Abstract
Bacteria associated with mites influence their fitness, nutrition and reproduction. Previously, we found Solitalea-like (Sphingobacteriales) and Candidatus Cardinium (Cytophagales) bacteria in the stored product mite Acarus siro L. by cloning and using pyrosequencing. In this study, taxon-specific primers targeting 16S rRNA gene were used to detect and quantify the bacteria in mites and eggs of three A. siro populations. The specific probes for fluorescent in situ hybridization (FISH) were used to localize Solitalea-like and Cardinium bacteria in mite bodies. The population growth as an indirect estimator of fitness was used to describe the mite-bacteria interactions on (1) control diet; (2) rifampicin supplemented diet; (3) tetracycline supplemented diet; (4) rifampicin pretreated mites; (5) tetracycline pretreated mites. Solitalea-like 16S rRNA gene sequences from A. siro formed a separate cluster together with sequences from Tyrophagus putrescentiae. qPCR analysis indicated that number of Solitalea-like bacteria 16S rRNA gene copies was ca. 100× higher than that of Cardinium and the numbers differed between populations. FISH analysis localized Solitalea-like bacteria in the parenchymal tissues, mesodeum and food bolus of larvae, nymphs and adults. Solitalea-like, but not Cardinium bacteria were detected by taxon-specific primers in mites and eggs of all three investigated populations. None of the antibiotic treatments eliminated Solitalea-like bacteria in the A. siro populations tested. Rifampicin pretreatment significantly decreased the population growth. The numbers of Solitalea-like bacteria did not correlate with the population growth as a fitness indicator. This study demonstrated that A. siro can host Solitalea-like bacteria either alone or together with Cardinium. We suggest that Solitalea-like bacteria are shared by vertical transfer in A. siro populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acarus siro L. is a cosmopolitan mite species and one of the most common pests of stored grains and flour (Palyvos et al. 2008; Athanassiou et al. 2011). Acarus siro is found also in stored cheese and meat products (McClymont-Peace 1983; Armentia et al. 1994; Melnyk et al. 2010). The importance of A. siro, as well as other stored product mites, is in contamination of stored food and human environment with their bodies, excretory products and substances of allergenic potential with risks to consumers (Marx et al. 1993; Sanchez-Borges et al. 2009, 2013) and workers in food production, e.g. farmers (Musken et al. 2000) and bakers (Armentia et al. 1997). Acarus siro transfers microorganisms on the body surface or in the mite bodies (Hubert et al. 2004, 2012, 2016; Erban et al. 2016b).
Except of gut-associated bacteria, the mites host intracellular symbiotic or parasitic bacteria, e.g. Candidatus Cardinium (Bacteroidetes, Cytophagales, Amoebophilaceae) and Wolbachia spp. (Alphaproteobacteria, Rickettsiales, Anaplasmataceae). Cand. Cardinium was originally described as a manipulator of reproduction in insects and mites (Nakamura et al. 2012). However, the underlying mechanisms of cytoplasmic incompatibility (in parasitoid wasps, planthoppers, phytoseiids and spider mites) or feminization (in false spider mites) are still unknown. With exception of one diplodiploid insect host, a planthopper (Nakamura et al. 2012), host reproductive manipulations have been described only in haplodiploid and parahaploid hosts (Weeks et al. 2001; Zchori-Fein et al. 2004; Breeuwer et al. 2011; Wu and Hoy 2012a). However, no relevant observation was reported in diplodiplod Acari. The ultrastructure observations repeatedly showed Cardinium bacteria living in almost every single tissue of their insect, proturan, mite and tick hosts. Therefore, Cardinium is not exclusively associated to the reproductive tissues (Hess and Hoy 1982; Kurtti et al. 1996; Bigliardi et al. 2006; Kitajima et al. 2007; Sacchi et al. 2008; Dallai et al. 2011). Though Cardinium endosymbionts are prevalent in arthropods including several diplodiploid members of Chelicerata, no reproductive manipulations were uncovered so far (Chang et al. 2010; Perlman et al. 2010; Stefanini and Duron 2012; Kopecky et al. 2013). Interestingly, a relevant role in host nutrition was suggested by analysis of the Cardinium genome (Penz et al. 2012). For example, C. hertigii (strain cEper1) may provide biotin for insect growth and metamorphosis that cannot be synthetized by its insect host, the parasitoid wasp Encarsia pergandiella (Penz et al. 2012). Santos-Garcia et al. (2014) described the genome of Cardinium strain C1 from Bemisia tabaci (cBtQ1) containing a plasmid that carries a unique combination of four gliding genes gldK, gldL, gldM and gldN. It has shed some light on behavior of several Bacteroidetes species, which spread within different host tissues (Santos-Garcia et al. 2014). Cardinium ubiquity within chelicerates might be justified by a long-term interaction (Stefanini and Duron 2012; Duron 2013) due to a similar, predominantly nutritional role in diplodiploid chelicerates (Fukuzawa et al. 2008).
In the previous screening of cloned 16S rRNA gene sequences of A. siro, Lepidoglyphus destructor, T. putrescentiae and Dermatophagoides farinae from laboratory populations of Crop Research Institute, Prague, Czechia, the sequences of Bacteroidetes were found in D. farinae and A. siro (Hubert et al. 2012). In A. siro, they represented 34 % (20/170) of the cloned sequences. The phylogenetic analyses of cloned 16S rRNA gene sequences indicated two clusters (1) ‘Candidatus Cardinium’ in D. farinae and (2) Solitalea-like clade within Sphingobacteriales in A. siro (Hubert et al. 2012). The genus Solitalea comprises free-living species found in soil samples (Weon et al. 2009). In another experiment, both Cardinium and Solitalea were found in the cloned universal bacterial 16S rRNA gene amplicon from the laboratory population of A. siro (Kopecky et al. 2014). The presence of Cardinium in this laboratory population and 12 other species of astigmatid mites was confirmed using Cardinium specific primers (Kopecky et al. 2013). Further, the pyrosequencing analysis of A. siro bacteriome showed that the bacteriome is formed by two dominant groups, i.e. Bartonella-like and Solitalea-like bacteria covering almost 75 and 25 % of sequences, respectively (Hubert et al. 2016). Additionally, Solitalea-like symbionts were identified in the cloned amplicons of bacterial 16S rRNA primers from Phillips and Dog populations of T. putrescentiae (Erban et al. 2016a). Using the taxon-specific primers, Solitalea-like bacteria were found in four populations of T. putrescentiae including the eggs indicating that the bacterium is transmitted vertically (Erban et al. 2016a). The results showed that T. putrescentiae populations varied in the amount of these bacteria. The data indicated that Cardinium and Solitalea-like bacteria can co-exist in both A. siro and T. putrescentiae (Erban et al. 2016a). However, the changes observed in the bacterial communities of A. siro laboratory populations may suggest that the two groups replace each other at the population level. The fact that the analyses were done on only one A. siro population was a weakness of the previous studies (Hubert et al. 2012, 2016; Kopecky et al. 2014).
