Abstract
Acute liver failure (ALF) is a life threatening disease for which only few treatment options exist. The molecular pathways of disease progression are not well defined, but the death receptor Fas (CD95/Apo-1) appears to play a pivotal role in hepatocyte cell death and the development of ALF. Here, we explored posttranscriptional gene silencing of Fas by RNAi to inhibit pathophysiological gene expression. For targeting Fas expression in mice, Fas siRNA was formulated with the liver-specific siRNA delivery system DBTC. Treatment of mice with DBTC/siRNAFas reduced Fas expression in the liver, but not in the spleen, lung, kidney or heart. Furthermore, silencing of Fas receptor was effective in blocking or reducing several aspects of ALF when it was tested in mice exposed to galactosamine/lipopolysaccharide (G/L), a well-known model of ALF. The application of DBTC/siRNAFas 48 h prior G/L exposure resulted in amelioration of hepatic perfusion, reduction of hepatocellular death and increase of survival rate. The administration of DBTC/siRNAFas formulation further diminished the inflammatory response upon G/L challenge, as indicated by a marked decrease of TNFα mRNA expression. However, IL-6 plasma concentration remained unaffectedly by DBTC/siRNAFas formulation. Since the specific silencing of hepatic Fas expression only partially protected from inflammation, but completely attenuated apoptotic and necrotic cell death as well as microcirculatory dysfunction, the development of therapeutic strategies with DBTC lipoplex formulations to treat ALF should be combined with anti-inflammatory strategies to reach maximal therapeutic efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute liver failure (ALF) is a clinical syndrome characterized by peripheral vasodilation, encephalopathy and coagulopathy culminating in multi-organ dysfunction and death [1]. In contrast with the well-documented morphological characterization of dying hepatocytes, the molecular pathways leading to the death of hepatocytes are not completely understood. A large amount of recent research indicates that a complex system, comprising death factors and death receptors, mediates cell death in liver disease [2]. One of these receptors is Fas (CD95/Apo-1) belonging to the tumor necrosis factor receptor family, which is-together with FasLigand (FasL)-an important inducer of apoptosis [3] and constitutively expressed in the liver [4]. Blockade of the Fas/FasLigand (FasL) system has been shown to ameliorate liver disease to various degrees [5, 6]. In detail, Kondo et al. [5] demonstrated by using FasL-neutralizing molecules a prevention of LPS-induced liver damage and Ksontini et al. [6] showed that an administration of soluble Fas immunoadhesion reduced liver injury in concanavalin A (ConA)-induced hepatitis. A recent method to block the Fas/FasL system represents the induction of RNA interference (RNAi) [7]. The RNAi is a powerful tool to silence gene expression post-transcriptionally [8]. It was shown that silencing of Fas expression with short interfering RNA (siRNA) holds therapeutic promise in the prevention of liver injury by protecting hepatocytes from cytotoxicity. However, siRNA therapeutics are hindered by a poor intracellular uptake and the limited blood stability of siRNA molecules, which limits effective delivery of the therapeutic siRNA to in vivo targets [9]. Here, we tested in vivo Fas siRNA formulated with the liver-specific siRNA delivery system, DBTC, which was developed by Silence Therapeutics GmbH (Berlin, Germany). We evaluated the liver-specificity of this new DBTC formulation and its capability to protect mice from galactosamine/lipopolysaccharide (G/L)-induced liver damage.
Materials and methods
Model of ALF and experimental groups
Male C57BL/6J (Charles River Laboratories, Sulzfeld, Germany) were used at 8–12 weeks of age with a body weight of approximately 20 g. Animals were provided with water and standard laboratory chow ad libitum. The experimental protocol was approved by the local committee (LALLF 7221.3-1.1-076/11) and all animals received human care according to the German legislation on protection of animals and the Guide for the Care and Use of Laboratory Animals (NIH publication 86-23 revised 1985).
