M T Grassi, S B da Silva, T Alessandre, S Hasenbein, R Connon, and M A Navarro-Silva. 2021. "Molecular and biochemical evaluation of effects of malathion, phenanthrene and cadmium on Chironomus sancticaroli (Diptera: Chironomidae) larvae." Ecotoxicology and Environmental Safety 211: 111953. https://doi.org/10.1016/j.ecoenv.2021.111953.
Rezaei Shadegan, M, and M Banaee. 2018. "Effects of dimethoate alone and in combination with Bacilar fertilizer on oxidative stress in common carp, Cyprinus carpio." Chemosphere 208: 101-107. https://doi.org/10.1016/j.chemosphere.2018.05.177.
Rudiansyah, M., Abdelbasset, W. K., Jasim, S. A., Mohammadi, G., Dharmarajlu, S. M., Nasirin, C., ... & Naserabad, S. S. 2022. Beneficial alterations in growth performance, blood biochemicals, immune responses, and antioxidant capacity of common carp (Cyprinus carpio) fed a blend of Thymus vulgaris, Origanum majorana, and Satureja hortensis extracts. Aquaculture, 738254.
Safari, R, S H Hoseinifar, M R Imanpour, M Mazandarani, H Sanchouli, and M Paolucci. 2020. "Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso)." Aquaculture 528: 735494. https://doi.org/10.1016/j.aquaculture.2020.735494.
Saffar Shargh, A., Zakipour Rahimabadi, E., Alizadeh, E., Gheybi, F. 2017. Amino acid and fatty acid profiles of materials recovered from Prussian carp, Carassius gibelio (Bloch, 1782), using acidic and basic solubilization/ precipitation technique. Caspian Journal of Environmental Sciences, 15(3), 285-294. https://doi.org/10.22124/cjes.2017.2469
Saravanan, M, K P Kumar, and M Ramesh. 2011. "Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane." Pesticide Biochemistry and Physiology 100(3): 206-211. https://doi.org/10.1016/j.pestbp.2011.04.002.
Shahbazi Naserabad, S., Mirvaghefi, A., Gerami, M. H., & Ghafari Farsani, H. 2015. Acute toxicity and behavioral changes of the gold fish (Carassius auratus) exposed to malathion and hinosan. Iranian Journal of Toxicology, 8(27), 1203-1208.
Shin, H S, J H Yoo, T S Min, K Y Lee, and C Y Choi. 2010. "The effects of quercetin on physiological characteristics and oxidative stress resistance in olive flounder, Paralichthys olivaceus." Asian-Australasian Journal of Animal Sciences 23(5): 588-597.
Shirazi, Zahra, and Fatemeh Khakdan. "In Silico Genome-Wide Identification and Characterization of Glutathione Peroxidase Gene Family in Wild Cherries (Prunus Avium L)." J. Plant Bioinform. Biotech. 1, no. 1 (2021): 60-72. https://doi.org/10.22034/jpbb.2021.289018.1008
Singh, U, and R S Pandey. 2021. "Fertilizer industry effluent induced hematological, histopathological and biochemical alterations in a stinging catfish, Heteropneustes fossilis (Bloch, 1794)." Environmental and Sustainability Indicators 10: 100110. https://doi.org/10.1016/j.indic.2021.100110.
Sohn, I D, Henry J B. 1969. Todd-Sunford Clinical Diagnosis by Laboratory Methods. 14th edn. 139–143. WB Saunders Company, London.
Solomon, Onoshe, Wasagu Rabiu Saidu Umar, Hassan Sanusi Wara, Abubakar Sadiq Yakubu, Madusolumou Michael Azubuike, Mbahi Asugu Mary, and Hitler Louis. 2018. "Antiulcerogenic Activity of Methanol Extract and Solvent Fractions of Stem Bark of Lannea Acida (A. Rich) against Ethanol-Induced Gastric Mucosal Injury in Albino Rats." Progress in Chemical and Biochemical Research 1, 29-39. https://doi.org/10.33945/SAMI/PCBR.2018.1.2939.
Srichaiyo, N, Tongsiri, S, Hoseinifar, SH, Dawood, MA, Esteban, MÁ, Ringø, E, and Van Doan, H. 2020. The effect of fishwort (Houttuynia cordata) on skin mucosal, serum immunities, and growth performance of Nile tilapia. Fish & Shellfish Immunology, 98, 193-200.
Tang, S M, X T Deng, J Zhou, Q P Li, X X Ge, and L Miao. 2020. "Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects." Biomedicine & Pharmacotherapy 121: 109604. https://doi.org/10.1016/j.biopha.2019.109604.
