Abstract
The objective of this study was to investigate whether four spider mite species, Tetranychus ludeni, T. phaselus, T. piercei and T. truncatus, currently with insignificant economic impact, have the potential to achieve the same status as T. urticae, which until now has been considered as the most serious tetranychid pest species in orchards and greenhouses. We investigated the effect of temperature on development, survival and oviposition at 11 constant temperatures ranging from 15 to 40 °C at intervals of 2.5 °C and estimated demographic parameters, such as the intrinsic rate of natural increase (r m), for these five species at five constant temperatures. Developmental time from egg to adult (female and male) decreased with increasing temperature from 15 to 32.5 °C in all five species, but increased slightly at 35 °C or higher, especially in T. ludeni and T. urticae. Using linear and non-linear developmental rate models, the lower thermal thresholds for egg-to-adult (female and male) and egg-to-egg development were found to range from 9.8 to 11.7 and from 9.8 to 11.4 °C, respectively. The intrinsic optimal temperature (T Φ) ranged from 18.0 to 27.4 °C for egg-to-female adult and from 23.9 to 27.2 °C for egg-to-egg development. The oviposition period and adult longevity were strongly affected by temperature. The r m-values increased with increasing temperature from 15 to 30 or 35 °C in all five species. The highest r m-values at each temperature were 0.114 day−1 at 15 °C for T. ludeni, 0.199 day−1 at 20 °C for T. urticae, 0.314 day−1 at 25 °C for T. ludeni, 0.451 day−1 at 30 °C for T. ludeni and 0.433 day−1 at 35 °C for T. truncatus. The total fecundity, net reproductive rate (R 0) and r m of T. ludeni were higher than those of T. urticae at all temperatures. T. piercei and T. truncatus showed higher r m-values at 30 and 35 °C than T. urticae. The results indicate that the former three species are better adapted to hot weather than T. urticae and have a high potential to become serious pests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are many studies showing a change in mite species composition in fruit tree orchards taking place during the past 50 years in Japan. Thus, in pear orchards, Amphitetranychus viennensis (Zacher), which was dominant until the 1960s, was replaced with Tetranychus urticae Koch (green form, G) and/or Tetranychus kanzawai Kishida (Nakagaki 1980; Uchida 1982; Takafuji and Kamibayashi 1984; Gotoh 1997; Kishimoto 2002). In apple orchards, Panonychus ulmi (Koch), which was a dominant species until 1950s, was displaced by T. urticae (G) (Fujibayashi and Kushida 1992). However, after using sex pheromone for disrupting the mating of fruit borers and leaf rollers application of pesticides has been drastically reduced and as a result natural enemies of spider mites began to increase and to effectively control T. urticae (G). Recently, previously dominant species, such as A. viennensis and P. ulmi, have increased again to become dominant instead of T. urticae (Okazaki 2000). These observations are experimentally confirmed in pear orchards, where agrochemical use was artificially managed: A. viennensis was dominant in agrochemical-free orchards, Panonychus citri (McGregor) was dominant in pesticide-free orchards, and T. urticae (G) was dominant in conventionally controlled orchards (Kishimoto 2002). Such changes in species composition are attributed to the negative effects of agrochemicals on the natural enemies attacking Tetranychus species and to the ability of spider mites to develop resistance against agrochemicals (van de Vrie et al. 1972; Trichilo and Wilson 1993; Goka 1999; Takafuji et al. 2000). On the other hand, tarsonemid mites have gradually become more serious pests after integrated pest management programs have been introduced in greenhouse crops (Mizukoshi and Goto 2003). Tarsonemid mites such as Phytonemus pallidus (Banks) and Polyphagotarsonemus latus (Banks) had once been simultaneously controlled with agrochemicals sprayed for controlling tetranychid mites and small insect pests as a side-effect, but recently application of insecticides and acaricides has been abandoned or reduced in order to protect the commercially produced natural enemies released for controlling spider mites and small insect pests. The enemies are Phytoseiulus persimilis Athias-Henriot, Neoseiulus californicus (McGregor) and Amblyseius swirskii Athias-Henriot. This indicates that a reduction in the use of agrochemicals can lead to an increase in minor or cryptic pests. Consequently, changes in the application of agrochemicals such as the number of sprayings or the chemical components applied (e.g., shifts from chemicals having broader spectrum to ones having target-specificity or a narrower spectrum) may influence spider mite composition in a field. This suggests that minor spider mite species (i.e., species that are usually not regarded as pests) inhabiting orchards and greenhouses, or neighboring areas, might become more dominant in environments with relatively low impact of agrochemicals, even if the mites have not yet developed resistance to such chemicals. However, the effect of agrochemicals on the minor species, the preference of natural enemies for minor species and the potential capacity of minor species for population increase at different temperatures remain unknown.