For induction of ALF, mice were injected with galactosamine (G; 720 mg/kg body weight intraperitoneally [bw ip]; Sigma) and lipopolysaccharide (L; 10 µg/kg bw ip, serotype 0128:B12; Sigma) and were studied 6 h thereafter. Concentrations of G/L were used in accordance with work published previously by our [10, 11] and other groups [12, 13]. For kinetic analysis of Fas silencing G/L-exposed animals were pre-treated with DBTC/siRNAFas (2.2 mg/kg bw; 0.1 ml/10 g bw) or equivalent volumes of saline (NaCl) either 24, 48, 72 or 96 h prior to ALF induction intravenously (i.v.) through jugular vein injection. The livers of these animals were analyzed for mRNA and protein expression of Fas. Since protein expression of Fas was maximally lowered at -48 h, we further used this time point for the readout of Fas silencing in G/L-induced ALF and analyzed the hepatic TNFα mRNA expression. For intravital fluorescence microscopy additional sets of G/L-exposed animals received 48 h prior induction of ALF (G/L) DBTC formulations of siRNA Fas (DBTC/siRNAFas + G/L), non-targeting siRNA of the luciferase gene (DBTC/siRNALuci + G/L) or equivalent volumes of saline (NaCl), as well as DBTC/siRNAFas/Luci alone which served as the controls. To test liver-specific Fas silencing by DBTC/siRNA the liver, spleen, lung, heart and kidney of these control mice were analyzed for Fas mRNA expression.
Oligonucleotides
The siRNA molecules (AtuRNAi) used in this study are blunt-ended, double-stranded RNA oligonucleotides (Table 1) stabilized by alternating 2′-O-methyl modifications on both strands as previously described [14, 15], and were synthesized by Biospring (Frankfurt a.M., Germany). For in vivo application, siRNAs were formulated with DBTC, a lipoplex system developed by Silence Therapeutics GmbH for targeting specific cell types in the liver.
Intravital fluorescence microscopy
For in vivo analysis of hepatocellular apoptosis, intrahepatic leukocyte accumulation, and sinusoidal perfusion failure, fluorescence microscopy was performed 6 h after G/L exposure in ketamine/xylazine-anesthetized animals (75/25 mg/kg bw ip) in accordance with work previously published by our group [10, 11, 16]. Further details are provided in supplemental Materials and methods.
Sampling and assays
After in vivo microscopy, animals were exsanguinated by puncture of the vena cava inferior for immediate separation of EDTA plasma. The degree of hepatic disintegration was assessed by spectrophotometric determination of plasma glutamate dehydrogenase (GLDH), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities using commercially available reaction kits (Roche Diagnostics, Mannheim, Germany). Interleukin 6 (IL-6) was analyzed with a commercially available enzyme-linked immunosorbent assay kit in accordance with the manufacturer’s instructions (Pierce, Biotechnology, Rockford, IL, USA). Tissues of liver, spleen, lung, heart and kidney were sampled for subsequent Real time-PCR, Western blot analysis and histology. In addition spleen tissue was sampled for flow cytometry analysis.
Flow cytometry analysis
For evaluation of Fas expression on splenocytes, single-cell suspensions were incubated with fixable viability stain 510 (1:1,000, BD Biosciences) and anti-CD16/CD32 (1:100, eBioscience) to exclude dead cells from analysis and to prevent nonspecific antibody binding. Subsequently, cells were stained with lineage specific markers anti-CD4 (1:100, GK1.5, eBioscience) -CD8 (1:100, 53-6.7, eBioscience), -CD11c (1:200, HL3, BD Pharmingen) -CD11b (1:100, M1/70, eBioscience), -CD19 (1:100, 1D3, eBioscience), -Ly6G (1:100, 1A8-Ly6 g, eBioscience). -Ly6C (1:100, AL-21, BD Pharmingen), -CD3 (1:100, 145-2C11 BD Pharmingen), and -NKp46 (1:100, 29A1.4, eBioscience). Fas expression was analyzed using anti-CD95 (1:150, Jo2, BD Pharmingen). Cells were analyzed by flow cytometry on a FACS LSR Fortessa (BD).
