Abstract
Tetranychus urticae Koch is one of the most common and harmful pests in vegetable production areas. Similar to other countries, control of T. urticae is mainly based on acaricides in Turkey. However, T. urticae rapidly develops resistance and failures in chemical control have occurred frequently. The toxicity of various acaricides was investigated in ten T. urticae populations collected from vegetable crops in Turkey. In addition, populations were screened for the presence of currently known target-site resistance mutations. It was shown that resistance to bifenthrin was the most widespread, but also half of the populations were resistant to abamectin and hexythiazox. Resistance mutations in the voltage-gated sodium channel (VGSC) and chitin synthase 1 were found in various populations. Moreover, for the first time, F1538I and L1024V VGSC mutations were reported for Turkish populations. Mutations that confer resistance to abamectin, bifenazate and METI-I acaricides such as pyridaben were not detected. These results will contribute to the design of an effective resistance management program in Turkey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vegetable production is economically very important for Turkey both for meeting the local demands as well as for export. Especially in the southern part of Turkey (Mediterranean region), which has a suitable climate allowing year-round cultivation, vegetable production spreads to huge areas comprising 170.000 ha in 2017 (TSI 2018). However, the climate also allows for the fast development of several plant pests. The two-spotted spider mite Tetranychus urticae Koch is one of the most common and harmful pests in vegetable production areas in Turkey. Although more and more farmers consider biological control as a valid option to keep spider mites below economic damage thresholds, control of T. urticae is still mainly based on the application of acaricides in Turkey (Çağatay et al. 2018). Turkey has the 10th biggest acaricide market in the world (Van Leeuwen et al. 2015) and acaricide usage in Turkey is increasing year by year (from 902 tonnes in 2006 to 2452 tonnes in 2017) (TSI 2018).
Tetranychus urticae is notorious for its ability to develop acaricide resistance very quickly (Van Leeuwen et al. 2010; Van Leeuwen and Dermauw 2016). Its short life cycle, arrhenotokous reproduction and high fecundity all contribute to resistance development. Resistance has often been reported to evolve only a few years after the introduction of a new acaricide (Van Leeuwen et al. 2009, 2010). Another reason for fast resistance development is the polyphagous nature of the species. T. urticae is encountered on many crops, resulting in high acaricide exposure. In addition, the evolution to polyphagy might have equipped spider mites with a unique detoxification toolkit (Dermauw et al. 2013), although other factors in resistance development might prevail in the broader context of arthropod pests (Dermauw et al. 2018).
Arthropods can develop resistance either by decreasing the pesticide quantity that can reach the target-site (pharmacokinetic mechanisms) or by altering the target-site of the pesticide (pharmacodynamic mechanisms) (Van Leeuwen et al. 2009; Van Leeuwen and Dermauw 2016). Among other pharmacokinetic mechanisms like e.g. cuticle thickening, the role of detoxification enzymes such as cytochrome P450-mono-oxygenases, glutathione S-transferases and carboxyl/choline esterases in resistance development is well studied. On the other hand, pharmacodynamic resistance mechanisms such as mutations that alter the structure or expression of the target-site are also well documented (Feyereisen et al. 2015). The acaricides tested in this study belong to different mode of action groups according to IRAC (Insecticide Resistance Action Committee) classification (Sparks and Nauen 2015). Bifenthrin and abamectin act on nervous systems of insect/mites, targeting voltage-gated sodium channels and glutamate-gated chloride channels, respectively (Lynagh and Lynch 2012; Dong et al. 2014). On the other hand, cyflumetofen, bifenazate and fenbutatin oxide inhibit mitochondrial electron transport and respiration at Complex II, Complex III and ATP synthase, respectively (Van Leeuwen et al. 2008, 2015; Hayashi et al. 2013). Although hexythiazox and spiromesifen both interfere with growth and development, they have different mode of actions. The former inhibits chitin synthesis (Demaeght et al. 2014) whereas the latter inhibits acetyl coenzyme A carboxylase, part of the first step in lipid biosynthesis (Bretschneider et al. 2007; Lümmen et al. 2014).
In Turkey, a number of resistance cases have been described and partially studied. For example, more then 10 years ago high levels of bifenthrin resistance was linked with increasing esterase activity in T. urticae populations sampled from cotton production areas (Ay and Gürkan 2005). Other studies have reported on chlorpyrifos and abamectin resistance in T. urticae populations collected from vegetable areas (Ay 2005; Ay et al. 2005; Sökeli et al. 2007). On the other hand, monitoring of T. urticae populations from strawberry did not reveal extreme resistance levels to abamectin, etoxazole, spiromesifen and tebufenpyrad (Yalçın et al. 2018). One of the most comprehensive studies conducted recently, investigated abamectin resistance incidence and mechanisms in a number of greenhouse T. urticae strains. It was revealed that resistance ratio’s extended from 200- to 400-fold for abamectin and resistant populations displayed increased esterase activity. However, mutations in the target-site of abamectin (glutamate-gated chloride channel, see Dermauw et al. 2012) were not detected (Çağatay et al. 2018). Finally, next to T. urticae, the resistance status of Turkish populations of the European red mite Panonychus ulmi (Kumral and Kovancı 2007; Çağatay et al. 2015) and the citrus red mite Panonychus citri (Döker and Kazak 2012) was also monitored.
Besides the fragmented toxicity screening studies mentioned above, there is no systematic study that aims to look at the overal susceptibility levels of the acaricides most frequently used in Turkey. In addition, a thorough molecular screening of the many known resistance mutations is still lacking. Furthermore, the efficiency of recently registered acaricides such as bifenazate and cyflumetofen has never been assessed on field-collected T. urticae strains from Turkey.
For this purpose, we investigate in this study the resistance levels for the most frequently used and newly registered acaricides and investigate the presence of well studied target-site resistance mutations. This may well lead to more effective resistance management strategies, based on rational decision making and molecular diagnostics.
