Abstract
The intensive use of pesticides is a common practice for the management of the two-spotted spider mite, Tetranychus urticae, in greenhouses and field farms of Ethiopia. However, incidence of resistance and possible resistance mechanisms in T. urticae populations from Ethiopia have not yet been studied. Here, we assessed the toxicity of various acaricides—bifenazate, abamectin, emamectin benzoate, profenofos, fenbutatin oxide, fenpyroximate, amitraz and chlorfenapyr—on T. urticae populations sampled from six flower greenhouse farms, three strawberry greenhouse farms, one field-grown vegetable farm and two wild populations. In parallel, all populations were screened for known target-site mutations. All tested populations were fully susceptible to bifenazate, abamectin, emamectin benzoate and profenofos, but resistant against fenbutatin oxide and fenpyroximate. Four populations showed considerable levels of resistance against amitraz and one population was resistant to chlorfenapyr. Several target-site mutations were identified in the tested populations, including G119S, A201S, T280A, G328A and F331W/C/Y in acetylcholinesterase and the F1538I and L1024V mutation in the voltage-gated sodium channel. The F1538I mutation was found in eight out of 12 populations, whereas the L1024V mutation was only found in two populations. The H92R mutation in the PSST subunit of complex I and the I1017F mutation in chitin synthase 1 was detected in half of the tested populations. The G326E and I321T mutations in the glutamate-gated chloride channel 3 were also detected, but more rarely, whereas mitochondrial cytochrome b mutations were not detected. The current study revealed multiple resistance patterns in Ethiopian T. urticae populations and together with the wide presence of target-site mutations, calls for the wise use of acaricides in the management of T. urticae in Ethiopia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite (TSSM), Tetranychus urticae Koch (Acari: Tetranychidae), is a cosmopolitan phytophagous pest and among the economically most important pests in a wide range of outdoor and protected crops (Ilias et al. 2014; Van Leeuwen et al. 2010). Its host range exceeds 1000 plant species (Migeon et al. 2010) and the species causes significant damage to ornamentals and greenhouse and outdoor vegetables (Jeppson et al. 1975; Kasap 2005; Regev and Cone 1976; Zhang 2003). Tetranychus urticae is particularly dominant in intensive, high-yield cropping systems, and affects crops by direct feeding. In severe infestations, it reduces the area of photosynthetic activity and causes leaf abscission (Gorman et al. 2002). Control of T. urticae is largely based on the use of different acaricides—this is also the case in Ethiopia—and several compounds with a different mode of action are available (Dekeyser 2005; Van Leeuwen et al. 2010). Because of its high reproductive potential, very short life cycle and arrhenotokous parthenogenesis, TSSM is often found difficult to manage in many countries and develops resistance very rapidly (Luczynski et al. 1990; Van Leeuwen et al. 2010).
Resistance to pesticides can evolve in arthropods in various ways. The amount of pesticide that reaches the target site can be decreased by metabolism or transport (metabolic resistance), often mediated by gene families such as cytochrome P450-monooxoygenases (P450s), glutathione S-transferases (GSTs) and carboxyl/choline esterases (CCEs) (Demaeght et al. 2013; Khalighi et al. 2016; Van Leeuwen and Dermauw 2016; Van Leeuwen et al. 2010; Wei et al. 2019). Alternatively, resistance can also develop by mutations in the target site, that alter the binding kinetics of pesticides (target-site resistance). In TSSM, various mutations have been associated with resistance, including point mutations in the voltage-gated sodium channel (VGSC), glutamate-gated chloride channel 1 and 3 (GluCl1 and GluCl3), acetylcholinesterase (AChE), chitin synthase 1 (CHS1) and mitochondrial cytochrome b (cytb) (Feyereisen et al. 2015; Van Leeuwen et al. 2010, 2020).
In Ethiopia, very limited studies have documented TSSM control failures (Abate 1987; Ayalew et al. 2006; Goftishu et al. 2016) and only few studies investigated the efficacy of available pesticides (Belay et al. 2018; Ebrahim and Wakgari 2019; Geleto et al. 2015; Negash et al. 2014). Moreover, except for Geleto et al. (2015), who sampled mites from tomato farms, the efficacy of acaricides was mainly tested on mites collected from wild plants.
In Ethiopia, greenhouse flower (mainly roses) and fruit (strawberry) farms are economically important, and the wide and intensive use of pesticides is a common practice in the control of TSSM. More than 10 years ago, the magnitude of the problems with spider mites and pesticides resistance in Ethiopian greenhouse farms was already indicated (Elings et al. 2009), but the incidence of resistance to various pesticides and their possible resistance mechanisms have not yet been studied. In the present study, the efficacy of eight commercially important acaricides was investigated for 12 Ethiopian T. urticae populations collected from different areas and farms and one susceptible reference population from Germany. The pesticides were selected based on mode of action and use in Ethiopia, including compounds that have been used for decades, as well as more recently introduced molecules. Therefore, the current study is designed to assess resistance status of TSSM and investigate the presence of known mutations associated with acaricide resistance. The toxicity data and distribution and frequency of target-site resistance mutations will contribute to develop effective resistance management strategies for the control of TSSM in Ethiopia.
Materials and methods
Mites
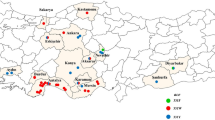
Populations were obtained from greenhouse and open field farms residing in close vicinity to Addis Ababa (Ziway [Batu], Koka, Ejersa, Bishoftu, Sebeta, Menagesha and Holeta) (Fig. 1; Table 1). Mites were also sampled from wild ornamental plants in Jimma and Bishoftu area. A reference susceptible strain from the Laboratory of Agrozoology, Ghent University, Belgium, was collected in 2019 from hop in Germany. The collected mite samples were transported to Jimma University, Biology Department, Plant Biotechnology Laboratory, and maintained on young haricot bean plants (Phaseolus vulgaris) in a climate-controlled room at 25 ± 2 °C, 70 ± 5% RH and L12:D12 photoperiod.