Real time-PCR (RT-PCR) analysis
Approximately 20 mg of tissue was homogenized in a Mixer Mill MM 301 (Retsch GmbH, Haan, Germany) using tungsten carbide beads (Qiagen). Total RNA was isolated via the Invisorb Spin Tissue RNA Mini Kit (Invitek, Berlin, Germany). Depending on the tissue, 25–100 ng total RNA was used for quantitative TaqMan Real Time (RT)-PCR with the amplicon sets (listed in Table 2) obtained from BioTez GmbH, Berlin, Germany: The TaqMan RT-PCR reactions were carried out with an ABI PRISM 7700 Sequence Detector [Software: Sequence Detection System v1.6.3 (ABI)] or StepOnePlus™ RT-PCR Sytem (ABI) using a standard protocol for RT-PCR as described previously [14] with primers (see Table 2) and probes at a concentration of 300 and 100 nM respectively. TaqMan data were calculated by using the comparative CT method.
Western blot analysis
Target protein expression was assessed by Western blotting of whole tissue lysates as described previously [17]. Snap frozen tissues were homogenized in a Mixer Mill and proteins were extracted in Riper-lysis buffer. Proteins were separated by SDS-PAGE and subjected to immune blotting as previously described [14] using the following antibodies: rabbit anti-Fas polyclonal antibody (1:500; M-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit anti-PTEN (138G6) monoclonal antibody (1:1,000; #9559, Cell Signaling Technology, Boston, MA, USA) for loading control. Immunoblots were analyzed by luminescence imaging using the Stella camera system and AIDA image analyzer software 4.25 from Raytest (Mannheim, Germany).
Histology and immunohistochemistry of liver tissue
For hematoxylin & eosin (H&E) staining liver tissue was fixed in 4 % phosphate-buffered formalin for 2–3 days and then embedded in paraffin. From the paraffin-embedded tissue blocks, 4 µm sections were put on glass slides and stained with H&E. For histomorphometric analysis of necrotic tissue images of twenty random low-power fields (100× magnification, Olympus BX 51, Hamburg, Germany) were acquired with a Color View II FW camera (Color View, Munich, Germany) and evaluated by means of an image analysis system (Adobe Photoshop, Adobe Systems, Uxbridge, UK). The quotient of the focal necrosis surface to the total liver section area was assessed and given in percent.
For immunohistochemical staining of Fas expression serial sections of liver tissue of DBTC/siRNAFas- and DBTC/siRNALuci-treated mice were incubated with primary antibodies overnight at 4 °C (polyclonal anti-Fas (1:50, M-20), Santa Cruz Biotechnology). Universal LSAB® kits (System-HRP and System-AP; DakoCytomation, Dako, Hamburg, Germany) were used according to the manufacturer´s instructions for the development of Fas with DAB chromogen. The sections were counterstained with hemalaun and analyzed with a light microscope (Olympus BX51, Hamburg, Germany).
Survival study upon G/L-induced liver injury
To verify the contribution of silencing of Fas expression to the survival in G/L-induced ALF, G/L-exposed animals and G/L-exposed animals with DBTC/siRNAFas treatment (n = 10 per group) were randomly numbered and mortality rate was assessed every hour within the first 12 h as well as at 15 and 24 h after G/L exposure. The final survival rate was determined at 24 h after G/L exposure.
Statistical analysis
All data are expressed as mean ± SEM. For statistics, one-way analysis of variance, including all groups, was used to assess significant differences between groups. Subsequently, post hoc pairwise comparison test including Bonferroni correction for multiple comparisons was applied to identify which group differs to each other. Data were considered significant if p < 0.05. Statistical analysis was performed using the SigmaStat software package (Jandel Scientific, San Rafael, CA, USA).
Results
DBTC/siRNAFas formulation shows high liver-specificity
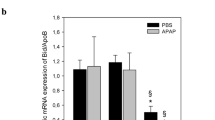
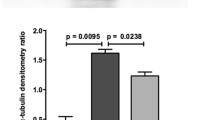
In order to test whether the DBTC/siRNAFas formulation specifically targets hepatic tissue, we evaluated mRNA and protein expression of Fas in liver (Fig. 1a, b), spleen, lung, heart and kidney (Fig. 2a–d) 48 h after i.v. injection. Here, silencing of Fas expression was restricted to the liver indicated by a significant decrease of hepatic Fas mRNA (Fig. 1a) and protein expression (Fig. 1b) compared to DBTC/siRNALuci control (Fig. 1a, b). In contrast, all other examined tissues (Fig. 2a–d) revealed almost equal Fas mRNA expression upon application of DBTC/siRNAFas when compared to both controls, i.e. NaCl and DBTC/siRNALuci.