Materials and methods
Strains
The susceptible strain German susceptible strain (GSS) is a reference laboratory strain (Stumpf et al. 2001) that was kindly provided by Dr. Ralf Nauen (Bayer Cropscience) and Prof. Dr. Recep Ay. Ten field strains (all red form) were collected from vegetable areas in the Southern part of Turkey during 2016–2017 (Table 1, Fig. 1). At least 1000 individuals were sampled and spider mite populations were subsequently transferred to clean kidney bean plants in order to allow the population to increase for bioassays and DNA extraction. Mites were propagated and maintained in a climatically controlled room at 26 ± 0.5 °C and 60 ± 2% RH with L16:D8 photoperiod. For species identification, the mitochondrial cytochrome oxidase subunit I gene (COI) was used. The partial COI fragment was amplified by PCR using the primers TuCOIF1 and TuCOIR1 and sequenced with the same primers (Supplementary Table 1). All COI sequences obtained in this study were submitted to the NCBI database (accession numbers MK508712-MK508722).
Phylogenetic analysis of COI sequences from 10 Turkish spider mite strains
COI sequences from 10 Turkish spider mite strains and the GSS strain were aligned with a selection of Tetranychidae COI sequences, previously analyzed in Navajas et al. (1998), Navajas and Boursot (2003), de Mendonça et al. (2011) and Matsuda et al. (2013), using MAFFT v7.416 (Katoh et al. 2017) and the ‘Auto’ strategy. A maximum likelihood (ML) phylogenetic analysis was performed with IQ-TREE (Nguyen et al. 2015) using default settings, the TIM + I + F + G4 model (identified to be the best-fit model by ModelFinder; Kalyaanamoorthy et al. 2017) and with 1000 ultrafast bootstraps. The resulting tree was midpoint rooted, optimized using MEGA7 (Kumar et al. 2016) and edited in CorelDRAW Home & Student X7.
Acaricides
Commercial formulations of all acaricides were used. Adult female mites were tested for cyflumetofen (Panula; 200 gL−1 SC), fenbutatin oxide (Acrimite; 550 gL−1 SC), abamectin (Agrimec; 18 gL−1 EC), bifenthrin (Talstar; 100 gL−1 EC) and bifenazate (Floramite; 240 gL−1 SC). The larval stage of mites were used for spiromesifen (Oberon; 240 gL−1 SC), whereas egg bioassays were conducted for hexythiazox (Nissorun; 50 gL−1 EC) (Table 2).
Bioassays
Toxicity bioassays on adult female mites were performed as previously described (Khajehali et al. 2011) with some modifications. Briefly, 20–25 adult female mite were transferred to the upper side of square-shaped kidney bean leaf discs placed on wet cotton, after which the disc with mites was sprayed in a Potter spray tower (Burckard Manufacturing, Rickmansworth, UK) at the rate of 2 mL per leaf disc at 1 bar. For larval and egg bioassays 10–15 adult female were allowed to lay eggs on the leaf disc for 24 h. For egg bioassays, leaf discs were sprayed immediately after adult females were removed whereas for larval bioassays leaf discs were sprayed directly after egg hatching (about 5 days after egg laying). After spraying, treated discs were transferred to a climatically controlled room and kept at 26 ± 0.5 °C and 60 ± 2% RH with L16:D8 photoperiod. Mortality was assessed after 24 h for adult bioassays (except fenbutatin oxide which was counted after 72 h) and after 5 days for egg (total eggs were counted before spraying) and larval bioassays. Mites that could not move when touched with a fine brush under a stereomicroscope were considered dead. Control discs were sprayed with deionized water and the observed mortality was always lower than 10%. The field dose (FD), 5 times the field dose (5FD) and one-fifth of the field dose (FD/5) were applied for all acaricides, as previoulsy described (Khajehali et al. 2011). Four replicates were used per concentration. The mortality rates were corrected using Abbott’s formula (Abbott 1925). Strains were classified as resistant if the observed mortality was lower than 50% at FD, and highly resistant when the observed mortality was lower than 50% at 5FD.
Screening for known mutations
Genomic DNA was extracted from approximately 100–150 adult female mites for each strain with the Qiagen DNeasy Blood & Tissue Kit following the manufacturer’s instructions. DNA extracts were stored at − 20 °C. The resulting DNA solution was used as template for PCR carried out in a TProfessional thermocycler (Biometra, Germany). Primers used for amplifying acaricide target-site regions know to bear resistance mutations and sequencing are listed in Table S1. PCR reactions were performed with Promega GoTaq® Flexi kit in 50 µL containing 3 µL of MgCl, 1 µL of dNTP, 10 µL of 5X Buffer, 2.5 µL of each primer, 0.25 µL Taq DNA polymerase and 2 µL template (between 70 and 130 ng µL−1). PCR was performed under the temperature cycling conditions of: 2 min at 94 °C, 35 cycles of 20 s at 94 °C, 30 s at 54 °C, 30 s at 72 °C, and followed by final extension of 5 min at 72 °C. For cytochrome b (cytb) gene amplification long-PCR (Expand Long Range PCR kit, Roche, Belgium) was used (Van Leeuwen et al. 2008). Full length cytb PCR amplicons were sequenced with four internal primers. All PCR products were purified using the EZNA Cycle-Pure kit (Omega Biotek, USA) according to the manufacturer’s instructions and sequenced at the LGC Sequencing Service (Berlin, Germany). The obtained sequence data were analyzed with BioEdit 7.0.5 software (Hall 1999). The mutations were classified as ‘not detected’, ‘present’ and ‘fixed’ based on visual inspection of sequencing chromatographs (Khajehali et al. 2011).