In this study, we analyzed Solitalea-like and Cardinium bacteria associated with three populations of the stored product mite A. siro. Conventional PCR and qPCR were used to analyse samples of adults/juveniles and eggs. Fluorescent in situ hybridization (FISH) was used to localize Solitalea-like bacteria inside the mite bodies. The antibiotic treatment was used to suppress the symbiotic bacteria thereby showing strength of interaction between the symbionts and their hosts. We investigated temporary variations of the intracellular symbiotic bacteria after rifampicin and tetracycline treatments and pre-treatments. This study aimed to answer the following questions: (1) Do Solitalea-like bacteria form one or more clusters when 16S rRNA gene sequences are analyzed? (2) Are Cardinium and Solitalea-like bacteria present in various populations of A. siro? (3) Are Solitalea-like bacteria present in mite body and in the eggs? And (4) do the Solitalea-like bacteria affect the fitness of A. siro?
Materials and methods
Mites
Acarus siro populations and sampling design are given in Table 1. The mites were cultivated on a 25 cm2 surface area in 70 mL tissue culture flasks (IWAKI flasks; Cat. No. 3100-025; Sterilin, Newport, UK). A constant 85 % RH was maintained with saturated KCl solutions in a Secador desiccator (Bel-Art Products, Pequannock, NJ, USA), which was in an air-conditioned, dark room maintained at 25 °C. The mites were reared on a wheat-derived diet designed for stored product mites (SPMd), which included a mixture of oat flakes, wheat germ and Pangamin-dried yeast extract (Rapeto, Bezdruzice, Czechia) (10:10:1 w/w) (Erban and Hubert 2008). The populations are maintained by transferring of ca. 1000 mites into the new diet and new flasks every month. The unsexed mites of known age were used for experiments. The mites were collected by a bush under STEMI 2000-C dissection microscope (Carl Zeiss, Jena, Germany). The samples of fresh mites were weighed using a microbalance, and a weight of 10 mg each was transferred to Eppendorf tubes in 6 replicates per population (LAB, ZVO, and LET in Table 1). The samples of mites were stored at −40 °C until they were used for the extraction of DNA. The eggs were obtained using a modified method of Stepien and Rodriguez (1973). The food with mites from rearing flasks was placed on mesh with a size of 176 µm under the water surface. All of the used mesh was polyamide (Silk & Progress, Brnenec, Czechia). The females deposited eggs on the water surface after 48 h. The water was collected and filtrated though the mesh manifold (Stepien and Rodriguez 1973) using a vacuum pump. The mesh sizes in the manifold were in decreasing order: 411, 300, 206, 176, 139, 109, 86, 42 µm diameter. Next, the eggs were cleaned with ddH20, Tween 20 (Cat No. P9416, Sigma-Aldrich, St Louis, MO, USA), bleach, and 80 % ethanol. The eggs were captured at 86 and 42 µm mesh and removed by pipetting into Eppendorf tubes and stored in 80 % ethanol. Each sample consisted of 50 eggs. The design included three replicates per LAB population.
DNA extraction
One mL of bleach (5 % solution of sodium hypochlorite) was added to the Eppendorf tubes with the mites or eggs and left for 1 min. The sample was centrifuged at 3000g for 1 min. The bleach was replaced by 1 mL of pure ethanol for 1 min and centrifuged again at 3000g for 1 min. The ethanol was replaced by phosphate-buffered saline (PBST: 3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, and 135 mM NaCl) with 0.05 % w/w Tween 20 detergent to remove the surface microflora. Washing by PBST and Tween with subsequent centrifugation was repeated 3×. The mites or eggs were then homogenized in 100 µL of PBST in Tissue Grinders (Cat No. 440613, Radnoti, Monrovia, CA, USA). DNA was extracted from mite homogenates using tissue Genomic DNA Mini Kit (Cat No. GT100, Geneaid, New Taipei City, Taiwan) following the manufacturer’s protocol. The eggs were extracted using the Exgene Genomic DNA micro Kit (Cat No. 118-050, Gene All, Biotechnology, Seoul, South Korea) according to the manufacturer’s protocol. The extracted DNA was stored at −28 °C until analysis.
Standard PCR conditions
Based on the alignment of 20 16S rRNA gene sequences from A. siro (Hubert et al. 2012), specific primers for Solitalea-like bacteria were designed (see Table 2). To validate the prepared templates, PCR amplification of the 16S rRNA gene was performed with universal bacterial primers (Barbieri et al. 2001). Amplification was conducted in a C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA) in a 25 µL PCR reaction mixture, containing 200 µM dNTPs, 3 mM MgCl2, primers (100 nM each), 1.25 unit Taq polymerase (Top-Bio, Prague, Czechia) and 100–300 ng template DNA (including mite genomic DNA). The amplification conditions were: 3 min at 94 °C, 30 cycles of 110 s at 94 °C, 110 s at 50 °C for eubacterial primers and 56 °C for Solitalea-like bacteria specific primers (see Table 2), and 60 s at 72 °C, and a final extension for 5 min at 72 °C. The resulting PCR products were purified with Wizard® SV Gel and the PCR product clean-up system Kit (Promega, Madison, WI, USA) and were cloned using the pGEM®-T Easy Vector (Promega). Selected clones were sequenced by Macrogen (Seoul, South Korea).