Preliminary observations have shown that Tetranychus ludeni Zacher, usually considered a minor pest in Japan, sometimes has outbreaks in commercial green pepper fields with ongoing IPM programs. Likewise, in Inner Mongolia, China, outbreaks of Tetranychus truncatus Ehara occur in corn cultivated without using any agrochemicals (Gotoh, personal observation). Therefore, the purpose of this study was to find out whether minor Tetranychus species have the potential to become important pests. We investigated the effect of temperature on development and demographic parameters of four minor species of the genus Tetranychus in Japan, and compared the values with those obtained for the serious pest, T. urticae (G), which was also included in this study. Tetranychus urticae is known as one of the most important pests on many crops worldwide. It attacks more than 1000 plant species, including vegetables, fruit trees and ornamentals (Bolland et al. 1998; Migeon and Dorkeld 2006–2013). Of the 13 Tetranychus species found in Japan, Tetranychus okinawanus Ehara and Tetranychus neocaledonicus Andre do not occur at the main island of Japan, Honshu, whereas Tetranychus ezoensis Ehara only infests coniferous trees. Life histories of Tetranychus parakanzawai Ehara and Tetranychus pueraricola Ehara & Gotoh have been studied by Gotoh and Gomi (2003) and Gotoh et al. (2004), respectively. Tetranychus mismaiensis Ehara & Gotoh is extremely rare in Japan and has only been collected once at Hokkaido, northern Japan. Tetranychus urticae, T. kanzawai and Tetranychus evansi Baker & Pritchard are all serious pests (Bolland et al. 1998). The remaining four species, currently regarded as minor pests, are T. ludeni, Tetranychus phaselus Ehara, Tetranychus piercei McGregor and T. truncatus. They have been found at least once at the main island, Honshu, where they infested a crop and/or occurred on weeds growing in or close to a field crop. However, biological information about the species is still scarce. Tetranychus ludeni occurs worldwide and infests more than 250 plant species. The species does not have diapause ability and all stages can be found throughout a year. Takafuji (1980) found three distinct population peaks (in June, August and November) on goldenrod, Solidago altissima L. Tetranychus phaselus is distributed within an area encompassing East Russia, China, Japan, Korea and Taiwan and appears on about ten plant species. Tetranychus piercei is known from south-eastern Asia and appears on about 30 plant species (Bolland et al. 1998). Tetranychus truncatus is known from Asia and infests more than 60 plant species. This is an extremely rare species in Japan, but it is a very severe pest on various crops such as corn, soybean, eggplant and cucumber in Thailand (Sakunwarin et al. 2003) and China (Pang et al. 2004). There are several studies on its life-history parameters (Sakunwarin et al. 2003; Pang et al. 2004) and its development at different temperatures, i.e., lower thermal threshold (Chao and Lo 1974; Fan et al. 2003; Sakunwarin et al. 2003).
Materials and methods
Mites
Table 1 summarizes information about the five Tetranychus species used in this study. Each species was maintained separately on down-side-up leaf discs (ca. 16 cm2) of common bean (Phaseolus vulgaris L.) placed on water-saturated polyurethane mats in plastic dishes (90 mm diameter, 20 mm depth) and kept at 25 ± 1 °C and under a 16:8 h light:dark photoperiod with 60–70 % relative humidity. Cultures of the five species had been kept under the same laboratory conditions for some years prior to the experiments which took place in 2010.
Immature development
Inseminated adult females obtained from the stock cultures of each species were transferred individually onto a leaf disc (2 × 2 cm) of common bean and kept at one of 11 constant temperatures, ranging from 15 to 40 °C with intervals of 2.5 °C, under a long-day photoperiod (16L:8D) with 60–70 % RH. Females were allowed to lay eggs for 24 h at 15–25 °C, for 12 h at 27.5–35 °C or for 6 h at 37.5–40 °C. Only one egg was left and reared on the leaf disc, and the developmental stages were recorded at the same time every day until all individuals reached the adult stage. Some eggs did not hatch and some immatures drowned—they were included in the calculation of the egg hatching rate and the survival rate, but were excluded from the calculation of developmental time.
Reproduction and female longevity
When a female teleiochrysalis appeared in the developmental experiments, two adult males obtained from the stock cultures were introduced onto the leaf disc (2 × 2 cm) for mating and then removed 48 h after emergence of the adult female as previous experiments have shown that females of the five species only mate once during lifetime (Gotoh unpubl). To determine the pre-oviposition period at the respective temperatures, we observed the leaf discs at 6 to 24-h intervals (depending on temperature—the shortest intervals were applied at the highest temperatures). Newly emerged females obtained from the above-mentioned experiments at 15, 20, 25, 30 and 35 °C, were used to assess their reproductive traits and longevity. The number of eggs laid by a female was recorded daily throughout her lifetime to determine oviposition period, total number of eggs laid per female, eggs laid per female per day, post-oviposition period and female longevity. Eggs laid were removed daily by means of tweezers. During the oviposition period, some adult females drowned, especially just after replacing the leaf discs, or were killed accidentally. These females were discarded from the analysis. Adult mites were transferred onto new leaf discs at 1 to 2-week intervals using a fine brush.