Results
Phylogenetic analysis of COI sequences from 10 Turkish spider mite strains
A maximum likelihood phylogenetic analysis clustered the COI sequences from the 10 Turkish spider mite strains within the T. urticae COI clade with high bootstrap support, strongly suggesting all spider mite strains are T. urticae strains. As COI sequences are not considered as the ideal marker sequence for distinguishing closely related spider mite species and should be combined with morphological characters (such as the shape of the aedeagus of spider mite males) (Ros and Breeuwer 2007; de Mendonça et al. 2011) a morphological determination should be performed to give a decisive answer with regard to species identification. In line with Navajas et al. (1998) and Kwon et al. (2015a), two lineages (I/group B and II/group A) can be distinguished in the T. urticae clade, with four Turkish strains belonging to lineage I/group B and six to lineage II/group A. Finally, in line with Hinomoto et al. (2001), Navajas and Boursot (2003) and Kwon et al. (2015a), COI sequences from the different color forms of T. urticae (red or green) did not cluster but were present in both lineages (Fig. 2).
Phylogenetic analysis of COI sequences from 10 Turkish spider mite strains. Maximum likelihood phylogenetic analysis of tetranychid COI nucleotide sequences. COI sequences were aligned using MAFFT (Katoh et al. 2017) and a phylogenetic analysis was performed using IQ-TREE (Nguyen et al. 2015). Only bootstrap values higher than or equal to 70% are shown. Tetranychus urticae strains discussed in this study are indicated in bold. Red color forms of T. urticae are indicated with a red circle, whereas green forms are indicated with a green dot. Two lineages can be distinguished within the T. urticae clade (lineage I/group B and lineage II/group A), with all 10 Turkish strains clustering in one of these two lineages
Resistance levels
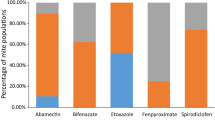
The observed mortality at the diagnostic screening concentrations (FD/5, FD, and 5FD) of assayed acaricides on 10 field strains sampled from important vegetable producing areas of Turkey are listed in Table 3. In total seven acaricides with different mode of action were tested on different developmental stages of mites. The susceptibility levels varied among strains over different products. Resistance to bifenthrin (nine out of 10 strains), abamectin (five out of 10 strains), hexythiazox (five out of 10 strains), fenbutation oxide (four out of 10 strains) was most commonly detected among the tested acaricides (exhibited less than 50% mortality at FD). Strains 1, 2 and 10 were multi-resistant to at least three acaricides. All strains were susceptible to bifenazate and cyflumetofen.
Resistance mutations
Various target-site mutations conferring resistance have been reported for spider mites resistant to acaricides belonging to different mode of action groups, reviewed in Van Leeuwen et al. (2010) and Van Leeuwen and Dermauw (2016). All mutations and resulting amino-acid substitutions are presented in Table 4.
The F1538I mutation in the voltage-gated sodium channel (VGSC), the target-site for pyrethroids, was found in eight out of 10 strains, but not fixed in strains 3, 4 and 6. The L1024V substitution in the VGSC was detected in strains 1 and 6 but was not fixed in both strains. A combination of F1538I + L1024V was found in strain 6, but was not fixed for both mutations. Another substitution, A1215D, was found in all strains except GSS. However, this mutation is no longer considered as a resistance mutation when present without F1538I (Riga et al. 2017). The chitin synthase 1 (CHS1) mutation, I1017F, was present in four out of 10 tested strains, of which in strain 1 and 2 the mutation was fixed while not being fixed in strain 4 and 10. None of the strains harboured known resistance mutations at conserved regions in mitochondrial cytb, the target-site of bifenazate. Mutations in the glutamate-gated chloride channel (GluCl), G314D in GluCl1 and G326E in GluCl3, which confer resistance to abamectin, or the H92R mutation in the PSST homologue of complex I (NADH: ubiquinone oxidoreductase), conferring resistance to Mitochondrial Electron Transport Inhibitors (Bajda et al. 2017), were also not found.
Discussion
There is a high tendency among farmers to use chemicals for spider mite control, due to the fast-acting features of acaricides and the relatively low cost compared to other management methods. However, a combination of favourable climate conditions, allowing multiple spider mite generations per season, and frequent and unconscious acaricide applications cause failure in chemical management of T. urticae populations due to the fast development of resistance. In this study, the efficacy of acaricides with different modes of action was assessed by using three diagnostic concentrations (5/FD, FD and 5FD). Ten spider mite strains were collected from important vegetable producing areas of Turkey. As a reference, a susceptible laboratory strain, GSS (from Germany) was also tested. A phylogenetic analysis (Fig. 2) strongly suggested that all 10 Turkish spider mite strains are T. urticae strains.