Quantitative PCR
DNA samples of the mites included control, and rifampicin or tetracycline treated and pre-treated mites. The samples were used for bacterial quantification via the quantitative PCR (qPCR). The primers are shown in Table 2. To avoid the influence of chloroplast DNA to analyse, we selected Com1 and 769R primers for quantification of total bacteria copies (Dorn-In et al. 2015). New taxon-specific primers were designed for quantification of Solitalea-like and Cardinium bacteria (Table 2). The qPCR standard was prepared from a cloned 16S rRNA gene amplicon (pGEM®-T Easy Vector, Promega) from the Solitalea and Cardinium specific primers. The competent bacterial cells with plasmids were inoculated in the LB medium (Himedia, Mumbai, India) with ampicillin 0.1 g/L (Cat No. A01104.0005, Duchefa Biochemie, Haarlem, The Netherlands) for 16 h at 37 °C. The plasmid was then purified with a Wizard Plus SV Mninipreps DNA purification system (Cat No. A1330, Promega) according to the manufacturer’s protocol. Plasmids were linearized by SacI restriction (Cat No. R6061, Promega) and cleaned with a Wizard SV gel and PCR Clean-Up system (Cat No. A9285). The concentration of the cleaned product was measured on P330 Implen NanoPhotometer (Munich, Germany) and adjusted to 10 ng of DNA for each reaction. The standard of DNA was diluted by 1/10. Amplifications were conducted using a StepOnePlus™ Real-Time PCR System (Life Technologies, Grand Island, NY, USA). SYBR green (Bio-Rad Laboratories, The Netherlands) was used as a double-stranded DNA (dsDNA) binding dye. Baseline and threshold calculations were performed with the StepOnePlus software. The amplification consisted of 40 cycles, including a denaturation of 30 s at 95 °C, annealing for 35 s at 54–60 °C (Table 2), and elongation for 45 s at 72 °C. Melting curves were recorded to ensure qPCR specificity. The qPCR measurements were conducted in duplicate. We used design for six biological replicates each analyzed in two technical repeats.
Phylogenetic analyses
Partial 16S rRNA gene sequences of Solitalea-like bacteria were assembled with CodonCode Aligner, v.1.5.2 (CodonCode, Dedham, MA, USA) and assigned to bacterial taxonomy using the Ribosomal database project naive Bayesian classifier (Wang et al. 2007). The sequences were aligned using SILVA Incremental Aligner v.1.2.11 (Pruesse et al. 2012). The best-fit model of nucleotide substitution was selected using jModelTest v.2.1.7 (Guindon and Gascuel 2003; Darriba et al. 2012). Based on the selection, a model GTR with a proportion of invariable sites (+I) and a gamma distribution with four rate categories (+G) was used to infer phylogeny through Bayesian analysis with PhyloBayes-MPI v.1.4e (Jow et al. 2002; Lartillot et al. 2009; Rodrigue and Lartillot 2014) and maximum-likelihood analysis in PhyML v.3.0 (Guindon et al. 2010). The phylograms were finalized using Figtree v.1.4.2 (http://tree.bio.ed.ac.uk/).
Fluorescent in situ hybridization
FISH was performed using specific bacterial probes (Table 2) on LAB population. From the control diet group, ca. 30 mites were transferred into 4 % formaldehyde and whole mite specimens were hybridized according to a previously described protocol (Perotti et al. 2007). For the hybridizations, samples were incubated at 45 °C in darkness for a maximum of 20 h and then washed for 1 h with the same hybridization buffer, followed by washing in PBTA (phosphate buffer with Triton X-100 plus sodium azide) at room temperature. Samples were mounted in PBS/glycerol and examined under a microscope.
The bacterial probes Cy3 and Cy5 (Eurofins Genomics, Ebersberg, Germany) used in this experiment (Table 2) were equimolar mixed in the hybridization buffer (following remarks of ProBase). No probe and competition suppression controls using excess unlabeled probes were performed. The hybridized specimens were visualized using a Confocal Zeiss LSM510 microscope (Carl Zeiss) with a Coherent Multiphoton laser. Length measurements were performed using the Zeiss LSM Image Examiner.
The growth experiment with antibiotics
Antibiotic diets preparation
The diet was derived from the house dust mite diet (HDMd), which is composed of dog food (Ontario-pet, Placek, Podebrady, Czechia), wheat germ, dried fish food (LonBio, Aqua Tropic Lonsky, Prague, Czechia), Mauripan-dried yeast extract (Rapeto, Bezdruzice, Czechia) and gelatin (Serva Electrophoresis, Heidelberg, Germany) (10:10:3:2:1 w/w) (Erban and Hubert 2008). The antibiotics rifampicin (Cat No. R3501; Sigma-Aldrich) and tetracycline (Cat No. T3258; Sigma-Aldrich) were diluted in HPLC-grade methanol and incorporated into the diet to obtain concentrations of 0 (control), 0.1, 1, 5, 10, 50 and 100 mg g−1. The resulting combination was properly mixed using a MS1 Minishaker (IKA, Staufen, Germany). The 50-mL centrifuge tubes containing the diet were covered with a filter, fixed with a vented cap and lyophilized in a PowerDry LL3000 lyophilizer (Thermo, Shanghai, China). The lyophilized material was ground into a powder in a pottery grinding mortar. Prior to the experiment, the material in the flasks was rehydrated 24 h before the experiment in desiccators using distilled water (Erban et al. 2012). The tested diets for the growth test were rifampicin or tetracycline diet at concentrations 0, 0.1, 1, 5, 10, 50 and 100 mg g−1 on LAB and ZVO populations of mites. The growth test started by adding 100 ± 5 mg of the experimental diet and 10 unsexed mites into IWAKI flask. The flasks with the diets and mites were incubated in the dark for 21 days in desiccator cabinets at 85 % RH and 25 °C. The experiment was terminated with the addition of Oudemans fluid (8 mL of acetic acid, 5 mL of glycerol and 87 mL of 70 % ethanol) to the flasks. Mites were counted under a dissection microscope (Carl Zeiss).
Antibiotic treated mite
Ca. 300 adults/juveniles were added to flasks with 100 ± 50 mg of HDMd, containing 5 mg g−1 of antibiotics or the control diet. The flasks were cultivated under the same conditions used for the population growth experiments. After 21 days, the mites were placed into sterile Eppendorf tubes. Samples of fresh weight of 10 ± 1 mg of mites were weighed using a microbalance. Six replicates per treatment were prepared. The samples were coded as LAB-Rif-Tre, LAB-Tet-Tre, ZVO-Rif-Tre and ZVO-Tet-Tre (Table 1). The samples were stored in a deep freezer at −40 °C until use.
Antibiotic pre-treated mites: Ten mite adults originated from previous mass production, i.e. diets with 5 mg g−1 of rifampicin or tetracycline and control were transferred into IWAKI flasks on HDMd 100 ± 5 mg and incubated for 21 days in the same conditions as growth experiments. The mites were collected during next moth according to the actual population density in the flask. The samples were coded as LAB-Rif-Pre, LAB-Tet-Pre, ZVO-Rif-Pre and ZVO-Tet-Pre (see Table 1). The samples were stored in a deep freezer at −40 °C until use.