Hatchability, survivability and sex ratio
To calculate age-specific survival rate (l x ) and fecundity rate (m x ) at 15, 20, 25, 30 and 35 °C, it was necessary to assess (1) egg hatchability, (2) the survival rate of immature stages and (3) the proportion of female offspring. To obtain these data, single female teleiochrysales were placed with two adult males on a leaf disc (ca. 16 cm2) of common bean for copulation. The females were allowed to lay eggs for 5 days after the pre-oviposition period. The eggs obtained from each female were kept to determine the above-mentioned parameters after reaching adulthood. As discussed by Sabelis (1981), the sex ratio of T. urticae gets increasingly female-biased within the first 5–6 days of egg laying. Though older females may tend to produce more female eggs than younger females (see also Riahi et al. 2011), it is not likely to affect the life-table parameters markedly as the majority of eggs are produced when the females are still young. Especially the intrinsic rate of natural increase (r m) depends on survival and fecundity rates during early ages (Birch 1948). We therefore consider the sex ratio determined on basis of eggs produced during the first 5–6 days of egg-laying as the most representative for the species in focus.
Life-table parameters
The r m, which expresses the maximum innate capacity of increase of a population living under optimal conditions, was estimated from the life-fecundity table according to the equation given by Birch (1948): \( \sum\nolimits_{x = 0}^{\infty } {l_{x} m_{x} e^{{ - r_{\rm m} x}} } = 1 \), where x is age in days, l x is the age-specific survival rates of females [(the fraction of females surviving at age x) × (rate of egg hatchability) × (survival rate of immature stages)] and m x is the expected number of daughters produced per female alive at age x [(age-specific oviposition) × (proportion of females)] (Sabelis 1985; Gotoh and Gomi 2003; Gotoh et al. 2010). The net reproductive rate (R 0) was calculated as \( R_{0} = \sum\nolimits_{x = 0}^{\infty } {l_{x} } m_{x} \), the mean generation time (t G ) in days as t G = ln R 0/r m, the finite rate of increase (λ) as \( \lambda = e^{r_{\rm m}} \), and the doubling time (t D ) in days as t D = (ln 2)/r m.
We also calculated r m by means of the approximate method suggested by Wyatt and White (1977) using the total number of eggs produced per female from day d to day D (M d ), where d is the duration of the preoviposition period at a given temperature and D = 2d. Thus, r m was estimated as \( r_{m} = c\ln M_{d} /d \) where c is a species specific correction factor equal to 0.749 for mites (Wyatt and White 1977). The BCaWW method (bias corrected and accelerated bootstrap) was used to calculate 95 % confidence intervals for r m obtained by means of Wyatt and White’s method (see also Lawo and Lawo 2011; the values were calculated based on their procedure using free R software).
Effect of temperature on mite developmental rate
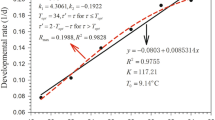
Developmental rates (calculated as 1/developmental duration) at different temperatures were used in linear and non-linear models. The thermal constant and lower thermal threshold were determined using the line-fitting method proposed by Ikemoto and Takai (2000). As the method assumes a linear increase in developmental rate with temperature, we excluded data obtained at temperatures above 30 °C from the analysis. The linear model by Ikemoto and Takai (2000) fitted to data is given as:
where D is the duration of development (days), T is environmental temperature (°C), t is the lower thermal threshold and k is the thermal constant.
The non-linear thermodynamics model describes the developmental rate over a wider range of temperatures and allows for estimating the optimum temperatures for development. The equation of the non-linear thermodynamics [Sharpe–Schoolfield–Ikemoto (SSI)] model can be expressed as follows (Ikemoto 2005, 2008; Shi et al. 2011):
where r represents the developmental rates (the dependent variables) at the absolute temperature ([T]) (the independent variable). All the other parameters are constants: [T L ], [T H ], and [T Φ] represent absolute temperatures—[T L ] and [T H ] represent temperatures below (L) and above (H) an optimum temperature, at which an enzyme is 50 % active—ΔH A , ΔH L , and ΔH H represent enthalpy changes, R is the universal gas constant, and ρ is the development rate at [T Φ]. [T Φ] is the intrinsic optimum temperature for development that exhibits the minimum effects on enzyme inactivation related to development at low and high temperature (Ikemoto 2005) and it is expressed as follows:
The SSI model was modified and developed as SSI-P, which runs on R statistical software by Shi et al. (2011). Finally, Ikemoto et al. (2013) improved their model and developed the program OptimSSI-P, which estimates T Φ along with its confidence limit.