Bifenthrin, a synthetic pyrethroid acaricide belonging to IRAC Group 3A, acts on voltage-gated sodium channels and causes repetitive neuronal discharge, membrane depolarization and the neuronal hyperexcitability (Dong et al. 2014; Sparks and Nauen 2015). Bifenthrin has been used in Turkey for more than 30 years and this study reveals the development of resistance as a result of this long-term usage. Almost 15 years ago, up to 600-fold resistance to bifenthrin was reported for a Turkish T. urticae strain (Ay and Gürkan 2005). At that time, bifenthrin resistance was found to be correlated with increased esterase hydrolysis in field collected strains (Ay and Gürkan 2005). Similar mechanisms were put forward for a strain from Belgium (Van Leeuwen et al. 2005; Van Leeuwen and Tirry 2007). In this recent re-assessment of bifenthrin resistance, all strains except strain 4, were found to be resistant to bifenthrin and half of the strains exhibited very high resistance levels (almost complete survival at 5FD). Various mutations in the VGSC have been described that confer pyrethroid resistance (Dong et al. 2014; Feyereisen et al. 2015). Among these mutations, the F1538I mutation in domain III segment 6 was found to be associated with high resistance levels to bifenthrin in spider mites (Davies et al. 2008; Tsagkarakou et al. 2009), was studied by molecular modeling (O’Reilly et al. 2006), and has been reported in many T. urticae strains worldwide (Khajehali et al. 2011; Ilias et al. 2014; Kwon et al. 2015b; Xu et al. 2018). The role of another mutation, A1215D, located in the intracellular linker between domains II and III, in pyrethroid resistance is not clear (Tsagkarakou et al. 2009; Khajehali et al. 2011). It has been suggested that the A1215D mutation might have a synergistic effect when it occurs in combination with other VGSC mutations (Van Leeuwen et al. 2010), but the mutation alone does not confer resistance (Riga et al. 2017). In addition, the L1024V mutation which was reported to cause knockdown resistance to fenpropathrin (Kwon et al. 2010a), was also screened in the present study. The A1215D mutation was found in all strains except GSS. All strains highly resistant to bifenthrin harboured the F1538I mutation (all being fixed except for strain 6), indicating that target-site resistance is probably a major resistance mechanism against bifenthrin. Especially because introgression of this mutation in a susceptible background conveyed a strong bifenthrin resistance phenotype (Riga et al. 2017). Of particular note, the highly resistant strain 6 was the only strain that contained both the F1538I + L1024V mutation as assessed on DNA of pooled mites. Whether both mutations occur in a single vgsc copy (haplotype), or the population consists of individuals with each of the mutations being present in a separate haplotype, remains to be tested. Both mutations give a very strong resistant phenotype when introgressed into a susceptible background (Riga et al. 2017), and thus could co-occur in populations under selection pressure, especially since fitness costs were not discovered (Bajda et al. 2018). Of particular note, it is the first time that the F1538I and L1024V mutations have been reported for Turkish T. urticae populations.
Abamectin is derived from the fermentation of Streptomyces avermitilis and it belongs to the avermectin subfamily of macrocyclic lactones (Fisher and Mrozik 1989). The mode of action of abamectin is the activation of glutamate-gated chloride channels (IRAC Group 6) (Lynagh and Lynch 2012) which is essential for hyperpolarization of a neuron or muscle (Wolstenholme 2012). Abamectin is one of the most popular acaricides in vegetable areas of Turkey, and resistance was previously documented (Çağatay et al. 2018). Based on Table 3, we can conclude that the registered FD does not control spider mites efficiently in the studied areas. Resistance to abamectin has been associated with cytochrome P450 mono-oxygenase mediated metabolism and mutations in the glutamate-gated chloride channel (GluCl), G314D in GluCl1 and G326E in GluCl3 (Stumpf and Nauen 2002; Kwon et al. 2010b; Dermauw et al. 2012; Riga et al. 2014; Mermans et al. 2017). Five strains were resistant to abamectin and mortality rates at FD were lower than 25% for these strains. However, none of the Turkish strains harbored any of the reported abamectin resistance mutations. Similarly, previous studies did also not detect these mutations in Turkish populations (Ilias et al. 2014; Çağatay et al. 2018), despite the high resistance levels to abamectin (Çağatay et al. 2018). Abamectin resistance mutations were not frequently observed in various strains collected from 27 countries (Ilias et al. 2014) and mutations were also not found in 26 strains from Washington, USA (Piraneo et al. 2015), whereas it has been reported as more common in seven strains from China (Xu et al. 2018). Although the presence of G326E mutation alone does not impose significant fitness costs, co-occurring G314D + G326E mutations were reported to cause consistent changes in life parameters of T. urticae which also might contribute to low frequency of GluCl mutations (Bajda et al. 2018). However, a recent study showed that resistance mutations (individually or a combination of both mutations) to abamectin do not confer high level of resistance on their own (Riga et al. 2017). Contribution of resistance mechanisms other than target-site mutations seems also to play major role in abamectin resistance as previously reported (Stumpf and Nauen 2002; Khajehali et al. 2011; Riga et al. 2014; Çağatay et al. 2018).
Other acaricides with a long history of use in Turkey are hexythiazox and fenbutatin oxide. Hexythiazox belongs to the mite growth inhibitors class and, similar to abamectin, was registered almost 30 years ago. Mite growth inhibitors (IRAC group 10) are effective against immature stages of mites and important tools for resistance management programs as they are considered as safe for beneficial insects and predatory mites as well as vertebrates (Aveyard et al. 1986; Douris et al. 2016). Although IRAC group 10 acaricides belong to different chemical classes, they have chitin synthase 1 as a common target site (Demaeght et al. 2014). An aminoacid substitution, I1017F, in the C-terminal transmembrane domain of CHS1 has been linked with mite growth inhibitor acaricide resistance (Van Leeuwen et al. 2012; Demaeght et al. 2014). While four strains were considered resistant, one strain (strain 1) showed exceptionally high resistance against hexythiazox. Interestingly, none of the field-collected populations had 100% mortality even at 5FD. The I1017F mutation was detected in four out of 10 strains. Strains with the I1017F mutation fixed also had the most resistant phenotypes. The CHS1 mutation has been previoulsy reported in only one strain from Turkey (Ilias et al. 2014), but without matching toxicity data. Although significant fitness cost has been reported in the presence of the I1017F mutation (Bajda et al. 2018), the mutation has been reported in T. urticae strains from different continents (Demaeght et al. 2014; Ilias et al. 2014; Osakabe et al. 2017; Adesanya et al. 2018; Herron et al. 2018). With regard to resistance management, our data suggest that more modern CHS1 inhibitors, like etoxazole that is in use throughout Europe, are not a good option to control T. urticae population in Turkey.