Acaricides
Commercial formulations of acaricides were used: bifenazate, fenbutatin oxide, abamectin, emamectin benzoate, amitraz, chlorfenapyr, profenofos and fenpyroximate (Supporting information, Table S1), and were purchased from local retailers and distributors in Addis Ababa and Adama town, or by FytoVanhulle (Belgium).
Bioassays
A leaf dip bioassay on adult female mites was performed for all tested pesticides, following the standard protocol (IRAC 2009). Specific diagnostic doses based on the field dose (FD, see Table S1) of each compound (FD/5, FD/2, FD, 2FD and 5FD) and a water control were prepared. Square bean leaf discs of about 9 cm2 were introduced into the prepared pesticide solution for 5 s and allowed to dry for 45–50 min. Similarly, bean leaf discs were introduced in sterile water for the control treatment. Each leaf disc was placed on moist cotton wool inside Petri dishes. The disc edges were covered with wetted tissue paper strips to prevent mites from escaping. Then, 10 female adults were placed on the upper side of the leaf discs. Every assay was replicated five times per concentration. Water was regularly added to the Petri dishes to keep the leaves green and turgid, and to prevent mite escape. Mite mortality was recorded after 48 h. Mites that were not able to respond or could not move when prodded with a fine brush were considered dead. Abbott’s formula (Abbott 1925) was used to correct mortality data. Control mortality never exceeded 5%. Populations were classified as ‘resistant’ or ‘highly resistant’ to a specific compound when mortality was lower than 50% at the field dose (FD) or 5× the FD (5FD), respectively.
Detection of resistance-associated mutations
Genomic DNA was extracted from approximately 100 pooled adult female mites per population. The Gentra Puregene Tissue Kit (Qiagen, Belgium) was used for the extraction of genomic DNA from mites preserved with 95% ethanol, following the manufacturer’s instructions. The resulting DNA solution was used as a template for subsequent PCR in a T-professional thermocycler (Biometra, Germany). Primers used for amplifying target-site regions known to carry resistance mutations are listed in Table 2, including references.We aimed to detect currently known and partially validated resistance mutations in spider mites from Ethiopian populations. The two primer pairs that were designed in this study were based on the AChE (tetur19g08500) and vgsc (tetur34g00970) sequence of the T. urticae London strain (Grbić et al. 2011, available at https://bioinformatics.psb.ugent.be/orcae/annotation/Tetur). The AChE primer set was designed to amplify a 965 bp region of the first exon of T. urticae AChE, comprising all AChE mutations that were previoulsy reported to be involved in OP resistance (Khajehali et al. 2010). The vgsc primer set was designed to amplify a 420 bp region of a vgsc exon carrying the super-kdr mutation (M918T, or a variant thereof, M918L), which was recently identified in pyrethroid resistant populations of T. urticae (Wu et al. 2019).
PCR reactions were performed with Promega GoTaq Flexi kit in 50 µL containing 3 µL of MgCl, 1 µL of dNTP, 10 µL of 5X Buffer, 2.5 µL of each primer (10 µM), 0.25 µL Taq DNA polymerase and 1 µL template DNA. PCR was performed as described in Inak et al. (2019) with minor modifications, under the temperature cycling conditions of 2 min at 95 °C, 40 cycles of 30 s at 95 °C, 30 s at 55 °C, 30 s at 72 °C, and followed by a final extension of 5 min at 72 °C. For complete cytb amplification, long range PCR (Expand Long Range dNTPack, Roche, Belgium) was used (Van Leeuwen et al. 2008). Full length cytb PCR amplicons were sequenced with four internal primers. All PCR products were purified using the EZNA Cycle-Pure kit (Omega Biotek, USA) according to the manufacturer’s instructions. The purified PCR product was then sequenced using the LGC sequencing service (Berlin, Germany). The obtained sequence data were analyzed with BioEdit v.7.0.5 software (Hall 1999). Finally, based on visual inspection of sequencing chromatograms, the mutations were classified as ‘not detected’, ‘present’ and ‘fixed’ (Khajehali et al. 2011). It should be noted that this method is semi-quantitative, and will only detect mutations with a relatively high frequency, as compared to assessing multiple single males or females. Thus, the early onset of resistance might not be detected with this method.
Results
Resistance levels
The mortality after 48 h at FD/5, FD/2, FD, 2FD and 5FD for the eight compounds on all assayed populations is presented in Table 3. Tetranychus urticae populations showed various levels of susceptibility to the tested acaricides. All T. urticae populations were fully susceptible (mortality > 50% at FD) to bifenazate, abamectin, emamectin benzoate and profenofos. Most of the populations were found resistant to at least three acaricides. Resistance (mortality < 50% at FD) against fenbutatin oxide and fenpyroximate was detected in all the populations collected from the farms and wild plants, whereas most of these populations were even highly resistant (mortality < 50% at 5FD) to these products. Four out of the 12 tested Ethiopian populations (Ejersa, Holeta-1, Holeta-2 and Menagesha) were found resistant to amitraz. Among the tested populations, only Holeta-2 was resistant to chlorfenapyr.