Statistical analysis
Data were analyzed by means of analysis of covariance (ANCOVA), using temperature as the covariate. The purpose was to quantify relationships between the response variables (eggs/female, hatchability, survival rate, female ratio, oviposition period, female longevity, egg-to-adult developmental time, total oviposition, net reproductive rate, intrinsic rate of natural increase, generation time and the finite rate of increase), and predictor variables, such as gender (G), temperature (T) and species (S), in order to identify which of the predictor variables contribute most to explain the variation in data. The full ANCOVA model for analysing all response variables includes the main effects of species (with five levels), gender (with two levels) and temperature (T and T 2) as well as all their first- and second-order interactions. Thus, the full model predicting egg-to-adult development has 30 parameters of which one expresses the intercept (denoted β 0) and the remaining 29 are associated with the predictor variables. The full model for the remaining response variables does not include gender and has therefore only 14 parameters plus β 0. In some instances, the model also involved third-order terms of temperature, but only the significant terms were included in the final model.
All response variables were analyzed by means of generalized linear models (McCullagh and Nelder 1989) using PROC GENMOD (Enterprise Guide 4.1, SAS Institute 2006). The advantage of GENMOD is that it permits data with non-normal distributions. Proportions (female ratio, hatchability and survival rate) are likely to be binomially distributed, discrete numbers (eggs/female) to be Poisson or negative binomially distributed, whereas the continuous variables (developmental duration, oviposition period, adult longevity, R 0, r m, t G and λ) are likely to be normally distributed. When needed, continuous variables were subjected to a logarithmic (ln) transformation in order to stabilize the variance, to make the residuals more normally distributed, and to ensure that the back-transformed values were non-negative.
To test for differences in temperature responses among species and between genders, we compared the deviance of the full model with the increase in deviance resulting from omitting species and gender from the full model, whereas the overall effect of temperature was tested by omitting this factor from the full model. The difference between the full model and a reduced model (i.e., a model with fewer factors) was tested by means of Manly’s (1990) test:
where D 1 and D 2 denote the deviance of the reduced and the full model, respectively, p 1 and p 2 are the number of parameters of the reduced and full model, and N is the total number of observations in the data set. The degrees of freedom for F are given as ν 1 = p 2 − p 1 and ν 2 = N − p 2 − 1. Large values of F indicate that the factor(s) omitted from the full model contributed significantly (P < 0.05) to explain variation in the dependent variable (y).
Results
Immature development
No eggs of T. ludeni (0/96) and T. urticae (0/96) hatched at 40 °C, whereas most of the hatched individuals did not reach to the adult stage in the other three species (Appendix 1). In T. ludeni, survival rate was also very low at 37.5 °C (10.5 %, n = 67). Immature survivorship (egg to adult) for the five Tetranychus species was similar between 15 and 35 °C ranging from 61.6 to 100 % (Appendix 1). Temperature strongly affected the egg-to-adult duration, and there was a significant difference between the five species with respect to how they responded to temperature (Tables 2, 3; Fig. 1). There was also a significant difference between genders with respect to developmental time in all species (Table 2). From 15 to 32.5 °C, the egg-to-adult developmental time of both sexes decreased with increasing temperature, whereas it became slightly longer at higher temperatures, especially in T. ludeni and T. urticae (Fig. 1; Appendix 1).
Effect of temperature on developmental time from egg to adult, mean duration of the oviposition period, adult longevity and total fecundity in the five Tetranychus species kept on common bean at various temperatures and a 16L:8D photoperiod. See Appendixes 1 and 2 for further information. Points show the sample averages with 95 % confidence limits. The heavy lines show the predictions based on the generalized linear models after eliminating non-significant terms. Thin lines show the 95 % confidence limits for the predicted line. The models as well as total sample sizes are given in Table 3
The linear model of Ikemoto and Takai (2000), when fitted to values of developmental rates, gave a close fit to the data in the temperature range between 15 and 30 °C (0.9723 ≤ r 2 ≤ 0.9904) (Table 4). The estimated lower thermal thresholds (t) for egg-to-adult and for egg-to-egg development were similar and the values ranged from 9.81 to 11.66 and from 9.81 to 11.35 °C, respectively, for the five Tetranychus species (Table 4). The thermal constant (k) for the respective stages ranged from 118.3 to 153.6 DD and from 130.7 to 170.4 DD, respectively (Table 4). The non-linear OptimSSI-P model, when fitted to values of developmental rates, gave a close fit to the data in the temperature range between 15 and 37.5 °C (0.0008 ≤ χ 2 ≤ 0.0041) (Table 4). The intrinsic optimum temperature (T Φ) ranged from 18.0 to 27.4 °C for egg-to-female adult, from 20.6 to 28.2 °C for egg-to-male adult and from 23.9 to 27.2 °C for egg-to-egg development (Table 4).