Fenbutatin oxide is a relatively slow-acting acaricide that targets mitochondrial ATPase and has been frequently used historically. Data on resistance screening to fenbutatin oxide is extremely limited for spider mite strains in Turkey. Although fenbutatin oxide is not registered in vegetables, four strains were resistant to fenbutatin oxide. Döker and Kazak (2012) reported 200-fold resistance to fenbutatin oxide in a field collected Panonychus citri strain from Turkey. A strain (MR-VL) collected from a greenhouse in Belgium showed high resistance levels to fenbutatin oxide (> 500-fold resistance) (Van Leeuwen et al. 2005), but at present very little is known about resistance mechanisms at play.
Cyflumetofen and bifenazate belong to the class of mitochondrial electron transport inhibitors (METIs), acting on Complex II (IRAC Group 25) and Complex III (IRAC Group 20) of the mitochondrial electron transport chain, respectively. They are relatively recently registered acaricides. Cyflumetofen was registered in Turkey only 2 years ago and, together with cyenopyrafen, is the first commercial acaricide that targets Complex II. A potential cross-resistance risk between cyenopyrafen and cyflumetofen has been reported previously (Khalighi et al. 2014, 2016). Hence, although cyenopyrafen is not registered in Turkey, selection with cyflumetofen could cause cross-resistance in the future. Although most of the populations were very susceptible to cyflumetofen, strain 3 had only 80% mortality at FD which could reflect the onset of decreased susceptibility in Turkish spider mite populations.
Although bifenazate was registered and used earlier than cyflumetofen, mortality rates were higher and it was the most effective acaricide tested against all strains. Even at FD/5 dose of bifenazate caused 100% mortality in almost every strain. This might reflect the rare use of bifenazate in vegetable production areas in southern Turkey as it is relatively expensive comparing to other registered acaricides. Not surprisingly, none of the reported resistance mutations in the Qo pocket of cytb gene (Van Leeuwen et al. 2008, 2011; Van Nieuwenhuyse et al. 2009) were detected.
Another group of acaricides targeting mitochondrial respiration are the METI-Is (mitochondrial electron transport inhibitors of complex I), with tebufenpyrad, fenpyroximate and pyridaben as frequently used compounds. Recently, a H92R mutation in the PSST subunit of complex I has been reported to be associated with resistance (Bajda et al. 2017). However, we did not find this mutation in any of the field-collected strains.
Spiromesifen is an insecticide/acaricide that belongs to spirocyclic tetronic and tetramic acid derivatives (IRAC group 23) which cause lipid biosynthesis reduction thorough inhibition of acetyl-CoA carboxylase (ACCase), similar to spirodiclofen (Bretschneider et al. 2007; Lümmen et al. 2014). Two out of 10 strains (6 and 10) showed < 60% mortality at FD to spiromesifen. However, resistance mutations in the carboxyltransferase domain of spider mite ACCase, the target of spiromesifen or any tetronic/tetramic acid derivative (Lümmen et al. 2014), have so far not been reported. Tetranychus urticae strains collected from melon and strawberry plants from Southern Turkey showed 20- and 10-fold resistance, respectively (Turan et al. 2016; Yalçın et al. 2018). Higher levels of resistance to spirodiclofen, another tetronic acid derivative registered for mite control, have been attributed to cytochrome P450 mono-oxygenase hydroxylation (Demaeght et al. 2013).
Finally, three out of 10 populations were multiresistant to abamectin, bifenthrin, fenbutation oxide and hexythiazox. Resistance to bifenthrin was the most widespread for spider mite populations collected from vegetables. Similar to the MR-VL strain in Van Leeuwen et al. (2005), multi-resistant strains in this study showed resistance against both bifenthrin and fenbutatin oxide, probably reflecting their long-term use and period of selection on spider mite populations.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. https://doi.org/10.1093/jee/18.2.265a
Adesanya AW, Morales MA, Walsh DB, Lavine LC, Lavine MD, Zhu F (2018) Mechanisms of resistance to three mite growth inhibitors of Tetranychus urticae in hops. Bull Entomol Res 108(1):23–34. https://doi.org/10.1017/S0007485317000414
Aveyard CS, Peregrine DJ, Bryan KMG (1986) Biological activity of clofentezine against egg and motile stages of tetranychid mites. Exp Appl Acarol 2:223–229. https://doi.org/10.1007/BF01193954
Ay R (2005) Determination of susceptibility and resistance of some greenhouse populations of Tetranychus urticae Koch to chlorpyrifos (Dursban 4) by the petri dish–Potter tower method. J Pest Sci 78(3):139–143. https://doi.org/10.1007/s10340-005-0084-7
Ay R, Gürkan MO (2005) Resistance to bifenthrin and resistance mechanisms of different strains of the two-spotted spider mite (Tetranychus urticae) from Turkey. Phytoparasitica 33(3):237–244. https://doi.org/10.1007/BF02979860
Ay R, Sökeli E, Karaca I, Gürkan MO (2005) Response to some acaricides of the two-spotted spider mite (Tetranychus urticae Koch) from protected vegetables in Isparta. Turk J Agric For 29(3):165–171
