Abstract
Wolbachia and Cardinium are maternally inherited intracellular bacteria that can manipulate the reproduction of their arthropod hosts, such as by inducing cytoplasmic incompatibility (CI). Although the reproductive alteration induced by Wolbachia or Cardinium have been well investigated, the effects of these two endosymbionts co-infecting the same host are poorly understood. We found that Tetranychus piercei McGregor is naturally infected with Wolbachia and Cardinium. We performed all possible crossing combinations using naturally infected and cured strains, and the results show that Wolbachia induced a weak level of CI, while Cardinium-infected and doubly infected males caused severe CI. Wolbachia and Cardinium could not rescue CI each other; however, Wolbachia boosted the expression of Cardinium-induced CI. Quantitative PCR results demonstrated that CI was associated with the infection density of Wolbachia and Cardinium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many arthropods are infected with diverse bacterial endosymbionts. These intracellular bacteria manipulate their host’s reproduction in such a way as to enhance their own spread through a population [38]. These manipulations include male killing, feminization, thelytokous parthenogenesis, and cytoplasmic incompatibility (CI, see below), and result in an increased proportion of infected females (the transmitting sex) in the host population. One of the most common of these endosymbionts is Wolbachia, which belong to the alpha subdivision of the phylum Proteobacteria and which are widespread among arthropods and nematodes. It has been estimated that more than 20 % of all arthropod species are infected with Wolbachia [37, 38]. CI is the most common effect of Wolbachia and has been described in arachnids and most groups of insects [7, 26, 37].
CI is a form of conditional infertility in which the cross between infected male and uninfected female are incompatible, whereas the reciprocal cross and self-crosses are compatible. In addition, bidirectional incompatibility can occur when two host lines are infected with different Wolbachia strains [2, 27]. The mechanisms of CI can be explained by the “modification–rescue” system [15, 36]. This system contains the Wolbachia-induced modification of sperm and the Wolbachia-induced rescue of that modification upon fertilization [36]. Following fertilization with a Wolbachia-modified sperm, the result is normal development in embryos from eggs harboring at least the same Wolbachia types as the father. Otherwise, abnormal mitosis occurs lacking the father’s Wolbachia type(s), which typically results in embryo’s death. Many studies have shown that incompatibility was manifested as a disruption of pronuclear chromatin condensation followed by missegregation of chromosomes during mitosis [9, 39]. The great majority of studies have shown that there is diversity among Wolbachia strains in both the modification and rescue function. A Drosophila study showed that several Wolbachia strains that cannot generate modifications in host sperm can still rescue the modifications caused by other strains as long as the two strains are sufficiently closely related [4]. Transfer of nine distantly related Wolbachia strains into the same host background showed that a given Wolbachia variant can possess multiple rescue determinants corresponding to different CI systems [43]. Some superinfections, i.e., infections with more than one strain of Wolbachia, can have additive effects. For example, a superinfected male is unidirectionally incompatible with both single-infected and uninfected females. The strength of CI caused by superinfected males is similar to the strength of CI from the strongest single infection when male is mated to singly infected or uninfected females [10, 20].
CI was once thought to be a unique phenotype of Wolbachia. Cardinium was the second bacterial lineage discovered to induce CI in arthropods [16]. Cardinium, which belong to the Cytophaga–Flavobacterium–Bacteroides phylum, have been found in 6–7 % of all arthropods [11, 35]. Cardinium-induced CI have been found in the parasitoid wasp Encarsia pergandiella, the spider mite Eotetranychus suginamensis, the spider mite Bryobia sarothamni, and the carmine spider mite Tetranychus cinnabarinus [12, 16, 24, 42]. The mechanism of Cardinium-induced CI is unclear.
Many factors, including host genotype, the bacterial strain, bacterial density, and sperm cyst infection, have been proposed to affect the expression of CI [22, 31, 32, 34]. Bacterial density has been found to strongly affect the strength of CI in insects [3, 8]. The strength of CI differs in the planthoppers Laodelphax striatellus and Sogatella furcifera, and appears to be due to different amounts of Wolbachia in males [21]. It has also shown a positive correlation between the density of Wolbachia within pole cells and CI levels in Drosophila [30]. The bug Orius strigicollis is superinfected with two strains of Wolbachia, wOus1 and wOus2. wOus1 interferes with the ability of wOus2 to cause CI by suppressing wOus2 densities [33]. In addition, Cardinium density also affects CI strength in the carmine spider mite T. cinnabarinus, and a threshold level of Cardinium density may be required for the induction of CI [42]. Although the factors that influence strength of Wolbachia-induced CI have been widely investigated, the CI induced by doubly infected Wolbachia and Cardinium has been rarely reported.