Reproduction
The pre-oviposition period decreased with increasing temperature from 15 to 35 °C for T. truncatus or to 30 °C for the other four species (Appendix 1). The oviposition period and adult longevity were also strongly affected by temperature (Appendix 2; Fig. 1). Total fecundity (eggs/female) was highest in T. ludeni at all five temperatures and lowest in T. phaselus at 15 and 35 °C, in T. piercei at 20 °C and in T. urticae at 30 °C (Fig. 1; Appendix 2). The difference between species with respect to total fecundity was highly significant (Table 2). For instance, at 20 °C total fecundity ranged from 32.2 eggs in T. piercei to 202.0 eggs in T. ludeni (Appendix 2).
The number of eggs laid during the first 5 days of the oviposition period, their hatchability, the survival rate of the immature stages and the proportion of female offspring are given in Appendix 3. The effect of temperature on these variables was also highly significant (P < 0.001) (Table 2).
Demographic parameters
The age-specific survival rate (l x ) started to drop at younger ages as the temperature increased from 15 to 35 °C (Table 5; Fig. 2). The age-specific fecundity rate (m x ) peaked at an earlier age and the oviposition period became shorter as the temperature increased. The peak oviposition rate was highest in T. ludeni at all five temperatures (Table 5). The age of the first oviposition decreased with increasing temperature for all five species.
The net reproductive rate (R 0), the intrinsic rate of natural increase (r m, day−1), the mean generation time (t G , days) and doubling time (t D , days) were all affected by temperature and there were significant differences among the five species with respect to temperature response (Tables 2, 3; Fig. 3; Appendix 4). The highest R 0 value was observed in T. ludeni at all five temperatures (Appendix 4). The species with the highest r m value varied with temperature: T. truncatus at 15 °C (0.1141 day−1) and at 35 °C (0.4330 day−1), T. urticae at 20 °C (0.1998 day−1), and T. ludeni at 25 °C (0.3138 day−1) and 30 °C (0.4514 day−1) (Appendix 4). The values of r m and λ increased with increasing temperature from 15 to 30 or 35 °C in all five species. Mean generation time (t G ) and doubling time (t D ) decreased with increasing temperature (Fig. 3; Appendix 4).
Effect of temperature on demographic parameters of five Tetranychus species. See Appendix 4 for further information. R 0, net reproductive rate; r m, intrinsic rate of natural increase; t G , mean generation time; λ, finite rate of increase. Points show the sample averages with 95 % confidence limits. The heavy lines show the predictions based on the generalized linear models after eliminating non-significant terms. Thin lines show the 95 % confidence limits for the predicted line. The models, as well as total sample sizes, are given in Table 3
Discussion
The present study shows that total fecundity, R 0 and r m of T. ludeni were higher than those of T. urticae at all five temperatures tested. It indicates that T. ludeni has a high potential to become a serious pest. T. piercei and T. truncatus showed higher r m-values at 30 and 35 °C than that of T. urticae, suggesting that these two species as well as T. ludeni are better adapted to hot weather than T. urticae (Appendix 4). The lower thermal thresholds in the five Tetranychus species were in the order T. urticae ≈ T. ludeni > T. truncatus > T. phaselus ≈ T. piercei (Table 4). T Φ, which is the most important parameter for development because it exhibits the minimum effects on enzyme inactivation related to development at low and high temperature (Ikemoto 2008), was higher in the four minor species than in T. urticae. Development and reproduction of arthropods are considered to be gradually inhibited at temperatures far from T Φ. As the ambient temperatures are expected to increase dramatically during the coming years due to global warming (Chen et al. 2013; Kiritani 2013; Estay et al. 2014), the species having a high T Φ are likely to increase their abundance in temperate regions and eventually replace T. urticae as major pests. Accordingly, T. ludeni, T. piercei and T. truncatus have the potential to become serious pests. Consequently, if the use of agrochemicals is restricted, we can expect that the invasion of these until now minor species into crop fields will be accelerated.
The thermal threshold (t) of development from egg to adult female has been found to range from 7.8 to 13.8 °C in the genus Tetranychus. In our study, thermal thresholds ranged from 9.8 to 11.7 °C (Table 4), which fall within the previously reported range. The thermal threshold of T. truncatus from Japan was found to be 10.9 °C, which is lower than the one found for the same species in Thailand (11.6 °C; Sakunwarin et al. 2003) and China (13.9 °C; Fan et al. 2003). The thermal threshold of T. urticae in this study (11.2 °C) was in-between the one obtained from an Australian population (7.8 °C; Davies et al. 2009) and the one from an Iranian population (13.8 °C; Riahi et al. 2013). Thus, the threshold values vary greatly among populations and probably reflect adaptations to the local climate.