Bajda S, Dermauw W, Panteleri R, Sugimoto N, Douris V, Tirry L, Osakabe M, Vontas J, Van Leeuwen T (2017) A mutation in the PSST homologue of complex I (NADH: ubiquinone oxidoreductase) from Tetranychus urticae is associated with resistance to METI acaricides. Insect Biochem Mol Biol 80:79–90. https://doi.org/10.1016/j.ibmb.2016.11.010
Bajda S, Riga M, Wybouw N, Papadaki S, Ouranou E, Fotoukkiaii SM, Vontas J, Van Leeuwen T (2018) Fitness costs of key point mutations that underlie acaricide target-site resistance in the two-spotted spider mite Tetranychus urticae. Evol Appl 11(9):1540–1553. https://doi.org/10.1111/eva.12643
Bretschneider T, Fischer R, Nauen R (2007) Inhibitors of lipid synthesis (acetyl-CoAcarboxylase inhibitors). In: Krämer W, Schirmer U (eds) Modern crop protection compounds. Wiley, Weinheim, pp 909–925
Çağatay NS, Salman SY, Yaman Y, Ay R (2015) Abamectin, chlorpyrifos ethyl and bifenthrin resistance in Panonychus ulmi Koch. (Acari:Tetranychidae) populations collected from apple orchards in Isparta. Turk Bullet Entomol 4(4):203–209. https://doi.org/10.16969/teb.22076
Çağatay NS, Menault P, Riga M, Vontas J, Ay R (2018) Identification and characterization of abamectin resistance in Tetranychus urticae Koch populations from greenhouses in Turkey. Crop Prot 112:112–117. https://doi.org/10.1016/j.cropro.2018.05.016
Davies TE, O’Reilly AO, Field LM, Wallace BA, Williamson MS (2008) Knockdown resistance to DDT and pyrethroids: from target-site mutations to molecular modelling. Pest Manag Sci 64(11):1126–1130. https://doi.org/10.1002/ps.1617
de Mendonça RS, Navia D, Diniz IR, Auger P, Navajas M (2011) A critical review on some closely related species of Tetranychus sensu stricto (Acari: Tetranychidae) in the public DNA sequences databases. Exp Appl Acarol 55(1):1–23. https://doi.org/10.1007/s10493-011-9453-5
Demaeght P, Dermauw W, Tsakireli D, Khajehali J, Nauen R, Tirry L, Vontas J, Lümmen P, Van Leeuwen T (2013) Molecular analysis of resistance to acaricidal spirocyclic tetronic acids in Tetranychus urticae: CYP392E10 metabolizes spirodiclofen, but not its corresponding enol. Insect Biochem Mol Biol 43(6):544–554. https://doi.org/10.1016/j.ibmb.2013.03.007
Demaeght P, Osborne EJ, Odman-Naresh J, Grbić M, Nauen R, Merzendorfer H, Clark RM, Van Leeuwen T (2014) High resolution genetic mapping uncovers chitin synthase-1 as the target-site of the structurally diverse mite growth inhibitors clofentezine, hexythiazox and etoxazole in Tetranychus urticae. Insect Biochem Mol Biol 51:52–61. https://doi.org/10.1016/j.ibmb.2014.05.004
Dermauw W, Ilias A, Riga M, Tsagkarakou A, Grbić M, Tirry L, Van Leeuwen T, Vontas J (2012) The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem Mol Biol 42(7):455–465. https://doi.org/10.1016/j.ibmb.2012.03.002
Dermauw W, Wybouw N, Rombauts S, Menten B, Vontas J, Grbic M, Clark RM, Feyereisen R, Van Leeuwen T (2013) A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc Natl Acad Sci USA 110:E113–E122. https://doi.org/10.1073/pnas.1213214110
Dermauw W, Pym A, Bass C, Van Leeuwen T, Feyereisen R (2018) Does host plant adaptation lead to pesticide resistance in generalist herbivores? Curr Opin Insect Sci 26:25–33. https://doi.org/10.1016/j.cois.2018.01.001
Döker İ, Kazak C (2012) Detecting acaricide resistance in Turkish populations of Panonychus citri McGregor (Acari: Tetranychidae). Syst Appl Acarol 17(4):368–377. https://doi.org/10.11158/saa.17.4.4
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS (2014) Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol 50:1–17. https://doi.org/10.1016/j.ibmb.2014.03.012
Douris V, Steinbach D, Panteleri R, Livadaras I, Pickett JA, Van Leeuwen T, Nauen R, Vontas J (2016) Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis. Proc Natl Acad Sci USA 113(51):14692–14697. https://doi.org/10.1073/pnas.1618258113
Feyereisen R, Dermauw W, Van Leeuwen T (2015) Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol 121:61–77. https://doi.org/10.1016/j.pestbp.2015.01.004
Fisher MH, Mrozik H (1989) Chemistry in ivermectin and abamectin. In: Campbell WC (ed) ivermectin and abamectin. Springer, New York, p 1e23
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hayashi N, Sasama Y, Takahashi N, Ikemi N (2013) Cyflumetofen, a novel acaricide–its mode of action and selectivity. Pest Manag Sci 69(9):1080–1084. https://doi.org/10.1002/ps.3470
Herron GA, Woolley LK, Langfield KL, Chen Y (2018) First detection of etoxazole resistance in Australian two-spotted mite Tetranychus urticae Koch (Acarina: Tetranychidae) via bioassay and DNA methods. Austral Entomol 57(3):365–368. https://doi.org/10.1111/aen.12290
Hinomoto N, Osakabe M, Gotoh T, Takafuji A (2001) Phylogenetic analysis of green and red forms of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), in Japan, based on mitochondrial cytochrome oxidase subunit I sequences. Appl Entomol Zool 36(4):459–464. https://doi.org/10.1303/aez.2001.459
Ilias A, Vontas J, Tsagkarakou A (2014) Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem Mol Biol 48:17–28. https://doi.org/10.1016/j.ibmb.2014.02.006
Kalyaanamoorthy S, Minh BQ, Wong TK, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14(6):587. https://doi.org/10.1038/nmeth.4285
