Abstract
We tested the impacts of pyrethroid pesticide deltamethrin and its amelioration by a flavonoid, quercetin, using tissue macromolecules (protein, amino acid, carbohydrate and glycogen) and antioxidant enzymes (superoxide dismutase, catalase and peroxidase) as biomarkers, on fish, Channa punctata, gill and liver. Our study proved that quercetin supplement alone, in the absence of pesticide, might be detrimental to fish health, in terms of depletion of major tissue macromolecules, but, such supplement may be beneficial to fish with pesticide associated oxidative stress. Multivariate analyses predicted that the antioxidant enzymes and lipid peroxidation were closely associated biomarkers; whereas tissue macromolecules formed a different cluster. Hence, oxidative stress biomarkers in fish can be considered a valuable tool in assessment of deltamethrin stress and its amelioration by quercetin. The work can pave the way for further research in establishing quercetin as a probable curative agent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The pyrethroid pesticide deltamethrin (DM) is widely used in India due to its high bio-efficacy and relatively low mammalian toxicity as compared to organochlorine, organophosphorus and carbamate pesticides (Devi and Gupta 2014). DM [S (a) cyano-3-phenoxybenzyl (1R, 3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-carboxylate] is broadly used in agronomy and forestry against varied insect pests (Marques et al. 2014), as well as against ectoparasites of poultry and farm animals (Whyte et al. 2014). However, non-target organisms, like fish, often end up as targets to this pesticide due to spray drift, leaching or rain (Kumari et al. 2007; Abhilash and Singh 2009). Fish do not have the enzyme system that hydrolyzes DM, making them sensitive to aqueous exposure (Sayeed et al. 2003). Furthermore, rapid gill absorption, lipophilicity, and the ability to target Na + channels of the nerve cell membrane contribute to its toxicity in fish (Widmark et al. 2011; Oliveira et al. 2012). DM is also known to produce reactive oxygen species (ROS) and cause oxidative stress by targeting the anti-oxidative defense system (ADS) of fish (Yonar and Sakin 2011).
The flavonoid quercetin (3, 5, 7, 3, 4-pentahydroxy flavon), is a semi essential food component abundantly found in glycosylated form in French beans, broccoli, apples and onions (Wach et al. 2007). The phenolic hydroxyl groups of quercetin confer strong antioxidant property, acting as a ROS scavenger (Boots et al. 2007). In rats, quercetin has been reported to be ameliorative to pesticide stress (Demir et al. 2011). However, no such reports have been found in fish. Interactions of antioxidants, such as, lycopin, ascorbic acid and Vit E, with pesticides, and possible reductions of pesticide induced stress has been reported (Vani et al. 2011; Yonar and Sakin 2011; Kalender et al. 2005). However, quercetin has not been tested as an antioxidant supplement against pesticides, in fish. The goal of this research is, therefore, to understand the impact of pesticide DM and its interaction with quercetin in fish. For this purpose, we analyzed the suitability of certain biochemical (tissue macromolecules and antioxidant enzymes) biomarkers. Further, we used multivariate analyses (MVA) to predict which biomarkers played a greater role in establishment of quercetin amelioration of DM toxicity. MVA are a collection of statistical tools that can help in better understanding and interpretation of complex data matrices and allows for their grouping based on similarities between them (Kannel et al. 2007). We used principal component analysis (PCA), factor analysis (FA), correlation matrix, and one-way analysis of variance (ANOVA) in this study.