The intrinsic rate of natural increase (r m) calculated by other authors for Tetranychus species are difficult to compare with our results, as differences could be due to the local strains used as well as to differences in experimental methodology, for instance with respect to the size and type of experimental arenas, host plants used, relative humidity, photoperiod and differences in calculation methods (Bonato 1999; Ferrero et al. 2007). Nevertheless, Sabelis (1985, 1991), in an extensive review of life-history parameters of tetranychid mites, found r m-values for Tetranychus mites to range from 0.200 to 0.336 day−1 at ca. 25 °C. The r m-values of the five Tetranychus species in this study fall within this range. The r m-values of T. truncatus increased with increasing temperature form 15 °C (0.1141 day−1) to 35 °C (0.4330 day−1) in contrast to the decline observed in Tetranychus merganser Boudreaux at the highest temperature (Ullah et al. 2011). The r m-value (0.275 day−1) and R 0-value (88.0) of the Japanese T. truncatus are noticeably higher than those of the Thai population, which were found as 0.173 day−1 and 37.39, respectively (Sakunwarin et al. 2003). This discrepancy could be attributed to differences in developmental time (12.5 vs 10.1 days; Thai vs Japanese population), peak oviposition (6.5 vs 11.1 eggs on day 15 for both), and total fecundity (65.6 vs 115.9 eggs). The r m-value (0.193 day−1) and R 0-value (21.7) of the Chinese strain of T. truncatus on common bean (Pang et al. 2004) are much lower than those of the Japanese strain in spite of the fact that the former was examined at 28 °C. The developmental time (9.4 days for female) of the Chinese T. truncatus strain is longer than the one found in our study at 27.5 °C (7.6 days). The reason for this difference is not clear because the peak oviposition age and oviposition rates were not reported by Pang et al. (2004). In general, the two parameters of paramount importance in determining the r m-value are developmental time and the peak oviposition rate (Snell 1978; Wrensch 1985).
The r m-value (0.294 day−1 at 25 °C) of T. urticae in our study is comparable to the value (0.292 day−1) reported by Kondo and Takafuji (1985) and the value (0.259 day−1) by Saito (1979), which are much higher than the r m-values reported by some other authors (0.144–0.188 day−1) (Bounfour and Tanigoshi 2001; Khanamani et al. 2013; Riahi et al. 2013). In all of the latter reports, the R 0-values are also much lower than the one found in our study. The r m-value of T. ludeni (0.314 day−1) is comparable to that of T. okinawanus (0.316 day−1) at 25 °C (Takafuji et al. 1996), which is the second highest value reported for a Tetranychus species so far. The reproductive traits of T. ludeni are similar to or higher than those of other pest mite species such as T. urticae, suggesting that T. ludeni, together with T. piercei and T. truncatus, have the potential to become serious pests.
In the present study, the five species were kept under the same laboratory conditions from 1 to 15 years prior to the experiments. We cannot preclude the possibility that the species have adapted to the laboratory conditions which might have affected their life-history characteristics. In fact, Tetranychus pacificus McGregor populations originating from various grapevine cultivars showed significant differences with respect to developmental time and juvenile survival, but no difference with respect to reproductive rate, when reared on a common host plant under the same laboratory conditions (Scranton et al. 2013). Tetranychus urticae has shown rapid adaptation to unfavourable host plants after 15 generations, causing an increase in juvenile survival and female fecundity but no variation in developmental time (Magalhaes et al. 2007). Therefore, further studies are required to clarify the effect of adaptation when laboratory populations have been subjected to the long-term exposure to laboratory conditions, for instance by comparing individuals from newly established cultures with individuals originating from populations reared under laboratory conditions for many years. In addition, research is needed to investigate the preference of natural enemies for feeding on the focal mite species and how agrochemicals influence their survival, development and reproduction in order to assess the risk of transforming these minor pests into major ones.