Katoh K, Rozewicki J, Yamada KD (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. https://doi.org/10.1093/bib/bbx108
Khajehali J, Van Nieuwenhuyse P, Demaeght P, Tirry L, Van Leeuwen T (2011) Acaricide resistance and resistance mechanisms in Tetranychus urticae populations from rose greenhouses in the Netherlands. Pest Manag Sci 67(11):1424–1433. https://doi.org/10.1002/ps.2191
Khalighi M, Tirry L, Van Leeuwen T (2014) Cross-resistance risk of the novel complex II inhibitors cyenopyrafen and cyflumetofen in resistant strains of the two-spotted spider mite Tetranychus urticae. Pest Manag Sci 70(3):365–368. https://doi.org/10.1002/ps.3641
Khalighi M, Dermauw W, Wybouw N, Bajda S, Osakabe M, Tirry L, Van Leeuwen T (2016) Molecular analysis of cyenopyrafen resistance in the two-spotted spider mite Tetranychus urticae. Pest Manag Sci 72(1):103–112. https://doi.org/10.1002/ps.4071
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 7:1870–1874. https://doi.org/10.1093/molbev/msw054
Kumral NA, Kovanci B (2007) Susceptibility of female populations of Panonychus ulmi (Koch) (Acari: Tetranychidae) to some acaricides in apple orchards. J Pest Sci 80(3):131–137. https://doi.org/10.1007/s10340-007-0163-z
Kwon DH, Clark JM, Lee SH (2010a) Cloning of a sodium channel gene and identification of mutations putatively associated with fenpropathrin resistance in Tetranychus urticae. Pestic Biochem Physiol 97(2):93–100. https://doi.org/10.1016/j.pestbp.2009.07.009
Kwon DH, Yoon KS, Clark JM, Lee SH (2010b) A point mutation in a glutamate-gated chloride channel confers abamectin resistance in the two-spotted spidermite, Tetranychus urticae Koch. Insect Mol Biol 19:583–591. https://doi.org/10.1111/j.1365-2583.2010.01017.x
Kwon DH, Kang TJ, Kim YH, Lee SH (2015a) Phenotypic-and genotypic-resistance detection for adaptive resistance management in Tetranychus urticae Koch. PLoS ONE 10(11):e0139934. https://doi.org/10.1371/journal.pone.0139934
Kwon DH, Clark JM, Lee SH (2015b) Toxicodynamic mechanisms and monitoring of acaricide resistance in the two-spotted spider mite. Pestic Biochem Physiol 121:97–101. https://doi.org/10.1016/j.pestbp.2014.12.011
Lümmen P, Khajehali J, Luther K, Van Leeuwen T (2014) The cyclic keto-enol insecticide spirotetramat inhibits insect and spider mite acetyl-CoA carboxylases by interfering with the carboxyltransferase partial reaction. Insect Biochem Mol Biol 55:1–8. https://doi.org/10.1016/j.ibmb.2014.09.010
Lynagh T, Lynch JW (2012) Ivermectin binding sites in human and invertebrate Cys-loop receptors. Trends Pharmacol Sci 33(8):432–441. https://doi.org/10.1016/j.tips.2012.05.002
Matsuda T, Fukumoto C, Hinomoto N, Gotoh T (2013) DNA-based identification of spider mites: molecular evidence for cryptic species of the genus Tetranychus (Acari: Tetranychidae). J Econ Entomol 106(1):463–472. https://doi.org/10.1603/EC12328
Mermans C, Dermauw W, Geibel S, Van Leeuwen T (2017) A G326E substitution in the glutamate-gated chloride channel 3 (GluCl3) of the two-spotted spider mite Tetranychus urticae abolishes the agonistic activity of macrocyclic lactones. Pest Manag Sci 73(12):2413–2418. https://doi.org/10.1002/ps.4677
Navajas M, Boursot P (2003) Nuclear ribosomal DNA monophyly versus mitochondrial DNA polyphyly in two closely related mite species: the influence of life history and molecular drive. Proc R Soc Lond B 270:124–127. https://doi.org/10.1098/rsbl.2003.0034
Navajas M, Lagnel J, Gutierrez J, Boursot P (1998) Species-wide homogeneity of nuclear ribosomal ITS2 sequences in the spider mite Tetranychus urticae contrasts with extensive mitochondrial COI polymorphism. Heredity 80(6):742–752. https://doi.org/10.1046/j.1365-2540.1998.00349.x
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274. https://doi.org/10.1093/molbev/msu300
O’Reilly AO, Khambay BP, Williamson MS, Field LM, Wallace BA, Davies TE (2006) Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem J 396(2):255–263. https://doi.org/10.1042/BJ20051925
Osakabe M, Imamura T, Nakano R, Kamikawa S, Tadatsu M, Kunimoto Y, Doi M (2017) Combination of restriction endonuclease digestion with the ΔΔCt method in real-time PCR to monitor etoxazole resistance allele frequency in the two-spotted spider mite. Pestic Biochem Physiol 139:1–8. https://doi.org/10.1016/j.pestbp.2017.04.003
Piraneo TG, Bull J, Morales MA, Lavine LC, Walsh DB, Zhu F (2015) Molecular mechanisms of Tetranychus urticae chemical adaptation in hop fields. Sci Rep 5:17090. https://doi.org/10.1038/srep17090
Riga M, Tsakireli D, Ilias A, Morou E, Myridakis A, Stephanou EG, Nauen R, Dermauw W, Van Leeuwen T, Paine M, Vontas J (2014) Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem Mol Biol 46:43–53. https://doi.org/10.1016/j.ibmb.2014.01.006
Riga M, Bajda S, Themistokleous C, Papadaki S, Palzewicz M, Dermauw W, Vontas J, Van Leeuwen T (2017) The relative contribution of target-site mutations in complex acaricide resistant phenotypes as assessed by marker assisted backcrossing in Tetranychus urticae. Sci Rep 7(1):9202. https://doi.org/10.1038/s41598-017-09054-y
Ros VI, Breeuwer JA (2007) Spider mite (Acari: Tetranychidae) mitochondrial COI phylogeny reviewed: host plant relationships, phylogeography, reproductive parasites and barcoding. Exp Appl Acarol 42(4):239–262. https://doi.org/10.1007/s10493-007-9092-z