Quantitative RT-PCR analysis of Fas mRNA expression (a) and light microscopic image (original magnification x400) of liver sections stained for Fas protein expression (brown) by immunohistochemistry (b) of C57BL/6J mice (n = 24). Signals were corrected to that of β-actin. Animals were pre-treated (-48 h) with either Fas siRNA (DBTC/siRNAFas; n = 8) or luciferase siRNA (DBTC/siRNALuci; n = 8) as control. Time-matched sham-treated animals with application of equivalent volumes of saline served as additional control (NaCl; n = 8). Values are given as mean ± SEM; ANOVA, post hoc pairwise comparison tests: *p < 0.05 versus NaCl, &p < 0.05 versus DBTC/siRNALuci (Color figure online)
Quantitative RT-PCR analysis of Fas mRNA expression in spleen (a), lung (b), heart (c) and kidney (d) of C57BL/6J mice (n = 24). Signals were corrected to that of β-actin. Animals were pre-treated (-48 h) with either Fas siRNA (DBTC/siRNAFas; n = 8) or luciferase siRNA (DBTC/siRNALuci; n = 8) as control. Time-matched sham-treated animals with application of equivalent volumes of saline served as additional control (NaCl; n = 8). Values are given as mean ± SEM
To exclude that Fas expression is silenced in immune cells upon DBTC/siRNAFas treatment CD4+-, CD8+-, CD3+-T cells, NK cells, B cells, dendritic cells, macrophages and neutrophils (Fig. 3a–h) were analyzed. All cell population’s revealed almost equal Fas expression upon application of DBTC/siRNAFas compared to both controls, i.e. NaCl and DBTC/siRNALuci.
Quantitative flow cytometry of splenic Fas expression in CD4+-T cells (a), CD8+-T cells (b), CD3+-T cells (c), NK cells (d), B cells (e), dendritic cells (f), macrophages (g), and neutrophils (h) of C57BL/6J mice (n = 22). Animals were pre-treated (-48 h) with either Fas siRNA (DBTC/siRNAFas; n = 8) or luciferase siRNA (DBTC/siRNALuci; n = 8) as control. Time-matched sham-treated animals with application of equivalent volumes of saline served as additional control (NaCl; n = 8). Values are given as mean ± SEM
Fas as well as TNF expression is markedly down regulated 48 h after DBTC/siRNAFas application in G/L-exposed mice
To investigate the optimal time point of DBTC/siRNAFas application mice were administrated with DBTC/siRNAFas either 96, 72, 48 or 24 h before G/L exposure. By doing so, we were able to show in livers of G/L-exposed mice that application of DBTC/siRNAFas at 24 h before G/L exposure resulted in a 2.6-fold reduction of mRNA Fas expression (Fig. 4a) with still unchanged Fas protein expression (Fig. 4b). However, pre-treatment with siRNA Fas -48 h, -72 h and -96 h before G/L exposure caused both a 9.3- up to 12.8-fold decrease of hepatic Fas mRNA expression (Fig. 4a) and a marked reduction of hepatic Fas protein expression (Fig. 4b) when compared to G/L-exposure alone. Because the application of siRNA 48 h before G/L-induced liver injury already resulted in marked decrease of Fas protein expression, we chose this time point of siRNA injection for subsequent experiments.