Materials and Methods
Channa punctata weighing 27.5 ± 0.1 g and measuring 17.53 ± 0.2 cm, were bought from a fishery, immediately transferred to a water tank in the laboratory and kept for 3 week’s acclimatization. The water in the tank was dechlorinated tap water having pH between 6.4 and 6.6, temperature 23.3–25.8°C, electrical conductivity 82.2–84.6 μS cm−1, and dissolved oxygen 6.9–7.3 mg L−1. The fish were fed a commercial fish diet (Tokyu fish pellet procured from Fish Aquarium Home, Laxmi Nagar, Delhi, and contained 32% protein, 4% fat, 5% fiber, 10% crude ash, 9% moisture and 31% nitrogen free extract). Tank water was aerated and changed regularly. Commercial grade deltamethrin 2.8% E.C. (Trade name Decis – Bayer Crop Science Agrochemical India Ltd) was obtained from an agrochemical retailer. Quercetin (Himedia, Mumbai) was procured from an authorized dealer. Based on the 96-h LC50 of 0.3 μL L−1 (v/v) calculated from a prior study, six experimental groups, each with three replicates and three fish in each replicate, were set up for this study. These were control (pesticide and quercetin-free tap water), ½ 96-h LC50 of deltamethrin (0.15 μL L−1, ½ DM), 1/10th 96-h LC50 of deltamethrin (0.03 μL L−1, 1/10 DM), quercetin (0.14 g L−1, Q), ½ 96-h LC50 of deltamethrin and Q (½ DM + Q) and 1/10th 96-h LC50 of deltamethrin and Q (1/10 DM + Q). The fish were fed twice daily throughout the test period. Water used for experiments had temperature (22–23°C), pH (6.4–6.7), dissolved oxygen (6.4–6.8 ppm), total hardness (225–282 ppm) and nitrite (385–410 ppm). Both DM and Q exposures to fish were waterborne and their co-exposures were at the same time. After 21 days, liver and gill tissues were dissected from the control and treated fishes for further biochemical estimation.
The deltamethrin and quercetin conc. in test water was confirmed weekly by gas chromatography (GC, Nucon 5765) equipped with 63Ni electron capture detector, as per Singh and Singh (2008) and Soleas et al. (1997), respectively. Briefly, 100 mL of sample was separately extracted with methylene chloride (DCM) for DM and ethyl acetate for Q, using a separatory funnel. Both the extracts were separately dried with sodium sulfate, evaporated in a rota evaporator and the solvent was exchanged into petroleum ether for DM or with bis(trimethylsilyl) trifluoroacetamide (BSTFA) for Q. For DM, the extract was concentrated to approximately 1 mL and adjusted to 2.0 mL with isooctane. For Q, the residue was derivatized with BSTFA by heating for 2 h at 70°C. The derivatized samples were analyzed in GC. For both DM and Q, the mean value was found to be within 5% of the desired value. Standard DM and Q were bought from USEPA and made up in isooctane and methanol, respectively. The target analytes were identified by comparing the retention time from the standard and calculated using the response factors from five level calibration curves of the standard. Procedural blanks (analyte concentrations were less than method detection limit), random duplicate samples (Standard deviation <5), and matrix spike recovery of 100 ± 20% was carried out for DM and 88.6 ± 12% for Q.
Total carbohydrate and glycogen were analyzed using Anthrone reagent (Carrol et al. 1956), protein by Lowry et al. (1951) and free amino acid using Ninhydrin reagent (Moore and Stein 1954). Lipid peroxidation was determined indirectly in terms of MDA content, which was measured by thiobarbituric acid (TBA) reaction at 532 nm (Ohkawa et al. 1979). Unit was presented as μM g−1 FW. Spectrophotometric analysis of total SOD (EC 1.15.1.1) (Beauchamp and Fridovich 1971), CAT (EC1.11.1.6) (Maehly and Chance 1955) and GPx (EC 1.11.1.9) (Rotruck et al. 1973) were performed. One unit of SOD was defined as the enzyme activity that inhibited the photoreduction of NBT to blue formazan by 50%, and expressed as units/g of protein. The specific activity of CAT or GPx was expressed as mM/min/g of protein. Protein levels were estimated by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
PCA, FA, correlation matrix, and ANOVA were carried out using SAS JMP (Version 12. SAS Institute Inc., Cary, NC, 1989–2015) statistical software. PCA is mostly used to identify dimensionality of a data set while endeavoring to keep the relationships in the original data intact. FA was done on PCA based on the maximum likelihood method. The normality of the data was examined by Shapiro Wilk W Test and presuming its normal distribution, the data were tested for correlations between them using Pearson’s correlation coefficient. ANOVA followed by Tukey-LSD, for multiple comparisons, was used to establish significant differences between control and trial groups, if any (at p < 0.05).