References
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Bolland HR, Gutierrez J, Flechtman CHW (1998) World catalogue of the spider mite family (Acari: Tetranychidae). Brill Academic, Leiden
Bonato O (1999) The effect of temperature on life history parameters of Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 23:11–19
Bounfour M, Tanigoshi LK (2001) Effect of temperature on development and demographic parameters of Tetranychus urticae and Eotetranychus carpini borealis (Acari: Tetranychidae). Ann Entomol Soc Am 94:400–404
Chao SRS, Lo PKC (1974) Biological studies of the spider mite, Tetranychus truncatus and its natural enemies. J Taiwan Agric Res 23:126–135
Chen CY, Chiu MC, Kuo MH (2013) Effect of warming with temperature oscillations on a low-latitude aphid, Aphis craccivora. Bull Entomol Res 103:406–413
Davies JT, Ireson JE, Allen GR (2009) Pre-adult development of Phytoseiulus persimilis on diets of Tetranychus urticae and Tetranychus lintearius: implications for the biological control of Ulex europaeus. Exp Appl Acarol 47:133–145
Estay SA, Lima M, Bozinovic F (2014) The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 123:131–140
Fan LQ, Shi CL, Wang YP (2003) Developmental threshold temperature and the effective accumulated temperature of Tetranychus truncatus Ehara. Plant Prot 29(6):32–34
Ferrero M, de Moraes GJ, Kreiter S, Tixier MS, Knapp M (2007) Life tables of the predatory mite Phytoseiulus longipes feeding on Tetranychus evansi at four temperatures (Acari: Phytoseiidae, Tetranychidae). Exp Appl Acarol 41:45–53
Fujibayashi Y, Kushida T (1992) Susceptibility of Panonychus ulmi (Koch) and Tetranychus urticae Koch to acaricides in the Tsugaru region, Aomori Prefecture. Ann Rept Plant Prot North Jpn 43:152–154 (in Japanese)
Goka K (1999) The effect of patch size and persistence of host plants on the development of acaricide resistance in the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Exp Appl Acarol 23:419–427
Gotoh T (1997) Annual life cycle of populations of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) in four Japanese pear orchards. Appl Entomol Zool 32:207–216
Gotoh T, Gomi K (2003) Life-history traits of the Kanzawa spider mite Tetranychus kanzawai (Acari: Tetranychidae). Appl Entomol Zool 38:7–14
Gotoh T, Suwa A, Kitashima Y, Rezk HA (2004) Developmental and reproductive performance of Tetranychus pueraricola Ehara and Gotoh (Acari: Tetranychidae) at four constant temperatures. Appl Entomol Zool 39:675–682
Gotoh T, Sugimoto N, Pallini A, Knapp M, Hernandez-Suarez E, Ferragut F, Ho CC, Migeon A, Navajas M, Nachman G (2010) Reproductive performance of seven strains of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) at five temperatures. Exp App Acarol 52:239–259
Ikemoto T (2005) Intrinsic optimum temperature for development of insects and mites. Environ Entomol 34:1377–1387
Ikemoto T (2008) Tropical malaria does not mean hot environments. J Med Entomol 45:963–969
Ikemoto T, Takai K (2000) A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ Entomol 29:671–682
Ikemoto T, Kurahashi I, Shi P-J (2013) Confidence interval of intrinsic optimum temperature estimated using thermodynamic SSI model. Insect Sci 20:420–428
Khanamani M, Fathipour Y, Hajiqanbar H (2013) Population growth response of Tetranychus urticae to eggplant quality: application of female age-specific and age-stage, two-sex life tables. Int J Acarol 39:638–648
Kiritani K (2013) Different effects of climate change on the population dynamics of insects. Appl Entomol Zool 48:97–104
Kishimoto H (2002) Species composition and seasonal occurrence of spider mites (Acri: Tetranychidae) and their predators in Japanese pear orchards with different agrochemical spraying programs. Appl Entomol Zool 37:603–615
Kondo A, Takafuji A (1985) Resource utilization pattern of two species of tetranychid mites (Acarina: Tetranychidae). Res Popul Ecol 27:145–157
Lawo JP, Lawo NC (2011) Misconceptions about the comparison of intrinsic rates of natural increase. J Appl Entomol 135:715–725
Magalhaes S, Fayard J, Janssen A, Carbonell D, Olivieri I (2007) Adaptation in a spider mite population after long-term evolution ona single host plant. J Evol Biol 20:2016–2027
Manly BFJ (1990) Stage-structured populations: sampling, analysis and simulation. Chapman & Hall, London, p 187
McCullagh P, Nelder JA (1989) Generalized linear models. Monographs on statistics and applied probability 37, 2nd edn. Chapman & Hall, Boca Raton
Migeon A, Dorkeld F (2006–2013) Spider mites web: a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 28 Feb 2014
Mizukoshi T, Goto M (2003) Control of cyclamen mite, Steniotarsonemus pallidus (Banks) (Acarina: Tarsonemidae), by pouring of hot water on strawberry. Ann Rept Plant Prot North Jpn 55:232–235 (in Japanese)