Sökeli E, Ay R, Karaca İ (2007) Determination of the resistance level of two-spotted spider mite (Tetranychus urticae Koch) populations in apple orchards in Isparta province against some pesticides. J Agric Sci 13(4):326–330
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128. https://doi.org/10.1016/j.pestbp.2014.11.014
Stumpf N, Nauen R (2002) Biochemical markers linked to abamectin resistance in Tetranychus urticae (Acari: Tetraychidae). Pestic Biochem Physiol 72:111–121. https://doi.org/10.1006/pest.2001.2583
Stumpf N, Zebitz CP, Kraus W, Moores GD, Nauen R (2001) Resistance to organophosphates and biochemical genotyping of acetylcholinesterases in Tetranychus urticae (Acari: Tetranychidae). Pestic Biochem Physiol 69(2):131–142. https://doi.org/10.1006/pest.2000.2516
Tsagkarakou A, Van Leeuwen T, Khajehali J, Ilias A, Grispou M, Williamson MS, Tirry L, Vontas J (2009) Identification of pyrethroid resistance associated mutations in the para sodium channel of the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Insect Mol Biol 18(5):583–593. https://doi.org/10.1111/j.1365-2583.2009.00900.x
Turan İ, Yorulmaz Salman S, Ay R (2016) Resistance levels against Abamectin and Spirodiclofen of populations of Tetranychus Urticae Koch (Acari:Tetranychidae) collected from melons greenhouses in Kumluca District of Antalya Province. Mehmet Akif Ersoy Üniversitesi Fen Bilimleri Enstitüsü Dergisi 7(1):254–261
Turkish Statistical Institue, 2018. http://www.tuik.gov.tr/. Accessed 12 Nov 2018
Van Leeuwen T, Dermauw W (2016) The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu Rev Entomol 61:475–498. https://doi.org/10.1146/annurev-ento-010715-023907
Van Leeuwen T, Tirry L (2007) Esterase-mediated bifenthrin resistance in a multiresistant strain of the two-spotted spider mite Tetranychus urticae. Pest Manag Sci 63(2):150–156. https://doi.org/10.1002/ps.1314
Van Leeuwen T, Van Pottelberge S, Tirry L (2005) Comparative acaricide susceptibility and detoxifying enzyme activities in field-collected resistant and susceptible strains of Tetranychus urticae. Pest Manag Sci 61(5):499–507. https://doi.org/10.1002/ps.1001
Van Leeuwen T, Vanholme B, Van Pottelberge S, Van Nieuwenhuyse P, Nauen R, Tirry L (2008) Mitochondrial heteroplasmy and the evolution of insecticide resistance: non-Mendelian inheritance in action. Proc Natl Acad Sci USA 105:5980–5985. https://doi.org/10.1073/pnas.0802224105
Van Leeuwen T, Vontas J, Tsagkarakou A, Tirry L (2009) Mechanisms of acaricide resistance in the two-spotted spider mite Tetranychus urticae. In: Ishaaya I, Horowitz AR (eds) Biorational control of arthropod pests. Springer, Amsterdam, pp 347–393
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40(8):563–572. https://doi.org/10.1016/j.ibmb.2010.05.008
Van Leeuwen T, Van Nieuwenhuyse P, Vanholme B, Dermauw W, Nauen R, Tirry L (2011) Parallel evolution of cytochrome b mediated bifenazate resistance in the citrus red mite Panonychus citri. Insect Mol Biol 20:135–140. https://doi.org/10.1111/j.1365-2583.2010.01040.x
Van Leeuwen T, Demaeght P, Osborne EJ, Dermauw W, Gohlke S, Nauen R, Grbic M, Tirry L, Merzendorfer H, Clark RM (2012) Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc Natl Acad Sci USA 109(12):4407–4412. https://doi.org/10.1073/pnas.1200068109
Van Leeuwen T, Tirry L, Yamamoto A, Nauen R, Dermauw W (2015) The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic Biochem Physiol 121:12–21. https://doi.org/10.1016/j.pestbp.2014.12.009
Van Nieuwenhuyse P, Van Leeuwen T, Khajehali J, Vanholme B, Tirry L (2009) Mutations in the mitochondrial cytochrome b of Tetranychus urticae Koch (Acari: Tetranychidae) confer cross-resistance between bifenazate and acequinocyl. Pest Manag Sci 65:404–412. https://doi.org/10.1002/ps.1705
Wolstenholme AJ (2012) Glutamate-gated chloride channels. J Biol Chem 287(48):40232–40238. https://doi.org/10.1074/jbc.R112.406280
Xu D, He Y, Zhang Y, Xie W, Wu Q, Wang S (2018) Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic Biochem Physiol 150:89–96. https://doi.org/10.1016/j.pestbp.2018.07.008
Yalçin K, Döker İ, Kazak C (2018) Acaricide resistance in Tetranychus urticae red form (Acari: Tetranychidae) collected from strawberry in southern Turkey: bioassay and biochemical studies. Syst Appl Acarol 23(12):2279–2287. https://doi.org/10.11158/saa.23.12.1
Acknowledgements
We would like to thank to Dr. Ralf Nauen (Bayer CropScience) and Prof. Dr. Recep Ay (Isparta University of Applied Sciences) for providing GSS as a reference strain. This work was supported by the Research Foundation Flanders (FWO) [Grants G009312 N and G053815 N], the Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program under Grant Agreement No 772026 (Polyadapt) and Grant Agreement No 773902 (SuperPests). WD is a post-doctoral fellow of the Research Foundation Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
İnak, E., Alpkent, Y.N., Çobanoğlu, S. et al. Resistance incidence and presence of resistance mutations in populations of Tetranychus urticae from vegetable crops in Turkey. Exp Appl Acarol 78, 343–360 (2019). https://doi.org/10.1007/s10493-019-00398-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-019-00398-w