Quantitative RT-PCR analysis of Fas mRNA expression (a) and representative Western Blot of Fas protein expression (b) in livers of C57BL/6J mice (n = 22). Animals were injected either with G/L alone for induction of ALF (-6 h; G/L, n = 6) or additionally pre-treated either -24 h (n = 4), -48 h (n = 3), -72 h (n = 3) or -96 h (n = 2) with Fas siRNA (DBTC/siRNAFas). Quantitative RT-PCR analysis of TNFα mRNA expression in livers of C57BL/6 J mice (c). Animals were injected either with G/L alone for induction of ALF (-6 h; G/L, n = 6) or additionally pre-treated -48 h with Fas siRNA (DBTC/siRNAFas; n = 4). Signals were corrected to that of PTEN. An additional group of animals received equivalent volumes of saline (NaCl; n = 4). Values are given as mean ± SEM; ANOVA, post hoc pairwise comparison tests: *p < 0.05 versus NaCl, #p < 0.05 versus G/L
Upon application of G/L and of DBTC/siRNAFas-48 h prior G/L exposure TNFα mRNA expression was analysed. Livers of G/L-treated mice exhibited a significant increase of TNFα mRNA expression compared to NaCl-treated mice, which is 3.7-fold reduced by application of DBTC/siRNAFas formulation when compared to G/L challenge alone (Fig. 4c).
DBTC/siRNAFas formulation ameliorated hepatic perfusion and reduced G/L-induced hepatocellular death
In vivo microscopy of livers in G/L-exposed mice and in mice, which received in addition siRNA formulation DBTC/siRNALuci as non-targeting control lipoplex revealed characteristic features of acute injury (Fig. 5a and upper right panel), including a pronounced perfusion failure of up to 72 % non-perfused sinusoids (Fig. 5a). Administration of the DBTC/siRNAFas formulation 48 h before G/L exposure resulted in a significant reduction of microvascular perfusion failure when compared to G/L-treated mice (Fig. 5a and lower right panel). However, values did not reach baseline values of healthy control mice. The administration of DBTC/siRNAFas and DBTC/siRNALuci formulation alone did not influence sinusoidal perfusion (Fig. 5a).
Representative fluorescence microscopic images (a, b, right panel, original magnification ×200) with quantitative analysis (left panel) of sinusoidal perfusion failure (a) and of apoptotic hepatocytes (b) as well as representative H&E-stained images (c; right panel; original magnification ×100) with quantitative analysis of cell death area (c; left panel) in C57BL/6J mice (n = 48). Animals were injected with G/L (n = 8) for induction of ALF (-6 h) and pre-treated with either Fas siRNA (-48 h; DBTC/siRNAFas + G/L; n = 8) or with luciferase siRNA (-48 h; DBTC/siRNALuci + G/L; n = 8). In addition, time-matched sham-treated animals with application of equivalent volumes of saline (NaCl; n = 8) or DBTC lipoplex formulation alone (DBTC/siRNAFas/Luci; n = 16) were studied. Values are given as mean ± SEM; ANOVA, post hoc pairwise comparison tests: *p < 0.05 versus NaCl, #p < 0.05 versus G/L
Analysis of apoptotic as well as necrotic cell death (Fig. 5b, c) showed a significant G/L-induced increase of hepatocellular apoptosis and necrosis, as indicated by a sharp rise in the number of bisbenzimide-stained hepatocytes with nuclear chromatin condensation and fragmentation (Fig. 5b and upper right panel) and by extensive areas of cell death with 34 % necrotic tissue (Fig. 5c and upper right panel). Only the treatment with DBTC/siRNAFas in G/L-exposed mice decreased the number of apoptotic hepatocytes by ~70 % (Fig. 5b and lower right panel) as well as caused a 41 % decrease of the necrotic area (Fig. 5c and lower right panel) which still exceeded the corresponding values found in animals treated only with saline (Fig. 5b, c). Further, G/L-treated mice and mice which additionally received the non-targeting siRNA formulation DBTC/siRNALuci revealed a 9.4- up to 10.4-fold rise of plasma activities of AST, ALT and GLDH when compared to mice treated only with NaCl (Fig. 6a–c). Pre-treatment with the DBTC/siRNAFas formulation markedly reduced plasma activities of AST, ALT and GLDH without reaching control levels (Fig. 6a–c). Again application of DBTC/siRNAFas as well as DBTC/siRNALuci formulation alone did not influence hepatocellular cell death as well as plasma liver enzyme activities (Figs. 5b, c, 6a–c).