Results and Discussion
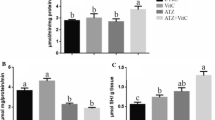
The measured concentrations of DM and Q were within 5% of the desired concentrations such that ½ DM was within 0.143–0.147 µL L−1, 1/10 DM was within 0.028–0.029 µL L−1and Q treatment was found to be between 0.135 and 0.138 g L−1 in respective test waters. Changes in major macromolecules relative to DM and Q exposure are shown in Fig. 1. There were significant decreases in carbohydrate (1a), glycogen (1b), protein (1c) and free amino acid (1d) levels on exposure to both doses of DM in gill and liver as compared to the control. DM influences the various biochemical systems that are involved in cycling of biomolecules, such as protein, carbohydrate and lipids, rendering them unsuitable for biochemical purposes. Proteins are cellular building blocks, the reduction of which in fish tissues, under DM stress, might hint towards increased proteolysis (Levine et al. 2000), impaired synthesis, increased oxidation of protein (Parvez and Raisuddin 2005), or serve as a compensatory mechanism to meet the energy requirements that is evident in DM stress (Kamalaveni et al. 2001; David et al. 2004). Carbohydrates are the energy source of cells, and DM stress leads to impaired carbohydrate metabolism (Kamalaveni et al. 2001). DM alters membrane permeability by lipid peroxidation (especially unsaturated lipids) (Sayeed et al. 2003). Interestingly, Q treatments alone had adverse effects on fish, significantly reducing the tissue carbohydrate, protein and free amino acid content, when compared to the control. Glycogen levels significantly increased in liver and remained unaltered in gills due to Q treatments. However, there were clear increments in these macromolecules when combinations of Q and DM treatments were applied (p < 0.05). The adverse effects of Q treatment alone, in the absence of pesticide, indicated that Q supplement may not be beneficial without oxidative stress (Conquer et al. 1998). Further, Q might be forming potentially toxic oxidation products (Boots et al. 2007) leading to macromolecular damage. The same cannot be said for glycogen content, as it is the storage form of carbohydrate and less susceptible to stress (Kieffer 2000).
Malondialdehyde (MDA) content generated as an indicator of lipid peroxidation (LPO) in fish tissues is shown in Fig. 2. While the control and Q treatments (in both the liver and gill tissues) generated similar patterns of MDA, both doses of DM in gill and 0.15 μL L−1 DM (½ DM) in liver significantly elevated LPO. Significant declines in MDA were observed in DM + Q treatments, with activity of liver LPO being statistically similar to the respective control values. Deltamethrin is known to induce oxidative stress in fish like C. punctata (Sayeed et al. 2003), Carassius auratus gibelio (Dinu et al. 2010), Cyprinus carpio (Yonar and Sakin 2011) and Danio rerio (Sharma and Ansari 2013). DM can interfere with the cellular detoxification mechanisms that counteract oxidative stress, and thus result in the generation of ROS (Yonar and Sakin 2011). Levels of LPO in liver was more than that in the gill. Fish liver is metabolically active and induces relatively high rates of mitochondrial ROS production. Consequently, more ROS-mediated lipid destruction can be observed in liver (Sayeed et al. 2003).
DM treatments induced SOD production while Q treatments in combination with DM lowered it (Fig. 3). Similarly, CAT (Fig. 4 ) and GPx (Fig. 5) activities were induced under DM treatments but Q supplements lowered their activities. SOD functions in the dismutation of the O2- to H2O2 and O2, to minimize the damage of ROS in the organism; while CAT is mainly responsible for the reduction of H2O2 and GPx reduces both H2O2 and organic peroxides by a glutathione-dependent reaction (Morales et al. 2004). Conversely, lowered SOD, CAT and GPx production might imply lowered ROS production after Q treatments. Quercetin is known to be a pro-oxidant that mobilizes anti-oxidative enzymes, opening the scope for detoxifying ROS. Additionally, mounting evidence supports quercetin as an agent of enhanced SOD and GPx activities (Amália et al. 2007).