Resistance mutation
Several target site mutations known to confer acaricide resistance (Feyereisen et al. 2015; Van Leeuwen and Dermauw 2016; Van Leeuwen et al. 2010) have been detected and are presented in Table 4. Among the known mutations in acetylcholinesterase (AChE), G119S, A201S, T280A, G328A and F331W/C/Y were found in some of the tested populations. The G119S mutation was detected in eight populations and was fixed in the Bishoftu-1 population. In addition, an F331 mutation was found in nine populations and fixed in four of these populations. A combination of mutations in AChE in many of the tested populations was observed. In the Bishoftu-1 population, four out of the six screened mutations (G119S, T280A, G328A and F331W/C/Y) were identified. Similarly, in Bishoftu-2, Holeta-2 and Menagesha populations, G119S, A201S and F331W/C/Y mutations were observed. A combination of two of the mutations (G119S and F331W/C/Y) was detected in Sebeta, Ziway-2, Ejersa, Koka and Holeta-1. Whether these mutations occur in a single haplotype, or reflect the presence of different alleles from the sampled population, was not investigated.
The H92R mutation in the PSST homologue of complex I was detected in half of the tested populations, whereas only in Bishoftu-1 this mutation was fixed.
Among the mutations in the glutamate-gated chloride channel, G1314D in GluCl1 was not detected, whereas G326E and I321T in GluCl3 were found, but not very frequently. The G326E mutation was present in four populations but only fixed in the Bishoftu-1 population. The recently discovered mutation I321T in GluCl3 (Papapostolou et al. 2020; Xue et al. 2020) was detected in the Bishoftu-1 population.
The F1538I mutation in the VGSC was found in eight populations, but was only fixed in four populations (Bishoftu-1, Sebeta, Holeta-2 and Menagesha). The L1024V mutation in the VGSC was rare and only detected in two populations (Bishoftu-1 and Holeta-1). A combination of the F1538I and L1024V mutation was detected in Bishoftu-1 and Holeta-1 populations. Last, none of the tested populations harbored the super-kdr mutation (M918T, or a variant thereof, M918L) in the VGSC (Nyoni et al. 2011; Wu et al. 2019).
The chitin synthase 1 (CHS1) mutation, I1017F, was detected in six populations and was fixed in three of these populations (Ziway-2, Ejersa and Sebeta). Several mutations (G126S + I136T, G126S + S141F, G126S + A133T, I260V + N326S, P262T and G132A) in cytochrome b (cytb) were screened in all populations but not detected.
Discussion
In this study, we used fixed acaricide diagnostic doses based on registered field rates in Ethiopia to characterize the resistance status of spider mite populations. However, resistance levels can only be accurately calculated when dose-response relationships are determined, and compared to the baseline response of a number of reference populations. It is clear that in some cases, such as for profenofos, better diagnostic doses could have been chosen. As full dose-response was not feasible, we took an arbitrary definition for susceptible (mortality > 50% at FD), resistant (mortality < 50% at FD) and highly resistant (mortality < 50% at 5FD). Whether this classification is directly associated with field performance of acaricides on these strains is doubtful, but it allows to describe differences between populations to some extent. Nevertheless, it is clear that if mortality is limited at 5FD, some levels of resistance must have evolved if this contrasts with patterns observed in other susceptible strains. As the Ghent reference strain shows only limited susceptibility to amitraz and chlorfenapyr in this setup, claims about resistance linked to poor field performance are almost impossible to make for these compounds.
All the tested populations in the current study were found fully susceptible to bifenazate, abamectin, emamectin benzoate and profenofos. Bifenazate is a recently introduced, highly effective miticide which belongs to the class of mitochondrial electron transport inhibitors (METIs), acting on complex III (IRAC Group 20) of the mitochondrial electron transport chain. Mutations in mitochondrial cytochrome b (cytb) were found to be associated with bifenazate resistance (Van Leeuwen et al. 2008, 2011; Van Nieuwenhuyse et al. 2009). Resistance of spider mites to bifenazate has been previously reported (Chen et al. 2019; Khajehali et al. 2011; Van Leeuwen et al. 2006, 2008; Xu et al. 2018). In Ethiopia, Elings et al. (2009) reported failure of field applications of bifenazate in some of the Ethiopian rose farms. However, toxicity tests were not conducted to supplement the observed field control failure of the acaricide. In the current study none of the tested mites from greenhouses and field farms had developed resistance to bifenazate, whereas also none of the previously reported cytb resistance mutations (Fotoukkiaii et al. 2020; Van Leeuwen et al. 2008, 2011; Van Nieuwenhuyse et al. 2009) were detected.