Wolbachia and Cardinium have been found to co-infect the same host species [11, 12, 35, 44], especially in the spider mite. The reproductive phenotype and interactions of a co-infection of Wolbachia and Cardinium have been studied in the spider mite B. sarothamni and the parasitic wasp Encarsia inaron [24, 40]. In B. sarothamni, Cardinium-infected males induced severe CI; however, CI could not be induced by doubly infected and Wolbachia-infected males. The different phenotypes caused by Cardinium are related to the different Cardinium strains infected in singly and doubly infected individuals [24]. However, in E. inaron, Wolbachia caused CI, but Cardinium did not [40].
In this study, to elucidate how double infection of Wolbachia and Cardinium affects CI, we investigated a species of spider mite that is known to have both infections, Tetranychus piercei McGregor, using isofemale lines obtained from naturally infected and cured individuals. Specifically, we attempted to determine whether (1) one or both of the bacteria caused CI, (2) one of the bacteria could affect the expression and rescue of CI of the other, and (3) bacterial density affected the expression and rescue of CI.
Materials and Method
Sample Collection and Culture
The spider mite Tetranychus piercei McGregor was collected from soybean [Glycine max (L.) Merr.] leaves in Yulong, Yunnan Province, southwest of China. The singly Cardinium-infected line was generated by treating doubly infected mites with high temperatures (34 ± 1 °C, L:D = 16:8, RH 60 %) for six successive generations [28]. Most F6 individuals were singly infected Cardinium, but a small proportion had two symbionts. Progeny of the singly Cardinium-infected F6 females were retained, and PCR screening procedure was repeated for the following generations to ensure stable transmission of the Cardinium. To obtain the singly Wolbachia-infected line, penicillin G (0.1 %, w/v) was used to treat doubly infected mites for four successive generations [18, 42]. Progeny of the singly Wolbachia-infected F4 females were retained, and PCR screening procedure was repeated for the following generations to ensure stable transmission of the Wolbachia. The uninfected line was generated by treating doubly infected mites with tetracycline solution (0.1 %, w/v) for six successive generations [42]. For the sixth and following generations, 45 individuals were taken from the population and checked by PCR to confirm that the line was uninfected. These lines were maintained in a mass-rearing environment without antibiotics for about six generations before use, to avoid the potential side effects of antibiotic treatment. Mites were reared on a leaf of the common bean (Phaseolus vulgaris L.) placed on a water-saturated sponge mat in Petri dishes (9 cm in diameter) at 25 ± 1 °C, 60 % r.h. and under 16 L:8D photoperiod.
Screening of Symbiont Infection
We used PCR to check Wolbachia and Cardinium infection status during the initiation of the laboratory cultures. DNA was extracted from single mites using the cetyltrimethylammonium bromide (CTAB) extraction method as described previously [23]. All PCR reactions were run in 25 μl buffer using the TAKARA Taq kit (No. R001B; Takara Co., Ltd., Shiga, Japan): 16.3 μl H2O, 2.5 μl 10× buffer, 1.5 μl of 2.5 mM dNTP, 1.5 μl of 25 mM MgCl2, 0.2 μl of 5 U/μl Taq, 2 μl sample, and 0.5 μl each of 20 μm forward and reverse primer. Wolbachia was detected by amplification of wsp and ftsZ genes by PCR, using the primers wsp-81F/wsp-691R [6] and ftsZ-F/ftsZ-R [1]. Reactions were cycled 35 times at 94 °C for 30 s, 52 °C for 45 s, and 72 °C for 1 min. The presence of Cardinium was detected using PCR amplification of a part of the 16S rDNA and gyrB. Cardinium 16S rDNA was amplified using the primers CLOf and CLOr [35]. The gyrB gene was amplified using primers from Groot and Breeuwer [14]. Each PCR was run for one cycle at 94 °C for 2 min, 35 cycles at 94 °C for 30 s, 57 °C for 30 s, 72 °C for 30 s and a final extension of 5 min at 72 °C. Reagent negative and positive controls were included in the reactions. The PCR products were electrophoresed in a 1.0 % agarose gel in TAE/EtBr for 30 min at 120 V, and then photographed on a UV transilluminator.