Topal, A., Atamanalp, M., Oruç, E., Beydemir, Ş., Işık, A. and Demir, Y., 2014. In vivo changes in carbonic anhydrase activity and histopathology of gill and liver tissues after acute exposure to chlorpyrifos in rainbow trout. Archives of Industrial Hygiene and Toxicology, 65(4).
Ullah, S, Z Li, Z Hasan, S U Khan, and S Fahad. 2018. "Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration." Ecotoxicology and Environmental Safety 161: 270-280. "https://doi.org/https://doi.org/10.1016/j.ecoenv.2018.06.002".
Wang, J, C Zhang, J Zhang, J Xie, L Yang, Y Xing, and Z Li. 2020. "The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio)." Fish physiology and biochemistry 46(2): 759-770.
Xiao, Y, L Zhou, T Zhang, C Qin, P Wei, L Luo, L Luo, G Huang, A Chen, and G Liu. 2020. "Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the TGF-β/AKT/mTOR signaling pathway." Life Sciences 250: 117552. https://doi.org/10.1016/j.lfs.2020.117552.
Xu, Z., Li, X., Yang, H., Liang, G., Gao, B., Leng, X., 2019. Dietary quercetin improved the growth, antioxidation, and flesh quality of grass carp (Ctenopharyngodon idella). Journal of the World Aquaculture Society 50, 1182–1195. https://doi.org/10.1111/jwas.12663
Xu, A, J Shang-Guan, Z Li, Z Gao, Y C Huang, and Q Chen. 2020. "Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus)." Aquaculture Reports 17: 100304. https://doi.org/10.1016/j.aqrep.2020.100304.
Yang, C, W Lim, and G Song. 2021. "Immunotoxicological effects of insecticides in exposed fishes." Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 247: 109064. https://doi.org/10.1016/j.cbpc.2021.109064.
Yousefi, M, H Ghafarifarsani, S H Hoseinifar, G Rashidian, and H V Doan. 2021. "Effects of dietary marjoram, Origanum majorana extract on growth performance, hematological, antioxidant, humoral and mucosal immune responses, and resistance of common carp, Cyprinus carpio against Aeromonas hydrophila." Fish & Shellfish Immunology 108: 127-133. https://doi.org/10.1016/j.fsi.2020.11.019.
Zeng, X, Z Du, X Ding, and W Jiang. 2021. "Protective effects of dietary flavonoids against pesticide-induced toxicity: A review." Trends in Food Science & Technology 109: 271-279. https://doi.org/10.1016/j.tifs.2021.01.046
Zhai SW, Liu SL. 2013. Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Italian Journal of Animal Science 12:523–527. https://doi.org/10.4081/ijas.2013.e85
Zhang, S, and I H Kim. 2020. "Effect of quercetin (flavonoid) supplementation on growth performance, meat stability, and immunological response in broiler chickens." LivestockScience 242: 104286. https://doi.org/10.1016/j.livsci.2020.104286.
Zhang, C, Q Zhang, Y Pang, X Song, N Zhou, J Wang, L He, J Lv, Y Song, Y Cheng, and X Yang. 2019. "The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin." Science of The Total Environment 653: 1426-1434. https://doi.org/10.1016/j.scitotenv.2018.11.063.
Zhang, J L, M Liu, W Cui, L Yang, and C N Zhang. 2020. "Quercetin affects shoaling and anxiety behaviors in zebrafish: Involvement of neuroinflammation and neuron apoptosis." Fish & Shellfish Immunology 105: 359-368. https://doi.org/10.1016/j.fsi.2020.06.058.
Zheng, G R, B Chen, J Shen, S Z Qiu, H M Yin, W Mao, H X Wang, and J B Gao. 2018. "Serum myeloperoxidase concentrations for outcome prediction in acute intracerebral hemorrhage." Clinica Chimica Acta 487: 330-336. https://doi.org/10.1016/j.cca.2018.10.026.
Author information
Authors and Affiliations
Contributions
SSN did the conceptualization of the paper. SSN conducted the methodology, field study, and sampling of the paper. AL and AD did the process of data collection. MN and TCC performed the statistical data by software. CN and AD evaluated the validation of data. SSN, DB, and MN wrote and prepared the original draft. TCC, MN, and DB performed writing-review and editing of the paper. SSN and CN had close supervision on the process of preparing the paper, too. CN and SSN did the project administration. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
All authors give consent for publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nasirin, C., Najm, M.A.A., Chen, TC. et al. The protective effects of quercetin on the physiological responses in malathion-exposed common carp, Cyprinus carpio. Trop Anim Health Prod 55, 22 (2023). https://doi.org/10.1007/s11250-022-03429-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03429-8