Globally, abamectin is extensively used to control mites and resistance has been documented in many regions of the world (Cagatay et al. 2018; Khajehali et al. 2011; Kwon et al. 2010a; Sato et al. 2005; Stumpf and Nauen 2002; Vassiliou and Kitsis 2013; Xu et al. 2018; Xue et al. 2020). In our study, toxicity tests did not reveal abamectin resistance. This might be partially due to the type of commercial formulation we used, which targets insect pests (aphids, leaf miner and bollworm) and on which the label indicated a field dose higher than the recommended dose used for the control of spider mites elsewhere (Inak et al. 2019). However, in the Holeta-2 population very low mortality was observed – 38 and 48% at 7.2 and 18 mg/L, respectively – showing resistance compared with the diagnostic dose in Inak et al. (2019). Moreover, low mortality (58%) at 36 mg/L was detected in this strain. Similarly, the Ziway-1 population exhibited only 61% mortality at 7.2 mg/L, and could be considered partially resistant, in comparison with previous studies. Nevertheless, also a previous study from tomato farms of Ethiopia confirmed the efficacy of abamectin against TSSM (Geleto et al. 2015). Abamectin targets the glutamate-gated chloride channel and resistance to abamectin has been associated with G314D in GluCl1 and G326E and I321T in GluCl3 (Dermauw et al. 2012; Kwon et al. 2010a; Mermans et al. 2017; Riga et al. 2014; Stumpf and Nauen 2002; Xue et al. 2020). Although toxicity tests in our study did not confirm resistance to abamectin, molecular diagnostics revealed the presence of the G326E mutation in GluCl3 in four populations and the I321T mutation in GluCl3 in one population. Hence, care should be taken when using abamectin, as resistance mutations can rapidly rise in frequency, potentially compromising future use of the product. On the other hand, as G326E was fixed in one population, the presence of the G326E mutation alone seems not sufficient to confer high resistance levels. This is in line with a previous study, in which marker-assisted backcrossing of the G326E mutation in a susceptible genetic background, revealed that the mutation alone or in combination with G314D in GluCl1, exhibited only a weak resistance phenotype in T. urticae. However, the recommended field dose of abamectin is tailored to insects and very high (see above) and the high concentrations might mask actual resistance levels. Similar to abamectin, all of the populations of TSSMs in our study were fully susceptible to emamectin benzoate. This acaricide is classified in the same group as abamectin, having a similar mode of action. Resistance to emamectin benzoate has been reported from Korea (Lee et al. 2003), yet was not observed in the current study.
A CHS1 mutation, I1017F, was detected in six out of 12 populations, being fixed in three of these six populations (Ziway-2, Ejersa and Sebeta). IRAC Group 10 acaricides (etoxazole, hexythiazox, clofentezine) are mite growth inhibitors and have CHS1 as a common target site (Demaeght et al. 2014). The I1017F substitution in the C-terminal transmembrane domain of CHS1 has been linked with resistance against these acaricides (Demaeght et al. 2014; Van Leeuwen et al. 2012) and CHS1 mutations in TSSM have been reported previously (Adesanya et al. 2018; Demaeght et al. 2014; Herron et al. 2018; Ilias et al. 2014; Inak et al. 2019; Osakabe et al. 2017). Hence, the presence of this mutation in most of the tested populations raises concern for the possible use of mite growth inhibitor acaricides in Ethiopian farms.
All populations collected from Ethiopia showed high levels of resistance against fenbutatin oxide, which targets mitochondrial ATPase. Spider mite populations from different areas have developed resistance against this compound (Doker and Kazak 2012; Goodwin et al. 1995; Gorman et al. 2002; Inak et al. 2019; Van Leeuwen et al. 2005). However, the resistance mechanism to this compound is not well understood and, at present, target-site mutations have not yet been documented.
Organophosphates (OPs) have been extensively used to control T. urticae. OP resistance has been reported since the 1940s in T. urticae populations (Khajehali et al. 2010), and resistance has been reported for various insect pests (Dong et al. 2014; Stumpf and Nauen 2001). However, previous findings from various parts of Ethiopia suggested the efficacy of profenofos for mite management (Belay et al. 2018; Ebrahim and Wakgari 2019). It was also evident that profenofos efficiently controlled spider mites in many regions (Venugopal et al. 2003). In Ethiopia, profenofos was registered for the control of pea aphids (Acyrthosiphon pisum) in pea fields (MOA, 2016). Acetylcholinesterase (AChE) mutations have been associated with OP resistance (Khajehali et al. 2010; Kwon et al. 2010b). The detection of the G119S, T201S, T280A, G328A and F331W/C/Y mutations in the AChE gene of some of the tested populations in Ethiopia suggests the presence of target-site resistance mechanisms against OPs. Why profenofos resistance is not detected in toxicity tests, at least in the few populations where mutations are fixed, is unclear. However, it could be related to the type of OP-compound, as the mutations have a different effect on different OPs (Khajehali et al. 2010; Zhang et al. 2017). In addition, the field dose indicated for insects is much higher than the recommended dose for spider mites (Herron et al. 1998), and even at ¼ of the field dose, mites that are resistant to other OP compounds might die.
Various mutations in the VGSC have been described to confer pyrethroid resistance. Among the known mutations in the VGSC, the F1538I mutation in domain III segment 6 was found to be associated with pyrethroid resistance and was reported worldwide in many TSSM populations (Davies et al. 2008; Dong et al. 2014; Feyereisen et al. 2015; Khajehali et al. 2011; Tsagkarakou et al. 2009; Xu et al. 2018). In the current study, the F1538I mutation was found in eight out of 12 populations. Moreover, the L1024V mutation, known to confer resistance against pyrethroids (Kwon et al. 2010b) was detected in two populations (Bishoftu-1 and Holeta-1). The observed combination of the F1538I and L1024V mutations might raise concern for strong phenotype resistance in these populations, although it needs to be confirmed that they occur in a single allele. Hence, the presence of F1538I mutation in most of the tested populations, together with L1024V, might indicate that target-site resistance is a major resistance mechanism against pyrethroids in Ethiopian TSSM populations.
Fenpyroximate is a mitochondrial electron transport inhibitor of complex I (METI-Is) of the respiratory chain (Dekeyser 2005) and was found to be very effective against all life stages of T. urticae and Panonychus citri (McGregor) (Motoba et al. 1992). However, widely distributed resistance of TSSM to fenpyroximate was observed in our study. Likewise, resistance against fenpyroximate has been previously reported (Jum et al. 1995; Kim et al. 2004; Sato et al. 2004; Stumpf and Nauen 2001; Van Pottelberge et al. 2009). The H92R mutation in the PSST subunit of complex I in T. urticae has been associated with resistance to fenpyroximate (Bajda et al. 2017), and was found in half of the tested populations. However, also the susceptible reference population shows limited mortality at 250 mg a.i./L. This could indicate that either the strain was contaminated, or that the leaf dip assay used in this study, providing only residual tarsal contact, is not suitable and comparable to previous work with fenpyroximate (Van Pottelberge et al. 2009). Nevertheless, the presence of resistance mutations does indicate that METIs should not be used in the near future.