The correlation matrix between different biomarkers in gill and liver tissues is shown in Table 1. Antioxidant enzymes and lipid peroxidation were positively correlated with each other, while being negatively correlated with major tissue macromolecules. Factor analysis was performed on the principal components (PC) and corresponding rotated varimax and factor loadings were presented for various biomarkers in fish tissues (Table 2). Eigen values >1.0 was considered significant (Kim and Mueller 1987) and equal number of varimax factors were obtained for two tissues through factor analysis performed based on maximum likelihood. Liu et al. (2003) classified factor loadings as strong if the corresponding loading values are >0.75 and moderate, if <0.75–0.5>. For gill, PC1 and PC2 explained 76.9% and 12.5% of the total variance, respectively. Here, Factor 1 and 2 contributed to 45.7% and 42.5%, respectively. Similarly, for liver PC1 and PC2 explained 78.3% and 13.2% of the total variance, respectively. Here, Factor 1 and 2 contributed to 51.8% and 40.2%, respectively. Multivariate analysis has been shown to be an effective predictor of the impact of contaminants in fish tissues (Mohanty and Samanta 2016). We found that in both gill and liver, the antioxidant enzymes and lipid peroxidation were predicted to be closely associated biomarkers, having highest association as predictors of impact, as against tissue macromolecule, which formed a separate cluster. Hence, oxidative stress biomarkers in fish can be considered a valuable tool for assessment of DM stress and its amelioration by Q. Further, biochemical studies demonstrated that quercetin supplement alone, in the absence of pesticide, may be detrimental to fish health in terms of major tissue macromolecular depletion. Q supplementation may be beneficial to fish with pesticide associated oxidative stress and has potential as a curative agent.
References
Abhilash PC, Singh N (2009) Pesticide use and application: an Indian scenario. J Hazard Mater 165:1–12
Amália PM, Possa MN, Augusto MC, Francisca LS (2007) Quercetin prevents oxidative stress in cirrhotic rats. Dig Dis Sci 52:2616–2621
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Boots AW, Li H, Schins RPF, Duffin R, Heemskerk JWM, Bast A, Haenen GRMM (2007) The quercetin paradox. FEBS Lett 222:89–96
Carrol NV, Longley RW, Roe JH (1956) Glycogen determination in liver and muscle by use of anthrone reagent. J Biol Chem 220:583–593
Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ (1998) Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr 128:593–597
David M, Mushigeri SB, Shivakumar R, Philip GH (2004) Response of Cyprinus carpio (Linn) to sublethal concentration of cypermethrin: alterations in protein metabolic profiles. Chemosphere 56:347–352
Demir F, Uzun FG, Durak D, Kalender Y (2011) Subacute chlorpyrifos-induced oxidative stress in rat erythrocytes and the protective effects of catechin and quercetin. Pestic Biochem Physiol 99:77–81
Devi SM, Gupta A (2014) Sublethal toxicity of commercial formulations of deltamethrin and permethrin on selected biochemical constituents and enzyme activities in liver and muscle tissues of Anabas testudineus. Pestic Biochem Physiol 115:48–52
Dinu D, Marinescu D, Munteanu MC, Staicu AC, Costache M, Dinischiotu A (2010) Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and intestine. Arch Environ Contam Toxicol 58:757–764
Kalender S, Ogutcu A, Uzunhisarcikli M, Açikgoz F, Durak D, Ulusoy Y, Kalender Y (2005) Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology 211:197–206
Kamalaveni K, Gopal V, Sampson U, Aruna D (2001) Effect of pyrethroids on carbohydrate metabolic pathways in common carp, Cyprinus carpio. Pest Manag Sci 57:1151–1154
Kannel PR, Lee S, Kanel SR, Khan SP (2007) Chemometric application in classification and assessment of monitoring locations of an urban river system. Anal Chim Acta 582:390–399
Kieffer JD (2000) Limits to exhaustive exercise in fish. Comp Biochem Phys A 126:161–179