Data analyses
Numbers of copies of bacteria, Solitalea and Cardinium 16S rRNA gene fragment
The design usually included six biological and two technical replicates. The data were log-transformed and analyzed by the Kruskal–Wallis nonparametric test, using Dunn potshot comparison and a Bonferroni correction.
Growth test
First, we compared the population growth of A. siro on HDMd diet, when the populations were factor, by t test. In the next step, the population numbers (N) of A. siro after 21 days of cultivation had a normal distribution, and data were analyzed by analysis of covariance (ANCOVA). Population numbers were dependent, and type of antibiotics, concentration, mite population and their interactions were independent variables. The ANCOVA models had higher R2 values when the antibiotic concentration (x) was transformed according to the formula ln[x + 1.10−7] than the models that were not transformed. Finally, the interaction between the final population number and the antibiotic concentration was analyzed separately for each antibiotic type and mite species using regression models. Effective doses of antibiotic concentrations that reduced the final population to 50 % (EC50) compared to the control were estimated from the model with 95 % confidence intervals.
To evaluate the effect of antibiotic pretreatment on the fitness of A. siro, final population numbers (N) were analyzed using ANOVA separately for each mite population. In the model, the dependent variable was the final population size, and the factors were the control, rifampicin and tetracycline treatment and pretreatment. A Tukey post host comparison of means was used to identify samples with different population growth. The analyses were conducted in XLSTAT 2007 (Addinsoft, New York, NY, USA) and QC-Expert (TriloByte Statistical Software, Pardubice, Czechia).
Results
Phylogenetic analysis of Solitalea-like bacteria
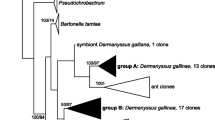
The 16S rRNA sequences classified as Solitalea-like group were obtained in previous studies by cloning of nearly full-length 16S rRNA gene amplified from total DNA isolated from the whole-body homogenates of mites using universal bacterial primers (Hubert et al. 2012; Kopecky et al. 2014; Erban et al. 2016a). A set of 110 Solitalea-like clones were analyzed together with the reference sequences including 191 type-strains of the order Sphingobacteriales, 27 closest sequences retrieved by BLAST search in GenBank database, and 29 other strains and clones classified within the genus Solitalea in the Ribosomal database project database. Phylogeny was inferred by Bayesian and Maximum-likelihood analyses employing the GTR model with a proportion of invariable sites (+I) and a gamma distribution with four rate categories (+G) chosen according to the Akaike information criterion. All Solitalea-like clones formed a new distinct cluster separated from the neighboring clusters of the genus Solitalea and of uncultured bacteria associated with amoebae and sweet water or soil habitats. The phylogenetic analysis did not show a close relatedness of the three clusters, but it supported the common node shared with the other Sphingobacteriaceae genera (Fig. 1).
Phylogenetic analysis of Solitalea-like clones from Acarus siro and Tyrophagus putrescentiae. Phylogeny was inferred by Bayesian analysis employing the GTR model with a proportion of invariable sites (+I) and a gamma distribution with four rate categories (+G). The initial alignment consisted of 110 nearly full-length sequences of the 16S rRNA gene from this study, 20 sequences from Hubert et al. (2012) and 247 reference sequences including 191 type-strains representing the order Sphingobacteriales, 27 closest sequences retrieved by BLAST search in the GenBank database, and 29 other sequences classified in the genus Solitalea in the Ribosomal database project database. Branch lengths correspond to mean posterior estimates of evolutionary distances (scale bar 0.3). Branch labels indicate the Bayesian posterior probability and supporting bootstrap value from maximum-likelihood analysis. The phylogram was rooted using Cytophaga fermentans sequence M58766 as an outgroup
Differences in Solitalea-like and Cardinium bacteria in populations of Acarus siro
Based on the conventional PCR using taxon-specific primers the presence of Solitalea-like and Cardinium bacteria differed in the DNA samples from A. siro populations (Table 3). Cardinium was identified in LET population only, whereas Solitalea-like bacteria was present in the DNA samples from all populations. The Cardinium presence was confirmed by qPCR in all populations. The numbers of bacterial 16S rRNA copies differed among the populations based on qPCR with universal Com1 and 769R primers (K33,2 = 17.573, P < 0.001) (Fig. 2a). The numbers decreased in the order from LET, LAB to ZVO. The numbers of Cardinium and Solitalea-like bacterial 16S rRNA gene copies also differed among populations (Fig. 2a). The Solitalea-like bacteria copy numbers differed among populations (K2,30 = 24.881, P < 0.001) (Fig. 2a). The LET population had 10× higher Solitalea-like copy numbers than LAB and ZVO populations. The differences in Cardinium copy numbers were significant among the populations (K2,33 = 31.135, P < 0.001). ZVO population had 10× and 100× higher Cardinium copy numbers than LET and LAB populations, respectively (Fig. 2a).
The quantification of bacteria, Solitalea-like and Cardinium 16S rRNA copies per one mite by qPCR in samples and the effect of antibiotic pretreatment to mite growth. The points are means, bars indicate standard deviations and the letters indicated significant differences (P < 0.05) based Kruskal–Wallis nonparametric test, using Dunn potshot comparison and a Bonferroni correction. a The numbers of Bacteria, Solitalea-like and Cardinium in samples of three populations of Acarus siro; b the effect of antibiotic pretreatment (5 mg g−1) on the final population density of A. siro reared on HDMd; c the effect of antibiotic treatment (5 mg g−1) and antibiotic pretreatment (5 mg g−1) to the numbers of bacteria and Solitalea-like bacteria in the samples of A. siro form the laboratory (LAB) and Zvoleneves (ZVO) populations. Let Lettuce populations, Con control (HDMd diets without any antibiotics), Tet tetracycline, Rif rifampicin, Tre treatment (antibiotic in the diet), Pre pretreatment (after antibiotic application on HDMd diet)
Localization of Solitalea-like bacteria by FISH and detection of bacteria in eggs using PCR in LAB population
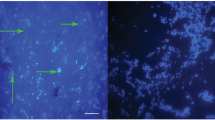
Cells of Solitalea-like bacteria were localized inside the digestive tract (i.e., cells of colon, diverticula, food bolus), reproductive tract and fat bodies in larvae, nymphs, males and females (Fig. 3). In multi-infected specimens, bacteria were observed at different locations. Cardinium bacteria were detected in reproductive tissues (Fig. 3b). In A. siro eggs, Solitalea-like bacteria were detected using the group-specific primers with the exception of LET population, whereas Cardinium-specific primers yielded no specific amplicon (Table 3).