Quantitative analysis of plasma activities of AST (a), ALT (b) and of GLDH (c) in C57BL/6J mice (n = 48). Animals were injected with G/L (n = 8) for induction of ALF (-6 h) and pre-treated with either Fas siRNA (-48 h; DBTC/siRNAFas + G/L; n = 8) or with luciferase siRNA (-48 h; DBTC/siRNALuci + G/L; n = 8). In addition, time-matched sham-treated animals with application of equivalent volumes of saline (NaCl; n = 8) or DBTC lipoplex formulation alone (DBTC/siRNAFas/Luci; n = 16) were studied. Values are given as mean ± SEM; ANOVA, post hoc pairwise comparison tests: *p < 0.05 versus NaCl, #p < 0.05 versus G/L
DBTC/siRNAFas formulation did not reduce the increased venular leukocytes adherence and IL-6 release upon G/L exposure
Analysis of venular leukocyte adherence (Fig. 7a) showed severe G/L-induced hepatic inflammation, as indicated by a high number of adherent leukocytes (Fig. 7a). Venular leukocyte-endothelial cell interaction could not be reduced by administration of the DBTC/siRNAFas formulation 48 h before G/L exposition. In line with this finding, IL-6 plasma concentration in G/L-exposed mice was 28-fold higher compared to NaCl-treated mice and was not reduced by pre-treatment with DBTC/siRNAFas (Fig. 7b).
Representative fluorescence microscopic images (a, right panel, original magnification ×200) with quantitative analysis of venular leukocyte adherence (a, left panel) as well as quantitative analysis of plasma concentrations of IL-6 (b) in C57BL/6J mice (n = 48). Animals were injected with G/L (n = 8) for induction of ALF (-6 h) and pre-treated with either Fas siRNA (-48 h; DBTC/siRNAFas + G/L; n = 8) or with luciferase siRNA (-48 h; DBTC/siRNALuci + G/L; n = 8). In addition, time-matched sham-treated animals with application of equivalent volumes of saline (NaCl; n = 8) or DBTC lipoplex formulation alone (DBTC/siRNAFas/Luci; n = 16) were performed. Values are given as mean ± SEM; ANOVA, post hoc pairwise comparison tests: *p < 0.05 versus NaCl. Survival rate (c) of C57BL/6J mice (n = 20) that were either injected with G/L alone (-6 h; G/L, n = 10) or additionally pre-treated with Fas siRNA (DBTC/siRNAFas + G/L) 48 h prior to the exposure to G/L. Mortality was assessed every hour within the first 12 h and after 15 h. The final survival rate was determined 24 h after G/L exposure
Increased survival rate by DBTC/siRNAFas pre-treatment upon G/L exposure
After application of G/L, all animals studied started to die at 6 h and were found dead within 7 h (Fig. 7c). After the additional pre-treatment with the DBTC/siRNAFas formulation, 4 out of 10 G/L-exposed mice survived (Fig. 7c; 40 %). Though not statistically significant, this reveals a survival advantage by silencing of Fas expression.
Discussion
Apoptosis of hepatocytes is known to be a major cause of hepatocellular injury in a variety of liver diseases. Though the pathogenesis of hepatocyte loss is not completely clarified, it is reported that the receptor Fas has an important role in regulating cell death [18, 19]. Thus, ligation of cell-membrane Fas triggers apoptosis in many cell types, including hepatocytes [3] and administration of anti-Fas antibody causes hepatocyte death and ALF [20]. Therefore, inhibition of Fas signaling by neutralization of FasL rescued mice from LPS-induced mortality, and Fas-null mice were resistant to LPS-induced hepatic cell death [5]. Comparably, Fas lpr mutant mice showed a decrease of ALF-associated apoptotic cell death and hepatic microcirculatory dysfunction [21]. Another possibility to block Fas signaling represents the silencing of Fas expression by using RNA interference (RNAi) technology [7]. Nevertheless, all these methods to block Fas have systemic effects and might stress the organisms additionally by activation of immune responses through toll-like receptors [22]. It is reported that several siRNA delivery systems are effective upon hydrodynamic injection [23, 24]. Although this method can reach the desired gene silencing effect, drawbacks exist such as non-selective delivery of the nucleotides, and clinical impracticality due to need for a large volume and high injection speed [25]. In order to overcome these drawbacks we tested Fas siRNA formulated with the liver-specific siRNA delivery system, DBTC, which was developed by Silence Therapeutics GmbH (Berlin, Germany) especially for in vivo applications. This formulation shows particularly high liver-specificity, as indicated by an absent silencing of Fas expression in other organs e.g. heart, lung, kidney, and spleen as well as various immune cell subsets of the spleen. Using this approach liver-specific silencing of Fas expression resulted in an amelioration of G/L-induced microcirculatory dysfunction with enhanced sinusoidal perfusion. Because the receptor Fas mainly triggers apoptotic cell death within ALF [18, 19] Fas silencing resulted in a significant reduction of hepatocellular apoptotic death.