Amitraz resistance in TSSM has been reported earlier (Al Antary et al. 2012). In the current study, four populations (Ejersa, Holeta-1, Holeta-2 and Menagesha) were found to be resistant to amitraz, whereas the remaining populations were susceptible for this compound. A similar study conducted by Geleto et al. (2015) reported amitraz resistance in mites collected from tomato farms. Unlike our findings, amitraz resistance was not detected in mites collected from wild plants as in the studies of Belay et al. (2018) and Ebrahim and Wakgari (2019). Chlorfenapyr was found to be effective against almost all of the tested populations, with only Holeta-2 showing resistance. Earlier studies also showed that TSSM could develop resistance to chlorfenapyr (Herron et al. 2004; Nicastro et al. 2013; Van Leeuwen et al. 2004).
Last, our study revealed that some of the Ethiopian TSSM populations are multi-resistant (chlorfenapyr, fenpyroximate, fenbutatin oxide and amitraz). Moreover, the efficacy of abamectin, OPs, METI-I acaricides and pyrethroids might be compromised as the target-site mutations known for the mechanisms of resistance of these pesticides are commonly existing in the Ethiopian T. urticae populations.
Although there is no direct correlation between field efficacy and the presence of resistance mutations at a certain frequency, their presence is probably indicative of wide acaricide use, at least historically, and care should be taken to develop an appropriate resistance management strategy.
References
Abate T (1987) New records of arthropod pests of grain legumes in ethiopia. Annual report of the Bean Improvement Cooperative (USA)
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Adesanya AW, Morales MA, Walsh DB, Lavine LC, Lavine MD, Zhu F (2018) Mechanisms of resistance to three mite growth inhibitors of Tetranychus urticae in hops. Bull Entomol Res 108:23–34. doi:https://doi.org/10.1017/s0007485317000414
Al Antary T, Lala A, K M, Abed-Wali M (2012) Response of seven populations of the two-spotted spider mite Tetranychus urticae Koch) for amitraz acaricide on cucumber (Cucumis sativus l.) under plastic houses in Jordan. Advances in Environmental Biology 6:951–954
Ayalew G, Mulatu B, Negeri M, Merene Y, Sitotaw L, Ibrahim A, Tefera T (2006) Review of research on insect and mite pests of vegetable crops in Ethiopia. Increasing crop production through improved plant protection Addis Ababa, Ethiopia, Plant Protection Society of Ethiopia, 47–65
Bajda S, Dermauw W, Panteleri R, Sugimoto N, Douris V, Tirry L, Osakabe M, Vontas J, Van Leeuwen T (2017) A mutation in the psst homologue of complex i (nadh:Ubiquinone oxidoreductase) from Tetranychus urticae is associated with resistance to meti acaricides. Insect Biochem Mol Biol 80:79–90. doi:https://doi.org/10.1016/j.ibmb.2016.11.010
Belay T, Goftishu M, Kassaye A (2018) Management of an emerging pest, Tetranychus urticae Koch (Acari: Tetranychidae), with pesticides in eastern ethiopia. Afr Crop Sci Jdoi. https://doi.org/10.4314/acsj.v26i2.10
Cagatay NS, Menault P, Riga M, Vontas J, Ay R (2018) Identification and characterization of abamectin resistance in Tetranychus urticae Koch populations from greenhouses in Turkey. Crop Prot 112:112–117. doi:https://doi.org/10.1016/j.cropro.2018.05.016
Chen JC, Gong YJ, Shi P, Wang ZH, Cao LJ, Wang P, Wei SJ (2019) Field-evolved resistance and cross-resistance of the two-spotted spider mite, Tetranychus urticae, to bifenazate, cyenopyrafen and syp-9625. Exp Appl Acarol 77:545–554. doi:https://doi.org/10.1007/s10493-019-00359-3
Davies TGE, O’Reilly AO, Field LM, Wallace BA, Williamson MS (2008) Knockdown resistance to ddt and pyrethroids: From target-site mutations to molecular modelling. Pest Manag Sci 64:1126–1130. doi:https://doi.org/10.1002/ps.1617
Dekeyser MA (2005) Acaricide mode of action. Pest Manag Sci 61:103–110
Demaeght P, Dermauw W, Tsakireli D, Khajehali J, Nauen R, Tirry L, Vontas J, Lummen P, Van Leeuwen T (2013) Molecular analysis of resistance to acaricidal spirocyclic tetronic acids in Tetranychus urticae: Cyp392e10 metabolizes spirodiclofen, but not its corresponding enol. Insect Biochem Mol Biol 43:544–554. doi:https://doi.org/10.1016/j.ibmb.2013.03.007
Demaeght P, Osborne EJ, Odman-Naresh J, Grbic M, Nauen R, Merzendorfer H, Clark RM, Van Leeuwen T (2014) High resolution genetic mapping uncovers chitin synthase-1 as the target-site of the structurally diverse mite growth inhibitors clofentezine, hexythiazox and etoxazole in Tetranychus urticae. Insect Biochem Mol Biol 51:52–61. doi:https://doi.org/10.1016/j.ibmb.2014.05.004
Dermauw W, Ilias A, Riga M, Tsagkarakou A, Grbic M, Tirry L, Van Leeuwen T, Vontas J (2012) The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem Mol Biol 42:455–465. doi:https://doi.org/10.1016/j.ibmb.2012.03.002