Crossing Experiment
The effects of Wolbachia and/or Cardinium on host reproduction were established by combining doubly infected, singly infected, and uninfected mites (see Table 1 for crosses and their possible effects). Single females in the teleiochrysalis stage (the last developmental stage before adult emergence) were placed with a 1 day-old adult virgin male from the same culture on the same leaf disk. Males were discarded 2 days after the females reached adulthood. The mated females were allowed to oviposit for 5 days. Both males and females were checked by PCR to confirm their infection status. The eggs on the leaf disks were checked daily to determine unhatchability, mortality in immature stages, and the sex ratio (% males).
Groups of crosses were tested for differences in investigated traits using the software package SPSS version 16.0 (Chicago, IL, USA). Tests were performed for individual bacterial effects and for interactions between Wolbachia and Cardinium (Table 1). Data were first tested for normality (Kolmogorov–Smirnov test) and homogeneity of group variances (Levene’s test). Which possible, square-root, logarithmic or arcsine transformations were performed to attain normality and homogeneity of variance. A one-way analysis of variance was performed for each trait (number of eggs laid, unhatched eggs, and sex ratio (% males), number of offspring, number of sons, number of daughters, and mortality) separately to determine whether there was heterogeneity among different crosses with respect to each trait. If heterogeneity was significant, pairwise comparisons were performed using Tukey post hoc tests.
Quantitative PCR of Bacteria
Wolbachia and Cardinium infection levels were determined by Q-PCR. Ten of doubly infected Wolbachia and Cardinium, singly Wolbachia-infected and singly Cardinium-infected mites (male and female) of 1 day-old were collected separately. Adult mites were prepared and individually subjected to DNA extraction using TaKaRa MiniBEST Universal Genomic DNA Extraction Kit Ver.4.0. The sample DNA extracted from mites was diluted to 1 ng/μl with deionized and distilled water for consistent Q-PCR assay. The wsp gene of Wolbachia and the 16S rDNA gene of Cardinium were quantified using the ABI PRISM 7300 Real-Time PCR System. The Wolbachia primers were designed specifically to amplify the 124 bp region of the Wolbachia wsp gene (WF5′-AGCAATCCTTTAGTAACAGAG-3′ and WR5′-ATTAGCACCATAAGAACCA-3′). The Cardinium primers were designed specifically to amplify the 88 bp region of the16S rDNA gene (CF5′-ATGGCATGTACAAAGGGAAGC-3′ and CR5′-TTGCAGACCTCAATCCGAAC-3′). SYBR green was used to monitor the amplification reaction. The 20 μl reaction mixture consisted of 10 μl 2× SYBR Premix Ex Taq, 0.4 μl of 10 μM of each primer, 0.4 μl 50× ROX Reference Dye, 2 μl DNA template and 6.8 μl dH2O. The Q-PCR cycling conditions included 1 cycle (30 s 95 °C) followed by 40 cycles (5 s 95 °C, 31 s 60 °C), and finally, 1 cycle (15 s 95 °C, 1 min 60 °C, 15 s 95 °C). Three replicates were run and averaged for each DNA sample. Negative controls were included in all amplification reactions. The PCR products of primers specific for the wsp gene of Wolbachia and the 16S rDNA gene of Cardinium were amplified by conventional PCR, then the PCR products was purified using the AxyPrep™ DNA Gel Extraction kit (AXYGEN) and cloned into a pGEM-T Easy vector (Promega). A series of DNA standards prepared from plasmid DNA was used and standard curves were plotted using a tenfold dilution series from 104 to 108 copies numbers. Ct values in each dilution were measured using a Q-PCR to generate the standard curves for wsp and 16S rDNA. The slopes of the standard curves for wsp and 16S rDNA were −3.44 and −3.45, respectively. From the slopes, a high amplification efficiency of 0.95 was determined for both wsp and 16S rDNA in the investigated range. The number of molecules in all samples is determined from the threshold cycles in the PCR based on a standard curve. Statistical analysis was performed using the Mann–Whitney U test.