Beside apoptotic also necrotic cell death dominantly influences the outcome of ALF-exposed animals [26]. In support of this, the present study shows an enormous rise of plasma activities of AST, ALT and GLDH as well as a marked increase of necrotic tissue area. The fact that Fas siRNA was also capable to reduce necrosis seems to be the consequence out of the anti-apoptotic efficacy of Fas silencing, since both modes of cell death are not strictly independent from each other. Instead, apoptotic cell death has frequently been shown to turn into necrosis [26] once the cell lost ATP being necessary for the machinery of apoptotic pathway signaling.
It is important to mention that in the present study silencing of Fas expression did not completely restore hepatic microcirculation and liver tissue integrity as well as prevent cell death. One explanation might be that not only Fas but also other death receptors such as TNFR and TRAIL participate in liver tissue damage upon ALF [27–29]. In that, Muto et al. showed already in 1988 that endotoxin-induced inflammation is mediated by the release of TNFα because patients with fulminate liver failure revealed significantly increased production of TNFα, whereas TRAIL signaling is rather prominent in patients with viral hepatitis [30].
Furthermore, it is known that beside its role in apoptosis Fas/FasL interaction may also contribute to non-apoptotic signaling, including NFκ-B activation [31–33] and cytokine release. In fact, we could show that ALF caused a huge rise in cytokine release and significant increase of TNFα mRNA expression. Treatment with the DBTC/siRNAFas formulation in G/L-exposed mice reduced the inflammatory response, as indicated by a marked decrease of TNFα mRNA expression. However, IL-6 plasma concentration and adherent venular leukoyctes remained unaffected by the Fas silencing. Hence, it can be hypothesized that both pathways Fas/FasL and TNFR/TNF contribute via a direct mechanistic link to apoptotic cell death in G/L-induced ALF, while other pathways such as TLR/LPS may mediate the inflammatory response [34]. Accordingly, McDonald et al. [34] demonstrated that endothelial TLR4 together with Kupffer cells coordinates neutrophil adhesion within liver sinusoids during endotoxemia. This might explain the still high venular leukocyte adherence despite DBTC/siRNAFas treatment in G/L-exposed mice and the remaining mortality of ~60 %.
In summary, the specific silencing of hepatic Fas expression only partially protected mice from inflammation, but completely from apoptotic and necrotic cell death as well as microcirculatory dysfunction. Thus, the development of therapeutic strategies with DBCT lipoplex formulations to treat ALF should combine anti-inflammatory and anti-apoptotic strategies to reach maximal therapeutic efficacy.