Doker I, Kazak C (2012) Detecting acaricide resistance in Turkish populations of Panonychus citri McGregor (Acari: Tetranychidae). Systematic Applied Acarology 17:368–377
Dong K, Du YZ, Rinkevich F, Nomura Y, Xu P, Wang LX, Silver K, Zhorov BS (2014) Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol 50:1–17. doi:https://doi.org/10.1016/j.ibmb.2014.03.012
Ebrahim Y, Wakgari M (2019) Full length research efficacy of pesticides against two-spotted spider mite (Tetranychus urticae Koch) (Acari: Tetranychidae) and its performance on some hosts in greenhouse at Haramaya, eastern Ethiopia. J Equity Sci Sust Dev 3(2):88–101
Elings B, Yilma Y, Dawd M (2009) On-farm evaluation of integrated pest management of red-spider mite in cut roses in Ethiopia. Final Report to the Ministry of Agriculture and Rural Development
Feyereisen R, Dermauw W, Van Leeuwen T (2015) Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pest Biochem Physiol 121:61–77. doi:https://doi.org/10.1016/j.pestbp.2015.01.004
Fotoukkiaii SM, Tan Z, Xue WX, Wybouw N, Van Leeuwen T (2020) Identification and characterization of new mutations in mitochondrial cytochrome b that confer resistance to bifenazate and acequinocyl in the spider mite Tetranychus urticae. Pest Manag Sci 76:1154–1163. doi:https://doi.org/10.1002/ps.5628
Geleto BG, Beyene Y, Azerefegne F, Gulich G (2015) Evaluation of acaricide resistance on two spotted spider mite (tetranychus urticae, coch) in the central rift valley of ethiopia. 2015 2, 8
Goftishu M, Dejene M, Kassaye A, Belay T (2016) Red spider mite, Tetranychus urticae Koch (Arachnida: Acari-Tetranychidae): A threatening pest to potato (Solanum tuberosum L.) production in eastern ethiopia. Pest Management Journal of Ethiopia 19:53–59
Goodwin S, Herron G, Gough N, Wellham T (1995) Relationship between insecticide-acaricide resistance and field control in Tetranychus-urticae (Acari, Tetranychidae) infesting roses. J Econ Entomol 88:1106–1112. doi:https://doi.org/10.1093/jee/88.5.1106
Gorman K, Hewitt F, Denholm I, Devine GJ (2002) New developments in insecticide resistance in the glasshouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest Manag Sci 58:123–130. doi:https://doi.org/10.1002/ps.427
Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osborne EJ, Dermauw W, Ngoc PCT, Ortego F (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492
Hall TA, Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. In: Nucleic acids symposium series, 1999. vol 41. [London]: Information Retrieval Ltd., c1979-c2000., p 95–98
Herron G, Rophail J, Wilson L (2004) Chlorfenapyr resistance in two-spotted spider mite (Acari: Tetranychidae) from Australian cotton. Experimental Applied Acarology 34:315
Herron GA, Edge VE, Wilson LJ, Rophail J (1998) Organophosphate resistance in spider mites (Acari: Tetranychidae) from cotton in Australia. Experimental Applied Acarology 22:17–30. doi:https://doi.org/10.1023/A:1006029307049
Herron GA, Woolley LK, Langfield KL, Chen YZ (2018) First detection of etoxazole resistance in Australian two-spotted mite Tetranychus urticae Koch (Acarina: Tetranychidae) via bioassay and DNA methods. Austral Entomol 57:365–368. doi:https://doi.org/10.1111/aen.12290
Ilias A, Vontas J, Tsagkarakou A (2014) Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem Mol Biol 48:17–28. doi:https://doi.org/10.1016/j.ibmb.2014.02.006
Inak E, Alpkent YN, Cobanoglu S, Dermauw W, Van Leeuwen T (2019) Resistance incidence and presence of resistance mutations in populations of Tetranychus urticae from vegetable crops in Turkey. Exp Appl Acarol 78:343–360. doi:https://doi.org/10.1007/s10493-019-00398-w
IRAC (2009) Susceptibility test methods series, version 3. Insecticide Resistance Action Committee. www.irac-online.org
Jeppson LR, Keifer HH, Baker EW (1975) Mites Injurious to Economic Plants, Univ of California Press
Jum BC, Young JK, Young JA, Jai KY, Jeong OL (1995) Monitoring of acaricide resistance in field-collected populations of Tetranychus urticae (Acari: Tetranychidae) in Korea. Korean Journal of Applied Entomology 34:40–45
Kasap I (2005) Life-history traits of the predaceous mite Kampimodromus aberrans (Oudemans) (Acarina: Phytoseiidae) on four different types of food. Biol Control 35:40–45. doi:https://doi.org/10.1016/j.biocontrol.2005.05.014
Khajehali J, Van Leeuwen T, Grispou M, Morou E, Alout H, Weill M, Tirry L, Vontas J, Tsagkarakou A (2010) Acetylcholinesterase point mutations in european strains of Tetranychus urticae (Acari: Tetranychidae) resistant to organophosphates. Pest Manag Sci 66:220–228. doi:https://doi.org/10.1002/ps.1884
Khajehali J, Van Nieuwenhuyse P, Demaeght P, Tirry L, Van Leeuwen T (2011) Acaricide resistance and resistance mechanisms in Tetranychus urticae populations from rose greenhouses in The Netherlands. Pest Manag Sci 67:1424–1433. doi:https://doi.org/10.1002/ps.2191