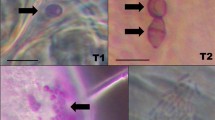
Localization of Solitalea-like bacteria in the body of Acarus siro using fluorescent in situ hybridization. a Single infection by Solitalea-like bacteria indicated by white arrows. b Multiple infection by Solitalea-like (green color, white arrows)/Cardinium bacteria (red color, black arrows). The Solitalea-like probe is Cy5, whereas Cy3 was a probe for Cardinium. c colon, fb food bolus, ov ovipositor, pc postcolon, pd diverticula
The growth test
Mite growth in control (HDM) diet
Acarus siro population densities on HDMd significantly differed between ZVO and LAB populations (T1,20 = 2.85; P = 0.01). The population density of ZVO populations was 1.4× higher compare to LAB population. The antibiotic supplemented to the diet suppressed the growth of mites (ANCOVA model: F10,133 = 37, P < 0.001). The population density was influenced by antibiotic concentration (F1,142 = 33, P < 0.001), but not by population (F1,142 = 0.82, P = 0.37) or type of antibiotic (F1,142 = 1.64, P = 0.20). However, the interaction of antibiotic concentration with population had a significant effect (F4,139 = 2.48, P < 0.05). The effect of antibiotics on A. siro population density is shown in Fig. 4. EC50 indicated that the LAB population was more susceptible to both antibiotics than ZVO (Fig. 4b). EC50 was found 13× and 40× higher in ZVO-Tet-Tre and ZVO-Rif-Tre than in LAB-Tet-Tre and LAB-Rif-Tre populations, respectively.
The effect of addition of the antibiotics tetracycline (Tet) or rifampicin (Rif) to HDMd on the final population size (N) of Acarus siro. The regression models described the interaction between ln(x + 1 × 10−7) transformed antibiotic concentration and population density (a). Fitted values of EC50 of antibiotic concentration reduced the final population density by 50 % compared to the control (b). LAB laboratory, ZVO Zvoleneves, LET Lettuce populations, Con control (HDMd diets without any antibiotics)
Effect of antibiotic pretreatment on the mite growth on the control (HDMd) diet
Pretreatment by antibiotics (5 mg g−1) significantly influenced the population growth of mites on the HDMd of LAB and ZVO populations (F2,32 = 15.489 and F2,31 = 26.844, both P < 0.001) (Fig. 2b). In the LAB population, rifampicin pretreatment (LAB-Rif-Pre) decreased the population density to 89 % of the control (LAB-Con). In the ZVO population, rifampicin pretreatment (ZVO-Rif-Pre) decreased the population density to 72 % of the control (ZVO-Con).
Effect of antibiotic treatment on numbers of bacteria and Solitalea-like bacteria
The numbers of bacteria and Solitalea-like bacteria 16S rRNA copies were compared in the mites on control diet, treated mites by 5 mg g−1 of rifampicin or tetracycline and in the mites pretreated by 5 mg g−1 of rifampicin or tetracycline. The effects of treatment and pretreatment to the numbers of bacteria and Solitalea-like bacteria were different. The pretreatment of mites significantly reduced the numbers of bacteria in both populations (K4,64 = 35.57 and 23.92, both P < 0.001, for LAB and ZVO, respectively) (Fig. 2b). However, no effect of antibiotic treatment and pretreatment was observed on Solitalea-like bacteria in both populations (K4,22 = 3.896, P = 0.44 and K4,22 = 7.225, P = 0.12, for LAB and ZVO, respectively) (Fig. 2b).
Discussion
Solitalea-like bacteria in astigmatid mites
Among the neighboring Solitalea taxa, according to our phylogenetic analysis (Fig. 1), the known Solitalea species S. koreenis and S. canadensis are considered free-living bacteria with representatives isolated from the soil (Weon et al. 2009). Solitalea has an ability to hydrolyze gelatin, starch and maltose (Weon et al. 2009; Liu et al. 2016). Members of another Sphingobacteriaceae genus, Pedobacter, were reported to be associated with the glassy-winged sharpshooter and bees (Mohr and Tebbe 2006; Lacava et al. 2007). Sphingobacterium spp. were reported from the gut of longhorn beetles (Batocera horsfieldi) and are suggested as symbionts and xylanase producers (Zhou et al. 2009). A hypothetical ability of Solitalea-like bacteria to hydrolyze starch-like substrates would correspond to the digestion of starch-type substrates in A. siro (Hubert et al. 2005; Erban et al. 2009). Bacteria could compete for or participate in utilization of plant starch from the grain diets. It would be supported by enzyme profiles of known Solitalea species (Weon et al. 2009; Liu et al. 2016). The important findings of this analysis is that Solitalea-like bacteria from two populations of A. siro and two populations of T. putrescentiae are closely related forming a cluster separated from other Sphingobacteriaceae genera. However for verification, future isolation and genome characterization of the Solitalea-like bacteria would be necessary.
Both Solitalea-like and Cardinium bacteria co-occur in mite populations
The present study has demonstrated the co-occurrence of Solitalea-like and Cardinium bacteria in all investigated populations of A. siro at the population and individual levels. At the population level, the numbers of Solitalea-like bacteria are much higher than those of Cardinium. Since Solitalea-like bacteria appear as new symbionts, to our knowledge, no information is available about the co-infection of these bacteria. Co-infection has been reported for Cardinium and Wolbachia (Weeks et al. 2003; Zchori-Fein and Perlman 2004; Gotoh et al. 2007; Duron et al. 2008). For example, a Tetranychus phaselus spider mite population was simultaneously infected by Cardinium and two distinct lineages of Wolbachia (Zhao et al. 2013). In some cases of multi-infested mites, only one symbiont is present in the eggs and is vertically transmitted. Both Wolbachia and Cardinium were found in the predatory mite Metaseiulus occidentalis, whereas in the eggs, only Cardinium were detected (Wu and Hoy 2012b). Here we found 100× lower numbers of Cardinium than of members of the Solitalea-like group in A. siro. In addition, we did not detect Cardinium in the eggs by taxon-specific primers, whereas Solitalea-like bacteria were present in the eggs. It means that Cardinium were rare in bodies of A. siro and under the detection limits in the mite bodies and eggs by conventional PCR. An alternative explanation of the absence in eggs might be that Cardinium were not vertically transmitted in A. siro populations used in the study. A further study employing high numbers of individuals would be necessary to solve the question regarding the vertical transmission.