References
Antoniades CG, Berry PA, Wendon JA, Vergani D (2008) The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol 49:845–861
Faubion WA, Gores GJ (1999) Death receptors in liver biology and pathobiology. Hepatology 29:1–4
Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L (1995) Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med 182:1223–1230
Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178
Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S (1997) Essential roles of the Fas ligand in the development of hepatitis. Nat Med 3:409–413
Ksontini R, Colagiovanni DB, Josephs MD, Edwards CK 3rd, Tannahill CL, Solorzano CC, Norman J, Denham W, Clare-Salzler M, MacKay SL, Moldawer LL (1998) Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J Immunol 160:4082–4089
Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J (2003) RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 9:347–351
Hannon GJ (2002) RNA interference. Nature 418:244–251
Jiang N, Zhang X, Zheng X, Chen D, Siu K, Wang H, Ichim TE, Quan D, McAlister V, Chen G, Min WP (2012) A novel in vivo siRNA delivery system specifically targeting liver cells for protection of ConA-induced fulminant hepatitis. PLoS One 7:e44138
Le Minh K, Klemm K, Abshagen K, Eipel C, Menger MD, Vollmar B (2007) Attenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of mice. Am J Pathol 170:1954–1963
Eipel C, Kidess E, Abshagen K, Leminh K, Menger MD, Burkhardt H, Vollmar B (2007) Antileukoproteinase protects against hepatic inflammation, but not apoptosis in the response of D-galactosamine-sensitized mice to lipopolysaccharide. Br J Pharmacol 151:406–413
Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang GZ, Takahashi K, Tamada Y, Yokochi T (1996) Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun 64:734–738
Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A (1995) Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 146:1220–1234
Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, Löffler K, Fechtner M, Röhl T, Fisch G, Dames S, Arnold W, Giese K, Klippel A, Kaufmann J (2006) RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther 13:1360–1370
Czauderna F, Fechtner M, Dames S, Aygün H, Klippel A, Pronk GJ, Giese K, Kaufmann J (2003) Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res 31:2705–2716
Schäfer T, Scheuer C, Roemer K, Menger MD, Vollmar B (2003) Inhibition of p53 protects liver tissue against endotoxin-induced apoptotic and necrotic cell death. FASEB J 17:660–667
Fehring V, Schaeper U, Ahrens K, Santel A, Keil O, Eisermann M, Giese K, Kaufmann J (2014) Delivery of therapeutic siRNA to the lung endothelium via novel Lipoplex formulation DACC. Mol Ther 22:811–820
Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456
Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH (1999) CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J Exp Med 189:441–446
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S (1993) Lethal effect of the anti-Fas antibody in mice. Nature 364:806–809
Kuhla A, Eipel C, Siebert N, Abshagen K, Menger MD, Vollmar B (2008) Hepatocellular apoptosis is mediated by TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of acute liver failure. Apoptosis 13:1427–1438
Karikó K, Bhuyan P, Capodici J, Weissman D (2004) Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol 172:6545–6549
Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A (2005) In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 106:2295–2301
Li X, Zhang JF, Lu MQ, Yang Y, Xu C, Li H, Wang GS, Cai CJ, Chen GH (2007) Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbecks Arch Surg 392:345–351
Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D (2004) Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther 11:675–682
Jaeschke H, Gujral JS, Bajt ML (2004) Apoptosis and necrosis in liver disease. Liver Int 24:85–89
Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R (1988) Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet 2:72–74
Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M (2005) Apoptosis in liver diseases—detection and therapeutic applications. Med Sci Monit 11:RA337–RA345
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43:S31–S44
Han LH, Sun WS, Ma CH, Zhang LN, Liu SX, Zhang Q, Gao LF, Chen YH (2002) Detection of soluble TRAIL in HBV infected patients and its clinical implications. World J Gastroenterol 8:1077–1080
Hu WH, Johnson H, Shu HB (2000) Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem 275:10838–10844
Ahn JH, Park SM, Cho HS, Lee MS, Yoon JB, Vilcek J, Lee TH (2001) Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-kappaB-dependent gene expression. J Biol Chem 276:47100–47106
Wajant H, Pfizenmaier K, Scheurich P (2003) Non-apoptotic Fas signaling. Cytokine Growth Factor Rev 14:53–66
McDonald B, Jenne CN, Zhuo L, Kimata K, Kubes P (2013) Kupffer cells and activation of endothelial TLR4 coordinate neutrophil adhesion within liver sinusoids during endotoxemia. Am J Physiol Gastrointest Liver Physiol 305:G797–G806
Acknowledgments
The authors cordially thank Berit Blendow, Dorothea Frenz, Maren Nerowski, and Eva Lorbeer-Rehfeldt (Institute for Experimental Surgery, University of Rostock, Germany) for excellent technical assistance.
Conflict of interest
Volker Fehring, Ute Schaeper, and Ulf Schulze-Topphoff are employees of and have stock options from Silence Therapeutics plc and declare competing financial interest. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuhla, A., Thrum, M., Schaeper, U. et al. Liver-specific Fas silencing prevents galactosamine/lipopolysaccharide-induced liver injury. Apoptosis 20, 500–511 (2015). https://doi.org/10.1007/s10495-015-1088-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1088-2