Results
Cytoplasmic Incompatibility of Doubly Infected and Singly Infected Lines
Effects of Wolbachia and Cardinium Alone
By comparing the four crosses in which we tested possible Wolbachia CI effects, we found that Wolbachia caused weak level of CI (Table 1a). In the incompatible cross (U×Iw), on average, 32.0 % of all eggs did not hatch, compared with 3.45–6.03 % in the other three crosses. The sex ratio of the offspring that did hatch in the incompatible cross approached 22.0 %; the number of eggs produced and the mortality were not significantly different among the four crosses.
In the case of Cardinium, the number of unhatched eggs was significantly different among the four crosses (Table 1b). In the incompatible cross U×Ic, approximate 61.9 % of all eggs did not hatch, compared with 3.5–5.3 % in the other crosses. The male ratio was significantly higher in the incompatible cross than in the other crosses. This was because of a decrease in the number of female progenies, as the number of male progenies, was not significantly different among the four crosses. Therefore, Cardinium is capable of causing severe CI.
Effects of Double Infection
Strong CI can be induced by doubly infected males (Iwc) (Table 1c). The unhatched eggs and the sex ratio (% males) were high in the incompatible cross (U×Iwc) which is significantly different from the other three crosses. The strength of CI induced by doubly infected males (Iwc) also was found to be higher than that induced by the singly infected males (Iw and Ic).
Interactions Between Wolbachia and Cardinium
In order to find out whether Wolbachia can influence the CI strength of Cardinium, different crosses were investigated. As shown in Table 1d, an average of 80 % of all eggs did not hatch in the cross Iw×Iwc, and the unhatched rate was significantly higher than the crosses U×Ic (61.9 %) and Iw×Ic (60.9 %). The presence of Wolbachia in both male and female of a cross seemed to change Cardinium-induced CI expression. The results indicated that Wolbachia can promote the strength of Cardinium-induced CI. In addition, the crosses Ic×Ic, Iwc×Ic, and Iwc×Iwc (Table 1e) showed that Wolbachia does not affect Cardinium-induced CI rescue. Moreover, there was no significant difference between the crosses U×Ic and Iw×Ic (Table 1d), which indicates that CI induced by Cardinium cannot be rescued by Wolbachia.
Similarly, the crosses (U×Iw, Ic×Iwc, Ic×Iw) and (Iw×Iw, Iwc×Iw, Iwc×Iwc) were investigated to find out whether Cardinium can influence the CI strength of Wolbachia (Table 1f, g). These crosses showed that Cardinium cannot change the strength of Wolbachia-induced CI and Wolbachia-induced CI rescue. In addition, a comparison of the crosses Iw×Iw, Iwc×Iw, and Iwc×Iwc (Table 1g) shows that CI induced by Wolbachia-infected males could not be rescued by Cardinium.
Wolbachia and Cardinium Densities in Singly and Doubly Infected Lines
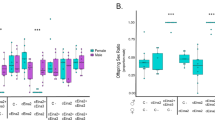
At day 1, the numbers of Wolbachia in the singly and doubly infected males were 0.55 and 0.47 × 106 (Fig. 1a). The density of Wolbachia in the doubly infected males was significantly lower than that in singly Wolbachia-infected males (P < 0.001). However, the numbers of Cardinium in the singly and doubly infected males were 0.49 and 0.57 × 106 (Fig. 1a), respectively. The number of Cardinium in the doubly infected males was significantly higher than that in singly Cardinium-infected males (P < 0.001). In the doubly infected males, the multiplication of Wolbachia was suppressed while the multiplication of Cardinium is promoted.
Infection densities of Wolbachia and Cardinium in singly infected and doubly infected males (a) and females (b). Iw densities of Wolbachia in singly Wolbachia-infected mites, Ic densities of Cardinium in singly Cardinium-infected mites, Dw densities of Wolbachia in doubly infected mites, and Dc densities of Cardinium in doubly infected mites. Asterisks indicate statistically significant differences (Mann–Whitney U test, P < 0.001) Error bars ±1 standard error
In 1 day-old mites, the densities of Wolbachia and Cardinium were clearly higher in females than that of males. The numbers of Wolbachia in the singly and doubly infected females were 1.29 and 1.10 × 106 (Fig. 1b), respectively. The densities of Wolbachia were lower in doubly infected females than in singly infected females. However, the numbers of Cardinium in the singly and doubly infected females were 1.38 and 1.41 × 106 (Fig. 1b), respectively. The densities of Cardinium were slightly higher in doubly infected females than in singly infected females, but it was not statistically significant (P > 0.05).