Changes in symbiotic bacterial populations during long-term growth in the rearing facility
Our data indicated the disappearance of Cardinium infection in the laboratory strain of A. siro and replacement by Solitalea-like bacteria during a long-term cultivation lasting for several years in the rearing facility. An investigation of the LAB population in 2010 indicated the presence of both Cardinium and Solitalea-like bacteria in that population (Kopecky et al. 2014). However, a more recent study of the same population in 2012 showed Solitalea-like and Bartonella-like bacteria predominating, whereas Cardinum was represented only by one sequence in a subsample dataset (Hubert et al. 2016). Later, in 2014, almost complete replacement of Cardinium by Solitalea-like bacteria was observed in this study. In summary, these results suggest that during 4 years of observation within one laboratory-reared population, Cardinium were replaced by another group of Solitalea-like bacteria. Further experiments are necessary to address such symbiont replacements in mites, and the physiological studies should uncover whether and how such replacements may or may not be beneficial for the host.
Localization of Solitalea-like and Cardinium bacteria
Based on the FISH localization, Solitalea-like bacteria were present in the digestive tract, reproductive organs and fat bodies. Such distribution is similar to the known distribution of Cardinium in Brevipalpus mites (Kitajima et al. 2007). We also found Solitalea-like bacteria in both sexes and all developmental stages. The presence of bacteria in the eggs provided the first evidence that these bacteria were vertically transmitted. The eggs were surface cleaned by bleaching a method recommended for symbiont detection in eggs by PCR (Wu and Hoy 2012b). The localization of Solitalea-like infection in reproductive organs, where the infection is transferred to the offspring and the presence of it in the eggs confirms transovarial transmission and rules out external contamination or horizontal transmission. Here we found Solitalea-like bacteria inside the gut, but also in the internal organs. Previously, it was found that Candidatus Erwinia dacicola resided intracellularly in the gastric ceca of the larval midgut but extracellularly in the lumen of the foregut and ovipositor diverticulum of adult olive flies (Bactrocera oleae) (Estes et al. 2009). Our results indicated that such distribution is also possible for Solitalea-like bacteria.
In specimens of A. siro with multiple infections of Solitalea-like bacteria and Cardinium, we observed a spatial separation of bacteria in their bodies. However, in some individuals, multiple infections were detected in reproductive organs. The reproductive organs are the source of the vertical transfer of bacteria, which is known to be the case for Cardinium (Ros et al. 2012).
Antibiotic treatment did not eliminate Solitalea-like bacteria
Antibiotic treatment is suggested as a useful tool for elimination of parasitic bacteria in mites and observation of cytoplasmic incompatibility, for instance, in Tetranychus urticae and T. turkestani (Breeuwer 1997). For example, tetracycline treatment eliminated Wolbachia and Cardinium in Neoseiulus paspalivorus females (Sourassou et al. 2014) and penicillin-cured females of Eotetranychus suginamensis were free of Cardinium (Gotoh et al. 2007). Tetracycline eliminated Cardinium in Brevipalpus phoenicis (Groot and Breeuwer 2006) and Tetranychus pierce (Zhu et al. 2012). Antibiotics added to the diet of Pieris rapae larvae (Lepidoptera) were used to detect previously unobserved bacterial species after perturbation of the gut bacterial community (Robinson et al. 2010). The dose 5 mg g−1 of tetracycline and rifampicin in the diet did not eliminate Solitalea-like bacteria in A. siro. Similar results were reported previsouly, when we found that Solitalea-like bacteria were still present in the clones of bacteria from A. siro after neomycin and streptomycin treatments (1 mg g−1) of A. siro (Kopecky et al. 2014). Here, we found that in antibiotic pretreated A. siro the total number of bacteria is 10× lower than on control diet. It means that the relative proportion of Solitalea-like bacteria is increasing in A. siro bacteriome after antibiotic treatment. We also observed different responses in terms of population growth suppression between populations and antibiotics. The fitted values of antibiotic concertation for 50 % suppression of the population growth of mites were at least 5× lower for ZVO population than that of LAB population. We observed 1.4× higher population growth, suggested as an indirect estimator of fitness (Erban and Hubert 2008), of ZVO population than of LAB population. The pretreatment of mites by rifampicin and tetracycline (5 mg g−1) did not increase fitness, contrary to the previously observed effect of streptomycin and neomycin (10 mg g−1) (Kopecky et al. 2014). Moreover, the pretreatment by rifampicin resulted in the decrease of population fitness in comparison to tetracycline pretreatment and the control situation in both A. siro populations. It seemed likely that rifampicin eliminated some beneficial symbionts in A. siro, whereas neomycin and streptomycin suppressed parasitic or pathogenic bacteria. ZVO population was found to have higher fitness, higher sensitivity to antibiotics and higher numbers of Solitalea-like bacteria. With respect to the higher effect of antibiotics on Solitalea-like bacterial numbers and fitness decrease, the suggestion is that the Solitalea-like group might be more important in the ZVO than in the LAB A. siro population.