Kim JO, Mueller CW (1987) Introduction to factor analysis: what it is and how to do it. Quantitative applications in the social sciences series. Sage University Press, Newbury Park
Kumari B, Madan VK, Kathpal TS (2007) Pesticide residues in rain water from Hisar, India. Environ Monit Assess 133:467–471
Levine RL, Wehr N, Williams JA, Stadman ER, Shacter E (2000) Determination of carbonyl groups in oxidized proteins. Methods Mol Biol 99:15–24
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis assessment of groundwater quality in a Blackfoot disease area in Taiwan. Sci Total Environ 313:77–89
Lowry OH, Rosenberg NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Maehly AC, Chance B (1955) Assay of catalase and peroxidase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 2. Academic Press, New York, pp 764–775
Marques A, Custo dio M, Guilherme Gaivao I, Santos MA, Pacheco M (2014) Assessment of chromosomal damage induced by a deltamethrin-based insecticide in fish (Anguilla anguilla L.) a follow-up study upon exposure and post-exposure periods. Pestic Biochem Physiol 113:40–46
Mohanty D, Samanta L (2016) Multivariate analysis of potential biomarkers of oxidative stress in Notopterus notopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere 155:28–38
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Morales AE, Pérez-Jiménez A, Hidalgo MC, Abellán E, Cardenete G (2004) Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp Biochem Phys C 139:153–161
Ohkawa H, Ohishi N, Tagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Chem 95:351–362
Oliveira C, Almeida J, Guilhermino L, Soares AMVM, Gravato C (2012) Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat Toxicol 124–125:209–216
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress- inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Pharmacol 20:112–117
Rotruck JJ, Pope AL, Ganthe HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Sharma DK, Ansari BA (2013) Effects of deltamethrin on CAT, LPO and GSH in tissues of zebra fish Danio rerio. Res J Environ Toxicol 7:38–46
Singh PB, Singh V (2008) Bioaccumulation of hexachlorocyclohexane, dichlorodiphenyltrichloroethane, and estradiol-17b in catfish and carp during the pre-monsoon season in India. Fish Physiol Biochem 34:25–36
Soleas GJ, Diamandis EP, Karumanchiri A, Goldberg DM (1997) A multiresidue derivatization gas chromatographic assay for fifteen phenolic constituents with mass selective detection. Anal Chem 69:4405–4409
Vani T, Saharan N, Mukherjee SC, Ranjan R, Kumar R, Brahmchari RK (2011) Deltamethrin induced alterations of hematological and biochemical parameters in fingerlings of Catla catla (Ham.) and their amelioration by dietary supplement of vitamin C. Pestic Biochem Physiol 101:16–20
Wach A, Pyrzynska K, Biesaga M (2007) Quercetin content in some food and herbal samples. Food Chem 100:699–704
Whyte SK, Westcott JD, Jimenez D, Revie CW, Hammell KL (2014) Assessment of sea lice (Lepeophtheirus salmonis) management in New Brunswick, Canada using deltamethrin (Alpha Max) through clinical field treatment and laboratory bioassay responses. Aquaculture 422–423:54–62
Widmark J, Sundström G, Ocampo Daza D, Larhammar D (2011) Differential evolution of voltage-gated sodium channels in tetrapods and teleost fishes. Mol Biol Evol 28:859–871
Yonar ME, Sakin F (2011) Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic Biochem Physiol 99:226–231
Acknowledgements
PB is thankful to UGC, India for BSR fellowship, Biotech Hub, Life Science & Bioinformatics, Assam University, for biochemical analysis facility and Bose Institute, Kolkata, India for GC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharjee, P., Das, S. Deltamethrin Induced Alteration of Biochemical Parameters in Channa punctata, Bloch and its Amelioration by Quercetin. Bull Environ Contam Toxicol 98, 763–769 (2017). https://doi.org/10.1007/s00128-017-2092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2092-8