Khalighi M, Dermauw W, Wybouw N, Bajda S, Osakabe M, Tirry L, Van Leeuwen T (2016) Molecular analysis of cyenopyrafen resistance in the two-spotted spider mite Tetranychus urticae. Pest Manag Sci 72:103–112. doi:https://doi.org/10.1002/ps.4071
Kim YJ, Lee SH, Lee SW, Ahn YJ (2004) Fenpyroximate resistance in Tetranychus urticae (Acari: Tetranychidae): Cross-resistance and biochemical resistance mechanisms. Pest Manag Sci 60:1001–1006. doi:https://doi.org/10.1002/ps.909
Kwon D, Clark J, Lee S (2010a) Extensive gene duplication of acetylcholinesterase associated with organophosphate resistance in the two-spotted spider mite. Insect molecular biology 19:195–204
Kwon DH, Seong GM, Kang TJ, Lee SH (2010b) Multiple resistance mechanisms to abamectin in the two-spotted spider mite. J Asia-Pac Entomol 13:229–232. doi:https://doi.org/10.1016/j.aspen.2010.02.002
Lee Y-S, Song M-H, Ahn K-S, Lee K-Y, Kim J-W, Kim G-H (2003) Monitoring of acaricide resistance in two-spotted spider mite (Tetranychus urticae) populations from rose greenhouses in korea. J Asia-Pac Entomol 6:91–96
Luczynski A, Isman MB, Raworth DA, Chan CK (1990) Chemical and morphological factors of resistance against the twospotted spider mite in beach strawberry. J Econ Entomol 83:564–569. doi:https://doi.org/10.1093/jee/83.2.564
Mermans C, Dermauw W, Geibel S, Van Leeuwen T (2017) A g326e substitution in the glutamate-gated chloride channel 3 (glucl3) of the two-spotted spider mite Tetranychus urticae abolishes the agonistic activity of macrocyclic lactones. Pest Manag Sci 73:2413–2418. doi:https://doi.org/10.1002/ps.4677
Migeon A, Nouguier E, Dorkeld F (2010) Spider mites web: A comprehensive database for the Tetranychidae. Springer, Dordrecht
Motoba K, Suzuki T, Uchida M (1992) Effect of a new acaricide, fenpyroximate, on energy-metabolism and mitochondrial morphology in adult female Tetranychus-urticae (2-spotted spider-mite). Pest Biochem Physiol 43:37–44. doi:https://doi.org/10.1016/0048-3575(92)90017-t
Negash R, Dawd M, Azerefegne F (2014) Pathogenecity of Beauveria bassiana and Metarhizium anisopliae, to the two spotted spider mites, Tetranychus urticae (Acari: Tetranychidae) at different temperatures and in greenhouse condition. Ethiop J Agric Sci 24:51–58
Nicastro RL, Sato ME, Arthur V, da Silva MZ (2013) Chlorfenapyr resistance in the spider mite Tetranychus urticae: Stability, cross-resistance and monitoring of resistance. Phytoparasitica 41:503–513. doi:https://doi.org/10.1007/s12600-013-0309-x
Nyoni BN, Gorman K, Mzilahowa T, Williamson MS, Navajas M, Field LM, Bass C (2011) Pyrethroid resistance in the tomato red spider mite, Tetranychus evansi, is associated with mutation of the para-type sodium channel. Pest Manag Sci 67:891–897
Osakabe M, Imamura T, Nakano R, Kamikawa S, Tadatsu M, Kunimoto Y, Doi M (2017) Combination of restriction endonuclease digestion with the delta delta ct method in real-time pcr to monitor etoxazole resistance allele frequency in the two-spotted spider mite. Pest Biochem Physiol 139:1–8. doi:https://doi.org/10.1016/j.pestbp.2017.04.003
Papapostolou KM, Riga M, Charamis J, Skoufa E, Souchlas V, Ilias A, Dermauw W, Ioannidis P, Van Leeuwen T, Vontas J (2020) Identification and characterization of striking multiple-insecticide resistance in a Tetranychus urticae field population from greece. Pest Manag Sci
Regev S, Cone WW (1976) Evidence of gonodotropic effect of farnesol in the twospotted spider mite, Tetranychus urticae, 2. Environmental Entomology 5:517–519. https://doi.org/10.1093/ee/5.3.517
Riga M, Tsakireli D, Ilias A, Morou E, Myridakis A, Stephanou EG, Nauen R, Dermauw W, Van Leeuwen T, Paine M, Vontas J (2014) Abamectin is metabolized by cyp392a16, a cytochrome p450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem Mol Biol 46:43–53. doi:https://doi.org/10.1016/j.ibmb.2014.01.006
Sato ME, Da Silva MZ, Raga A, De Souza MF (2005) Abamectin resistance in Tetranychus urticae koch (Acari: Tetranychidae): Selection, cross-resistance and stability of resistance. Neotrop Entomol 34:991–998. doi:https://doi.org/10.1590/s1519-566x2005000600016
Sato ME, Miyata T, Da Silva M, Raga A, De Souza MF (2004) Selections for fenpyroximate resistance and susceptibility, and inheritance, cross-resistance and stability of fenpyroximate resistance in Tetranychus urticae Koch (Acari: Tetranychidae). Appl Entomol Zoolog 39:293–302. doi:https://doi.org/10.1303/aez.2004.293
Stumpf N, Nauen R (2001) Cross-resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor-acaricide resistance in Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 94:1577–1583. doi:https://doi.org/10.1603/0022-0493-94.6.1577
Stumpf N, Nauen R (2002) Biochemical markers linked to abamectin resistance in Tetranychus urticae (Acari: Tetranychidae). Pest Biochem Physiol 72:111–121. doi:https://doi.org/10.1006/pest.2001.2583