References
Armentia A, Fernandez A, Perez-Santos C, de la Fuente R, Sanchez P, Sanchis F, Mendez J, Stolle R (1994) Occupational allergy to mites in salty ham, chorizo and cheese. Allergol Immunopathol 22:152–154
Armentia A, Martinez A, Castrodeza R, Martinez J, Jimeno A, Mendez J, Stolle R (1997) Occupational allergic disease in cereal workers by stored grain pests. J Asthma 34:369–378
Athanassiou CG, Kavallieratos NG, Sciarretra A, Palyvos NE, Trematerra P (2011) Spatial associations of insects and mites in stored wheat. J Econ Entomol 104:1752–1764
Barbieri E, Paster BJ, Hughes D, Zurek L, Moser DP, Teske A, Sogin ML (2001) Phylogenetic characterization of epibiotic bacteria in the accessory nidamental gland and egg capsules of the squid Loligo pealei (Cephalopoda: Loliginidae). Environ Microbiol 3:151–167
Bigliardi E, Sacchi L, Genchi M, Alma A, Pajoro M, Daffonchio D, Marzorati M, Avanzati AM (2006) Ultrastructure of a novel Cardinium sp. symbiont in Scaphoideus titanus (Hemiptera: Cicadellidae). Tissue Cell 38:257–261
Breeuwer JAJ (1997) Wolbachia and cytoplasmic incompatibility in the spider mites Tetranychus urticae and T. turkestani. Heredity 79:41–47
Breeuwer H, Ros VID, Groot TVM (2011) Cardinium: the next addition to the family of reproductive parasites. In: Zchori-Fein E, Bourtzis K (eds) Manipulative tenants: bacteria associated with arthropods. CRC Press, Boca Raton, pp 207–224
Chang J, Masters A, Avery A, Werren JH (2010) A divergent Cardinium found in daddy long-legs (Arachnida: Opiliones). J Invertebr Pathol 105:220–227
Dallai R, Mercati D, Giusti F, Gottardo M, Carapelli A (2011) A Cardinium-like symbiont in the proturan Acerella muscorum (Hexapoda). Tissue Cell 43:151–156
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Dorn-In S, Bassitta R, Schwaiger K, Bauer J, Holzel CS (2015) Specific amplification of bacterial DNA by optimized so-called universal bacterial primers in samples rich of plant DNA. J Microbiol Methods 113:50–56
Duron O (2013) Lateral transfers of insertion sequences between Wolbachia, Cardinium and Rickettsia bacterial endosymbionts. Heredity 111:330–337
Duron O, Hurst GDD, Hornett EA, Josling JA, Engelstadter J (2008) High incidence of the maternally inherited bacterium Cardinium in spiders. Mol Ecol 17:1427–1437
Erban T, Hubert J (2008) Digestive function of lysozyme in synanthropic acaridid mites enables utilization of bacteria as a food source. Exp Appl Acarol 44:199–212
Erban T, Erbanova M, Nesvorna M, Hubert J (2009) The importance of starch and sucrose digestion in nutritive biology of synanthropic acaridid mites: alpha-amylases and alpha-glucosidases are suitable targets for inhibitor-based strategies of mite control. Arch Insect Biochem Physiol 71:139–158
Erban T, Rybansky J, Hubert J (2012) The efficacy of four avermectins on the synanthropic mite Lepidoglyphus destructor under laboratory conditions. Exp Appl Acarol 58:43–50
Erban T, Klimov PB, Smrz J, Phillips TW, Nesvorna M, Kopecky J, Hubert J (2016a) Populations of stored product mite Tyrophagus putrescentiae differ in their bacterial communities. Front Microbiol 7:1046
Erban T, Rybanska D, Harant K, Hortova B, Hubert J (2016b) Feces derived allergens of Tyrophagus putrescentiae reared on dried dog food and evidence of the strong nutritional interaction between the mite and Bacillus cereus producing protease bacillolysins and exo-chitinases. Front Physiol 7:53
Estes AM, Hearn DJ, Bronstein JL, Pierson EA (2009) The olive fly endosymbiont, ‘Candidatus Erwinia dacicola’, switches from an intracellular existence to an extracellular existence during host insect development. Appl Environ Microbiol 75:7097–7106
Fukuzawa AH, Vellutini BC, Lorenzini DM, Silva PI Jr, Mortara RA, da Silva JM, Daffre S (2008) The role of hemocytes in the immunity of the spider Acanthoscurria gomesiana. Dev Comp Immunol 32:716–725
Gotoh T, Noda H, Ito S (2007) Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98:13–20
Groot TVM, Breeuwer JAJ (2006) Cardinium symbionts induce haploid thelytoky in most clones of three closely related Brevipalpus species. Exp Appl Acarol 39:257–271
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Hess RT, Hoy MA (1982) Microorganisms associated with the spider mite predator Metaseiulus (= Typhlodromus) occidentalis: electron microscope observations. J Invertebr Pathol 40:98–106
Hubert J, Stejskal V, Munzbergova Z, Kubatova A, Vanova M, Zdarkova E (2004) Mites and fungi in heavily infested stores in the Czech Republic. J Econ Entomol 97:2144–2153
Hubert J, Doleckova-Maresova L, Hyblova J, Kudlikova I, Stejskal V, Mares M (2005) In vitro and in vivo inhibition of alpha-amylases of stored-product mite Acarus siro. Exp Appl Acarol 35:281–291
Hubert J, Kopecky J, Perotti MA, Nesvorna M, Braig HR, Sagova-Mareckova M, Macovei L, Zurek L (2012) Detection and identification of species-specific bacteria associated with synanthropic mites. Microb Ecol 63:919–928
Hubert J, Kopecky J, Sagova-Mareckova M, Nesvorna M, Zurek L, Erban T (2016) Assessment of bacterial communities in thirteen species of laboratory-cultured domestic mites (Acari: Acaridida). J Econ Entomol. 109:1887–1896
Jow H, Hudelot C, Rattray M, Higgs P (2002) Bayesian phylogenetics using an RNA substitution model applied to early mammalian evolution. Mol Biol Evol 19:1591–1601
Kitajima EW, Groot TV, Novelli VM, Freitas-Astua J, Alberti G, de Moraes GJ (2007) In situ observation of the Cardinium symbionts of Brevipalpus (Acari: Tenuipalpidae) by electron microscopy. Exp Appl Acarol 42:263–271
Kopecky J, Perotti MA, Nesvorna M, Erban T, Hubert J (2013) Cardinium endosymbionts are widespread in synanthropic mite species (Acari: Astigmata). J Invertebr Pathol 112:20–23
Kopecky J, Nesvorna M, Mareckova-Sagova M, Hubert J (2014) The effect of antibiotics on associated bacterial community of stored product mites. PLoS ONE 9:e112919
Kurtti TJ, Munderloh UG, Andreadis TG, Magnarelli LA, Mather TN (1996) Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. J Invertebr Pathol 67:318–321
Lacava PT, Parker J, Andreote FD, Dini-Andreote F, Ramirez JL, Miller TA (2007) Analysis of the bacterial community in glassy-winged sharpshooter heads. Entomol Res 37:261–266
Lartillot N, Lepage T, Blanquart S (2009) PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 25:2286–2288
Liu FF, Kulinich A, Du YM, Liu L, Voglmeir J (2016) Sequential processing of mannose-containing glycans by two alpha-mannosidases from Solitalea canadensis. Glycoconj J 33:159–168