Discussion
Cytoplasmic Incompatibility
Both of Wolbachia and Cardinium can cause CI in our study. CI was expressed as a reduction in egg hatchability and a male-biased sex ratio in crosses between uninfected females and infected males in our testing crosses. This is concordant with the female mortality type of CI [7, 29]. Wolbachia caused a weak level of CI. The wsp gene sequence (unpublished) of this Wolbachia indicates that the strain belongs to the Ori subgroup. The Wolbachia belonging to the Ori subgroup were shown to cause a wide range of CI in Chinese populations of T. urticae [41]. Moreover, Cardinium-infected males caused severe CI, and the CI induced by doubly infected males was more severe than that caused by males singly infected with Cardinium or Wolbachia. The males singly infected by Cardinium also caused severe CI in the spider mite B. sarothamni; however, singly Wolbachia-infected and doubly infected males did not induce CI [24]. On the contrary, in doubly infected E. inaron, Wolbachia, and not Cardinium, caused CI of the female mortality type [40].
Symbionts Affect Each Other in the Expression and Rescue of CI
CI induced by singly Cardinium-infected males cannot be rescued by singly Wolbachia-infected females, and CI induced by singly Wolbachia-infected males cannot be rescued by singly Cardinium-infected females. These results are consistent with the result of studies in B. sarothamni and E. inaron [24, 40]. However, the rate of unhatched eggs was significantly higher in the Iw×Iwc cross than in the U×Ic cross. This suggests that Wolbachia increased the severity of Cardinium-induced CI. Although multiple infections of Wolbachia and other symbionts are common and the relative contributions of Wolbachia and Cardinium to CI modification and rescue have been studied in a doubly infected E. inaron [40], to the best of our knowledge, the present results are the first to show that Wolbachia can increase the severity of Cardinium-induced CI.
Factors Affect the Levels of CI
Host genotype has been proposed to affect expression of CI [17]. In our experiments, singly infected lines were generated from doubly infected isofemale lines to rule out effects of host genotype. Bacterial strains have also been shown to be important in the expression of CI [4], and it is possible that Wolbachia has evolved a new phenotype that is beneficial for Cardinium-induced CI in T. piercei McGregor. Transferring Wolbachia into other Cardinium-infected T. piercei McGregor would be necessary to verify this new phenotype.
A likely explanation is that Wolbachia promoted the strength of Cardinium-induced CI by promoting Cardinium densities when doubly infected males mated to uninfected females. Cardinium density has been suggested to be a critical factor for CI intensity in T. cinnabarinus, and a threshold level of Cardinium density may be required for the induction of CI [42]. We found that the multiplication of Wolbachia is suppressed while the multiplication of Cardinium is promoted in doubly infected males (Fig. 1a). It may also explain why the CI induced by doubly infected males was more severe than the CI induced by males singly infected with Cardinium or Wolbachia. Maintenance of infection by multiple CI-inducing symbionts is therefore often evolutionarily favored [29]. The synergy between Wolbachia and Cardinium appears to agree with their general co-occurrence in the host tissues. Cohabitation in the same host provides ample opportunities for interactions among symbionts that can either facilitate or limit symbiotic existence. For example, in the sweet potato whitefly Bemisia tabaci, secondary symbionts were found to share bacteriocytes with primary symbiont, which allowed them to be vertically transmitted by “hitching a ride” with primary symbiont [13]. In this way, symbionts co-infecting the same host can better manipulate the reproduction of their host.
In addition, the Wolbachia density in doubly infected males was lower than that in males singly infected with Wolbachia, although the rate of unhatched eggs in the Ic×Iwc cross was similar to that in the I×Iw cross. The severity of Wolbachia-induced CI in doubly infected males was not affected by the Wolbachia density. Similarly, in three Drosophila species (D. simulans Hawaii, D. sechellia, and D. auraria), CI was positively correlated with bacterial density, but in all the other Drosophila species including D. simulans Riverside, CI was not correlated with bacterial density [5]. There are two possible explanations for this result. On the one hand, a threshold level of bacterial density is required for inducing CI. Below the threshold density of Wolbachia, the penetrance of CI began to fall off. Densities above the threshold appeared to have no additional effect on the strength of CI. The threshold density could vary between different Wolbachia strains [25]. In doubly infected males, the Wolbachia density may have not been below the threshold density, so the level of CI did not fall. On the other hand, because of the selective pressure acting on both partners, various mechanisms should have evolved to control infection density within an appropriate range, which leads to the idea that both symbiont and host genotype may contribute to the regulation of infection density [19]. For example, in the adzuki bean beetles Callosobruchus chinensis, the host genotypes could suppress Wolbachia density by becoming co-infected with different Wolbachia strains [17].