Tsagkarakou A, Van Leeuwen T, Khajehali J, Ilias A, Grispou M, Williamson MS, Tirry L, Vontas J (2009) Identification of pyrethroid resistance associated mutations in the para sodium channel of the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Insect Mol Biol 18:583–593. doi:https://doi.org/10.1111/j.1365-2583.2009.00900.x
Van Leeuwen T, Demaeght P, Osborne EJ, Dermauw W, Gohlke S, Nauen R, Grbic M, Tirry L, Merzendorfer H, Clark RM (2012) Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc Natl Acad Sci USA 109:4407–4412. doi:https://doi.org/10.1073/pnas.1200068109
Van Leeuwen T, Dermauw W (2016) The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu Rev Entomol 61:475–498
Van Leeuwen T, Dermauw W, Mavridis K, Vontas J (2020) Significance and interpretation of molecular diagnostics for insecticide resistance management of agricultural pests. Current Opinion in Insect Science 39:69–76
Van Leeuwen T, Stillatus V, Tirry L (2004) Genetic analysis and cross-resistance spectrum of a laboratory-selected chlorfenapyr resistant strain of two-spotted spider mite (Acari: Tetranychidae). Exp Appl Acarol 32:249–261
Van Leeuwen T, Van Nieuwenhuyse P, Vanholme B, Dermauw W, Nauen R, Tirry L (2011) Parallel evolution of cytochrome b mediated bifenazate resistance in the citrus red mite Panonychus citri. Insect Mol Biol 20:135–140. doi:https://doi.org/10.1111/j.1365-2583.2010.01040.x
Van Leeuwen T, Van Pottelberge S, Tirry L (2005) Comparative acaricide susceptibility and detoxifying enzyme activities in field-collected resistant and susceptible strains of Tetranychus urticae. Pest Manag Sci 61:499–507. doi:https://doi.org/10.1002/ps.1001
Van Leeuwen T, Van Pottelberge S, Tirry L (2006) Biochemical analysis of a chlorfenapyr-selected resistant strain of Tetranychus urticae Koch. Pest Management Science: Formerly Pesticide Science 62:425–433
Van Leeuwen T, Vanholme B, Van Pottelberge S, Van Nieuwenhuyse P, Nauen R, Tirry L, Denholm I (2008) Mitochondrial heteroplasmy and the evolution of insecticide resistance: Non-mendelian inheritance in action. Proc Natl Acad Sci USA 105:5980–5985. doi:https://doi.org/10.1073/pnas.0802224105
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem Mol Biol 40:563–572. doi:https://doi.org/10.1016/j.ibmb.2010.05.008
Van Nieuwenhuyse P, Van Leeuwen T, Khajehali J, Vanholme B, Tirry L (2009) Mutations in the mitochondrial cytochrome b of Tetranychus urticae Koch (Acari: Tetranychidae) confer cross-resistance between bifenazate and acequinocyl. Pest Manag Sci 65:404–412. doi:https://doi.org/10.1002/ps.1705
Van Pottelberge S, Van Leeuwen T, Nauen R, Tirry L (2009) Resistance mechanisms to mitochondrial electron transport inhibitors in a field-collected strain of Tetranychus urticae Koch (Acari: Tetranychidae). Bull Entomol Res 99:23–31. doi:https://doi.org/10.1017/s0007485308006081
Vassiliou VA, Kitsis P (2013) Acaricide resistance in Tetranychus urticae (Acari: Tetranychidae) populations from Cyprus. J Econ Entomol 106:1848–1854. doi:https://doi.org/10.1603/ec12369
Venugopal V, Natou V, Prasad P (2003) Evaluation of new acaricides against red spider mite, Tetranychus cinnabarinus (Boisduval) on okra. Pestology 27:29–34
Wei P, Li JH, Liu XY, Nan C, Shi L, Zhang YC, Li CZ, He L (2019) Functional analysis of four upregulated carboxylesterase genes associated with fenpropathrin resistance in Tetranychus cinnabarinus (Boisduval). Pest Manag Sci 75:252–261. doi:https://doi.org/10.1002/ps.5109
Wu M, Adesanya AW, Morales MA, Walsh DB, Lavine LC, Lavine MD, Zhu F (2019) Multiple acaricide resistance and underlying mechanisms in Tetranychus urticae on hops. J Pest Sci 92:543–555
Xu DD, He YY, Zhang YJ, Xie W, Wu QJ, Wang SL (2018) Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in china. Pest Biochem Physiol 150:89–96. doi:https://doi.org/10.1016/j.pestbp.2018.07.008
Xue W, Snoeck S, Njiru C, Inak E, Dermauw W, Van Leeuwen T (2020) Geographical distribution and molecular insights into abamectin and milbemectin cross-resistance in european field populations of Tetranychus urticae. Pest Manag Sci 76:2569–2581
Zhang Y, Yang B, Li J, Liu M, Liu Z (2017) Point mutations in acetylcholinesterase 1 associated with chlorpyrifos resistance in the brown planthopper, Nilaparvata lugens Stål. Insect molecular biology 26:453–460
Zhang Z-Q (2003) Mites of greenhouses. Identification, Biology and Control, Cabi
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simma, E.A., Hailu, B., Jonckheere, W. et al. Acaricide resistance status and identification of resistance mutations in populations of the two-spotted spider mite Tetranychus urticae from Ethiopia. Exp Appl Acarol 82, 475–491 (2020). https://doi.org/10.1007/s10493-020-00567-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00567-2