Nakagaki S (1980) Seasonal prevalence of spider mites in pear orchard with reference to analysis of population density. Bull Ibaraki Fruit Tree Res Stn 8:37–51 (in Japanese)
Okazaki K (2000) IPM program by multiple mating disruptor in apple orchard. Agric Tech 59:409–413 (in Japanese)
Pang BP, Zhou XR, Shi L, Mu HB (2004) Performance of Tetranychus truncatus Ehara (Acarina: Tetranychidae) reared with different host plants. Acta Entomol Sin 47:55–58
Riahi E, Nemati A, Shishehbor P, Saeidi Z (2011) Population growth parameters of the two-spotted spider mite, Tetranychus urticae, on three peach varieties in Iran. Acarologia 51:473–480
Riahi E, Shishehbor P, Nemati AR, Saeidi Z (2013) Temperature effects on development and life table parameters of Tetranychus urticae (Acari: Tetranychidae). J Agric Sci Technol 15:661–672
Sabelis MW (1981) Biological control of two-spotted spider mites using phytoseiid predators. Part I. Modelling the predator–prey interaction at the individual level. Agricultural Research Reports, PUDOC, Wageningen, p 242
Sabelis MW (1985) Reproductive strategies. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control. Elsevier, Amsterdam, pp 265–278
Sabelis MW (1991) Life-history evolution of spider mites. In: Schuster R, Murphy PW (eds) The acari. Reproduction, development and life-history strategies. Chapman & Hall, London, pp 23–49
Saito Y (1979) Comparative studies on life histories of three species of spider mites (Acarina: Tetranychidae). Appl Entomol Zool 14:83–94
Sakunwarin S, Chandrapatya A, Baker GT (2003) Biology and life table of the cassava mite, Tetranychus truncatus Ehara (Acari: Tetranychidae). Syst Appl Acarol 8:13–24
SAS (2006) SAS Enterprise Guide 4.1. SAS Institute Inc. SAS Campus Drive, Cary NC 27513
Scranton K, Stavrinides M, Mills NJ, de Valpine P (2013) Small-scale intraspecific life history variation in herbivorous spider mites (Tetranychus pacificus) is associated with host plant cultivar. PLoS One 8(9):e72980
Shi P-J, Ikemoto T, Egami C, Sun Y, Ge F (2011) A modified program for estimating the parameters of the SSI model. Environ Entomol 40:462–469
Snell TW (1978) Fecundity, developmental time, and population growth rate. Oecologia 32:119–125
Takafuji A (1980) Population dynamics of some phytophagous mites and their predators on goldenrod Solidago altissima L. I. Seasonal trends in population numbers and spatial distributions. Res Popul Ecol 21:197–216
Takafuji A, Kamibayashi M (1984) Life cycle of a non-diapausing population of the two-spotted spider mite, Tetranychus urticae Koch in a pear orchard. Res Popul Ecol 26:113–123
Takafuji A, Yokotsuka T, Goka K, Kishimoto H (1996) Ecological performance of the spider mite, Tetranychus okinawanus Ehara (Acari, Tetranychidae), a species newly described from Okinawa Islands (1). J Acarol Soc Jpn 5:75–81
Takafuji A, Ozawa A, Nemoto H, Gotoh T (2000) Spider mites of Japan: their biology and control. Exp Appl Acarol 24:319–335
Trichilo PJ, Wilson LT (1993) An ecosystem analysis of spider mite outbreaks: physiological stimulation or natural enemy suppression. Exp Appl Acarol 17:291–314
Uchida M (1982) Ecological studies on the abundance and diapauses of spider mites and the damage caused by the spider mites in Japanese pear orchards. Spec Bull Tottori Fru Tree Exp Stn 2:1–63 (in Japanese)
Ullah MS, Moriya D, Badii MH, Nachman G, Gotoh T (2011) A comparative study of development and demographic parameters of Tetranychus merganser and Tetranychus kanzawai (Acari: Tetranychidae) at different temperatures. Exp Appl Acarol 54:1–19
Van de Vrie M, McMurtry JA, Huffaker CB (1972) Ecology of tetranychid mites and their natural enemies: a review III. Biology, ecology, and pest status, and host–plant relations of tetranychids. Hilgardia 41:343–432
Wrensch DL (1985) Reproductive parameters. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control. Elsevier, Amsterdam, pp 165–170
Wyatt IJ, White PF (1977) Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J Appl Ecol 14:757–766
Acknowledgments
The authors are grateful to Dr. T. Ikemoto for providing us R statistical software, and to Dr. Y. Kitashima and Mr. R. Sugawara, Ibaraki University, for their kind help in this research.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
See Table 6.
Appendix 2
See Table 7.
Appendix 3
See Table 8.
Appendix 4
See Table 9.
Rights and permissions
About this article
Cite this article
Gotoh, T., Moriya, D. & Nachman, G. Development and reproduction of five Tetranychus species (Acari: Tetranychidae): Do they all have the potential to become major pests?. Exp Appl Acarol 66, 453–479 (2015). https://doi.org/10.1007/s10493-015-9919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-9919-y