The density of Cardinium in doubly infected females was not significantly different from that in females singly infected with Cardinium (Fig. 1b), which is different from the case in males. The biological effects of symbionts may largely depend on their infection densities. A reduced infection density may result in imperfect vertical transmission and a consequent loss of infection. High infection density may also have pathological effects on the host, and hence have negative effects on their fitness [17]. In the parasitoid wasp Nasonia vitripennis, bacterial density was correlated with compatibility differences between males and females. Males from strains with high bacterial densities were incompatible with females from strains with lower densities [8]. However, this was not the case in our study. More work is needed to investigate the relations between degree of CI and density of Wolbachia and Cardinium in doubly infected females.
In brief, in the same host body, various interactions are expected to occur between coexisting symbionts. We investigated the interactions of both Wolbachia and Cardinium in the spider mite T. piercei, and found that the level of CI induced by Wolbachia was not related to Wolbachia density, but that Wolbachia promoted the strength of Cardinium-induced CI. These findings should help to design further studies of the interactions between Wolbachia and Cardinium in doubly infected hosts and help to develop a method for controlling arthropod pests using these two endosymbionts.
References
Baldo L, Dunning Hotopp JC, Jolley KA et al (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Barr AR (1980) Cytoplasmic incompatibility in natural populations of a mosquito, Culex pipiens L. Nature 283:71–72
Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ (2006) The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathog 2:e43
Bourtzis K, Dobson SL, Braig HR, O’Neill SL (1998) Rescuing Wolbachia have been overlooked. Nature 391:852–853
Bourtzis K, Nirgianaki A, Markakis G, Savakis C (1996) Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144:1063–1073
Braig HR, Zhou W, Dobson SL, O’Neill SL (1998) Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180:2373–2378
Breeuwer JAJ (1997) Wolbachia and cytoplasmic incompatibility in the spider mites Tetranychus urticae and T. turkestani. Heredity 79:41–47
Breeuwer JAJ, Werren JH (1993) Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135:565–574
Callaini G, Riparbelli MG, Giordano R, Dallai R (1996) Mitotic defects associated with cytoplasmic incompatibility in Drosophila simulans. J Invertebr Pathol 67:55–64
Dobson SL, Marsland EJ, Rattanadechakul W (2001) Wolbachia-induced cytoplasmic incompatibility in single-and super-infected Aedes albopictus (Diptera: Culicidae). J Med Entomol 38:382–387
Duron O, Hurst GDD, Hornett EA, Josling JA, Engelstädter J (2008) High incidence of the maternally inherited bacterium Cardinium in spiders. Mol Ecol 17:1427–1437
Gotoh T, Noda H, Ito S (2006) Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98:13–20
Gottlieb Y, Ghanim M, Gueguen G et al (2008) Inherited intracellular ecosystem: symbiotic bacteria share the bacteriocytes of whiteflies. FASEB J 22:2591–2599
Groot TVM, Breeuwer JAJ (2006) Cardinium symbionts induce haploid thelytoky in most clones of three closely related Brevipalpus species. Exp Appl Acarol 39:257–271
Hoffmann A, Turelli M (1997) Cytoplasmic incompatibility in insects. In: O’Neill SL, Hoffmann AA, Werren JH (eds) Influential passengers. Oxford University Press, Oxford, pp 42–80
Hunter MS, Perlman SJ, Kelly SE (2003) A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc R Soc Lond B 270:2185–2190
Kondo N, Shimada M, Fukatsu T (2005) Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol Lett 1:488–491
Morimoto S, Kurtti TJ, Noda H (2006) In vitro cultivation and antibiotic susceptibility of a Cytophaga-like intracellular symbiote isolated from the tick Ixodes scapularis. Curr Microbiol 52:324–329
Mouton L, Henri H, Bouletreau M, Vavre F (2003) Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol Ecol 12:3459–3465