Marx JJ Jr, Twiggs JT, Ault BJ, Merchant JA, Fernandez-Caldas E (1993) Inhaled aeroallergen and storage mite reactivity in a Wisconsin farmer nested case-control study. Am Rev Respir Dis 147:354–358
Matalon Y, Katzir N, Gottlieb Y, Portnoy V, Zchori-Fein E (2007) Cardinium in Plagiomerus diaspidis (Hymenoptera: Encyrtidae). J Invertebr Pathol 96:106–108
McClymont-Peace D (1983) Reproductive success of the mite Acarus siro L. on stored cheddar cheese of different ages. J Stored Prod Res 19:97–104
Melnyk JP, Smith A, Scott-Dupree C, Marcone MF, Hill A (2010) Identification of cheese mite species inoculated on Mimolette and Milbenkase cheese through cryogenic scanning electron microscopy. J Dairy Sci 93:3461–3468
Mohr KI, Tebbe CC (2006) Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ Microbiol 8:258–272
Musken H, Franz JT, Wahl R, Paap A, Cromwell O, Masuch G, Bergmann KC (2000) Sensitization to different mite species in German farmers: clinical aspects. J Investig Allergol Clin Immunol 10:346–351
Nakamura Y, Yukuhiro F, Matsumura M, Noda H (2012) Cytoplasmic incompatibility involving Cardinium and Wolbachia in the white-backed planthopper Sogatella furcifera (Hemiptera: Delphacidae). Appl Entomol Zool 47:273–283
O’Neill SL, Giordano R, Colbert AME, Karr TL, Robertson HM (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA 89:2699–2702
Palyvos NE, Emmanouel NG, Saitanis CJ (2008) Mites associated with stored products in Greece. Exp Appl Acarol 44:213–226
Penz T, Schmitz-Esser S, Kelly SE, Cass BN, Muller A, Woyke T, Malfatti SA, Hunter MS, Horn M (2012) Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS Genet 8:e1003012
Perlman SJ, Magnus SA, Copley CR (2010) Pervasive associations between Cybaeus spiders and the bacterial symbiont Cardinium. J Invertebr Pathol 103:150–155
Perotti MA, Allen JM, Reed DL, Braig HR (2007) Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J 21:1058–1066
Pruesse E, Peplies J, Glockner FO (2012) SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829
Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J (2010) Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb Ecol 59:199–211
Rodrigue N, Lartillot N (2014) Site-heterogeneous mutation-selection models within the PhyloBayes-MPI package. Bioinformatics 30:1020–1021
Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ (2012) Diversity and recombination in Wolbachia and Cardinium from Bryobia spider mites. BMC Microbiol 12:S13
Sacchi L, Genchi M, Clementi E, Bigliardi E, Avanzati AM, Pajoro M, Negri I, Marzorati M, Gonella E, Alma A, Daffonchio D, Bandi C (2008) Multiple symbiosis in the leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): details of transovarial transmission of Cardinium sp. and yeast-like endosymbionts. Tissue Cell 40:231–242
Sanchez-Borges M, Suarez-Chacon R, Capriles-Hulett A, Caballero-Fonseca F, Iraola V, Fernandez-Caldas E (2009) Pancake syndrome (oral mite anaphylaxis). World Allergy Organ J 2:91–96
Sanchez-Borges M, Suarez Chacon R, Capriles-Hulett A, Caballero-Fonseca F, Fernandez-Caldas E (2013) Anaphylaxis from ingestion of mites: pancake anaphylaxis. J Allergy Clin Immunol 131:31–35
Santos-Garcia D, Rollat-Farnier P-A, Beitia F, Zchori-Fein E, Vavre F, Mouton L, Moya A, Latorre A, Silva FJ (2014) The genome of Cardinium cBtQ1 provides insights into genome reduction, symbiont motility, and its settlement in Bemisia tabaci. Genome Biol Evol 6:1013–1030
Sourassou NF, Hanna R, Breeuwer JAJ, Negloh K, de Moraes GJ, Sabelis MW (2014) The endosymbionts Wolbachia and Cardinium and their effects in three populations of the predatory mite Neoseiulus paspalivorus. Exp Appl Acarol 64:207–221
Stefanini A, Duron O (2012) Exploring the effect of the Cardinium endosymbiont on spiders. J Evol Biol 25:1521–1530
Stepien Z, Rodriguez JG (1973) Collecting large quantities of acarid mites. Ann Entomol Soc Am 66:478–480
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Weeks AR, Marec F, Breeuwer JAJ (2001) A mite species that consists entirely of haploid females. Science 292:2479–2482
Weeks AR, Velten R, Stouthamer R (2003) Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc Lond B 270:1857–1865
Weon HY, Kim BY, Lee CM, Hong SB, Jeon YA, Koo BS, Kwon SW (2009) Solitalea koreensis gen. nov., sp. nov. and the reclassification of [Flexibacter] canadensis as Solitalea canadensis comb. nov. Int J Syst Evol Microbiol 59:1969–1975
Wu K, Hoy MA (2012a) Cardinium is associated with reproductive incompatibility in the predatory mite Metaseiulus occidentalis (Acari: Phytoseiidae). J Invertebr Pathol 110:359–365
Wu K, Hoy MA (2012b) Extended starvation reduced and eliminated Wolbachia, but not Cardinium, from Metaseiulus occidentalis females (Acari: Phytoseiidae): a need to reassess Wolbachia’s status in this predatory mite? J Invertebr Pathol 109:20–26
Zchori-Fein E, Perlman SJ (2004) Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13:2009–2016
Zchori-Fein E, Perlman SJ, Kelly SE, Katzir N, Hunter MS (2004) Characterization of a ‘Bacteroidetes’ symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus Cardinium hertigii’. Int J Syst Evol Microbiol 54:961–968
Zhao D-X, Chen D-S, Ge C, Gotoh T, Hong X-Y (2013) Multiple infections with Cardinium and two strains of Wolbachia in the spider mite Tetranychus phaselus Ehara: revealing new forces driving the spread of Wolbachia. PLoS ONE 8:e54964
Zhou J, Huang H, Meng K, Shi P, Wang Y, Luo H, Yang P, Bai Y, Zhou Z, Yao B (2009) Molecular and biochemical characterization of a novel xylanase from the symbiotic Sphingobacterium sp. TN19. Appl Microbiol Biotechnol 85:323–333
Zhu L-Y, Zhang K-J, Zhang Y-K, Ge C, Gotoh T, Hong X-Y (2012) Wolbachia strengthens Cardinium-induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Curr Microbiol 65:516–523
Acknowledgments
The authors are obligated to the referees for valuable comments of the draft. This study was supported by the project of the Ministry of Agriculture of the Czech Republic RO0415 and by Czech Science Foundation (GA CR) as the project number GA15-09038S. MAP was supported by The Royal Society, UK. We thank Martin Markovic for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hubert, J., Kopecky, J., Nesvorna, M. et al. Detection and localization of Solitalea-like and Cardinium bacteria in three Acarus siro populations (Astigmata: Acaridae). Exp Appl Acarol 70, 309–327 (2016). https://doi.org/10.1007/s10493-016-0080-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0080-z