Mouton L, Henri H, Bouletreau M, Vavre F (2005) Multiple infections and diversity of cytoplasmic incompatibility in a haplodiploid species. Heredity 94:187–192
Noda H, Koizumi Y, Zhang Q, Deng K (2001) Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem Mol Biol 31:727–737
Reynolds KT, Hoffmann AA (2002) Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res 80:79–87
Ros VID, Breeuwer JAJ (2007) Spider mite (Acari: Tetranychidae) mitochondrial COI phylogeny reviewed: host plant relationships, phylogeography, reproductive parasites and barcoding. Exp Appl Acarol 42:239–262
Ros VID, Breeuwer JAJ (2009) The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. Heredity 102:413–422
Sinkins SP, Braig HR, O’Neill SL (1995) Wolbachia pipientis: bacterial density and unidirectional cytoplasmic incompatibility between infected populations of Aedes albopictus. Exp Parasitol 81:284–291
Stouthamer R, Breeuwer J, Hurst G (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Tram U, Ferree PM, Sullivan W (2003) Identification of Wolbachia-host interacting factors through cytological analysis. Microbes Infect 5:999–1011
Van Opijnen T, Breeuwer JAJ (1999) High temperatures eliminate Wolbachia, a cytoplasmic incompatibility inducing endosymbiont, from the two-spotted spider mite. Exp Appl Acarol 23:871–881
Vautrin E, Charles S, Genieys S, Vavre F (2007) Evolution and invasion dynamics of multiple infections with Wolbachia investigated using matrix based models. J Theor Biol 245:197–209
Vavre F, Fleury F, Varaldi J, Fouillet P, Boulétreau M (2000) Evidence for female mortality in Wolbachia-mediated cytoplasmic incompatibility in haplodiploid insects: epidemiologic and evolutionary consequences. Evolution 54:191–200
Veneti Z, Clark ME, Karr TL, Savakis C, Bourtzis K (2004) Heads or tails: host-parasite interactions in the Drosophila–Wolbachia system. Appl Environ Microbiol 70:5366–5372
Veneti Z, Clark ME, Zabalou S, Karr TL, Savakis C, Bourtzis K (2003) Cytoplasmic incompatibility and sperm cyst infection in different Drosophila–Wolbachia associations. Genetics 164:545–552
Watanabe M, Miura K, Hunter M, Wajnberg E (2011) Superinfection of cytoplasmic incompatibility-inducing Wolbachia is not additive in Orius strigicollis (Hemiptera: Anthocoridae). Heredity 106:642–648
Weeks AR, Tracy Reynolds K, Hoffmann AA (2002) Wolbachia dynamics and host effects: what has (and has not) been demonstrated? Trends Ecol Evol 17:257–262
Weeks AR, Velten R, Stouthamer R (2003) Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc Lond B 270:1857–1865
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42:587–609
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
Werren JH, O’Neill SL (1997) Inherited microorganisms and arthropod reproduction. In: O’Neill SL, Hoffmann AA, Werren JH (eds) Influential passengers. Oxford University Press, Oxford, pp 1–41
Werren JH, Windsor D, Guo L (1995) Distribution of Wolbachia among neotropical arthropods. Proc R Soc Lond B 262:197–204
White JA, Kelly SE, Perlman SJ, Hunter MS (2009) Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 102:483–489
Xie RR, Chen XL, Hong XY (2011) Variable fitness and reproductive effects of Wolbachia infection in populations of the two-spotted spider mite Tetranychus urticae Koch in China. Appl Entomol Zool 46:95–102
Xie RR, Zhou LL, Zhao ZJ, Hong XY (2010) Male age influences the strength of Cardinium-induced cytoplasmic incompatibility expression in the carmine spider mite Tetranychus cinnabarinus. Appl Entomol Zool 45:417–423
Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I et al (2008) Multiple rescue factors within a Wolbachia strain. Genetics 178:2145–2160
Zchori-Fein E, Perlman SJ (2004) Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13:2009–2016
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, LY., Zhang, KJ., Zhang, YK. et al. Wolbachia Strengthens Cardinium-Induced Cytoplasmic Incompatibility in the Spider Mite Tetranychus piercei McGregor. Curr Microbiol 65, 516–523 (2012). https://doi.org/10.1007/s00284-012-0190-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0190-8