Abstract
The effect of five constant temperatures (16, 20, 24, 28 and 32 °C) on the development, survival and reproduction of Tetranychus cinnabarinus (Boisduval) [= Tetranychus urticae Koch (red form)] fed on cassava leaves was examined in the laboratory at 85% relative humidity. Development time of various immature stages decreased with increasing temperature, with total egg-to-adult development time varying from 27.7 to 6.7 days. The lower thermal threshold for development was 10.8 °C and the thermal constant from egg to adult was 142.4 degree-days. Pre- and post-oviposition period and female longevity all decreased as temperature increased. The longest oviposition period was observed at 20 °C with 20.4 days. Under different temperatures, mated females laid, on average, 1.0, 2.9, 4.7, 4.7 and 4.9 eggs per day, respectively. The maximum fecundity (81.5 eggs per female) was at 28 °C and the intrinsic rate of increase (r m ) was highest (0.25) at 32 °C. The results of this study indicate that T. cinnabarinus population could increase rapidly when cassava leaves serve as a food source. At the appropriate temperature T. cinnabarinus could seriously threaten growth of cassava.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassava, Manihot esculenta Crantz (Euphorbiaceae), also known as manioc, tapioca, and yuca, is a widely grown drought-tolerant crop that can be cultivated on marginal soils and can produce high yields given in favorable growing conditions. The carbohydrate storage capacity of its roots, including 30–35% starch, provides a major source of calories for humans living in tropical regions (Howeler et al. 2013). Nowadays, cassava is one of the most widely cultivated tropical crops, with the primary focus of much research aimed at conferring disease tolerance and increasing yield (Bredeson et al. 2016). In 2005, China’s cassava planting area was 0.6 million hectares with production totaling 11 million metric tons. This total yield accounted for approximatley 35% of China’s total bioethanol production capacity (Baeyens et al. 2015). Because suitable growing conditions for cassava are mainly confined to South and Southwest China, increased ethanol production from this crop will depend on increasing yield in these regions (Chen et al. 2016).
Approximately 45 species of phytophagous mites feed on cassava in the Americas, Africa and Asia. Climatic conditions in several Asian cassava growing countries are favorable for rapid dissemination and outbreaks of Mononychellus tanajoa, the cassava green mite. The Tetranychus, or red spider mite, complex predominates in Asia, where more than 10 species are reported from cassava (Bellotti 2008).
The carmine spider mite, Tetranychus cinnabarinus (Boisduval) [= Tetranychus urticae Koch (red form); Auger et al. 2013] (Acari: Tetranychidae), is a polyphagous species with nearly 100 host plant species, and is a serious pest in many cropping systems worldwide including various vegetables and fruits, cotton, and ornamentals (Luo et al. 2014). It can quickly adapt to different hosts, developing damaging populations on a variety of plants (Shen et al. 2016).
Temperature is usually the environmental factor with the greatest effect on developmental rate of immature mites and other poikilotherms. To quantify the effect of temperature on mite development, life stages of a given species may be held at constant temperatures and the resultant development times can be used to estimate developmental rate curves (Southwood 1978). From these developmental rate curves, models can be formulated to predict development time as a function of temperature. These models are useful in making pest management decisions and can be used as components of more comprehensive models for predicting population dynamics (Riahi et al. 2013). Development of T. cinnabarinus has been studied by many authors. Thus, Wu et al. (1988) and Gao et al. (2012) investigated its development at five temperatures, 15, 20, 25, 30 and 35 °C, finding a negative relationship between temperature and development of T. cinnabarinus, as development of each immature stage was shortened with increasing temperature.
At the same time, there are several studies that have developed life tables for T. cinnabarinus fed on different hosts, such as Solanum melongena (Biswas 2004), Dianthus caryophyllus (Tello et al. 2009), three varieties of muskmelon, Cucumis melo (Peralta and Tello 2011), eight strawberry cultivars (Kazak and Kibritci 2008), and different strains of Morus alba (Tao et al. 2005). However, few researchers have focused on T. cinnabarinus feeding on cassava (Bellotti 2008; Pan et al. 2011). Initial investigations of cassava planted in Dongxiang County, Jiangxi province of China, indicate that T. cinnabarinus is a serious pest (Peng et al. 2014). The present study examined development and reproduction of T. cinnabarinus on cassava at five temperatures (16, 20, 24, 28 and 32 °C) under laboratory conditions. Response variables included development, survival and fecundity rate, and life table parameters, which are important for predicting population dynamics of T. cinnabarinus under field conditions as well as for developing effective monitoring programs and management strategies for the pest.
Materials and methods
Mite colony
Tetranychus cinnabarinus was originally collected from cassava in Dongxiang, Jiangxi province, China, and was acclimated and propagated on cassava leaves for more than 1 year. In this study, mites were maintained in rearing units (McMurtry and Scriven 1964), consisting of a Petri dish (5 cm diameter) with a water-soaked sponge (3 cm diameter, 1 cm thick). A piece of filter paper (3 cm diameter) with plastic film (2 cm diameter) was placed on top of the sponge. The rearing units were kept in climate-controlled incubators (RXZ-260B; Ningbo Dongnan Instrument, China) at 25 ± 0.5 °C, 16D:8L photoperiod and 85% RH with the aid of an ultrasonic humidifier (D205; Beijing Yadu Science and Technology, China).
Development of immature stages
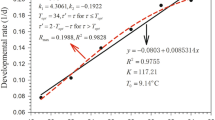
To obtain synchronized eggs for the experiments, 100 adult female T. cinnabarinus were taken from a stock colony and placed in the rearing units with fresh cassava leaves. The rearing units with the mites were maintained at 25 ± 0.5 °C, 85% RH and 16D:8L photoperiod. Eggs laid by the female of T. cinnabarinus within 6 h were transferred to new rearing units with a fine camel hair brush, one egg in each unit. Thereafter, these new units were placed in climate-controlled incubators and the development of immature stages was recorded at 85% RH and five constant temperatures ranging from 16 to 32 °C, at 4 °C interval. Every 2 days, cassava leaves were replaced to provide abundant fresh food for T. cinnabarinus in each rearing unit. The developmental stage of each individual was recorded every 12 h until they molted to adults. The relationship between temperature (T) and developmental rate (V), defined as the reciprocal of developmental time (days), was determined by a linear regression model: V = a + bT, where a and b are regression coefficients and estimated by means of least-squares regression. The lower thermal threshold for development (C) was estimated by extrapolating the regression line to the x-axis. The thermal constant K (sum of degree-days required for development to maturity, DD) was estimated for each case as the reciprocal slope of the regression line. Standard errors of C and K were computed as in Campbell et al. (1974).
Effect of temperature on longevity and reproduction
Newly-molted adult female T. cinnabarinus at 16, 20, 24, 28 and 32 °C were singly transferred into rearing units, together with five young males. Egg laying and survival of females were recorded daily and leaves were changed every 2 days. Males that escaped from the rearing units or died were replaced by new ones. Females that happened to drowned in the wet filter paper or died because of improper handling were excluded from data analysis. Parameters noted at each temperature were: pre-oviposition, oviposition and post-oviposition period, adult female longevity, average oviposition rate per female per day (daily fecundity), total oviposition per female (fecundity) and offspring sex ratio (% daughters).
Life table parameters
A life table was constructed from the observed survival and fecundity rates for individuals. Parameters at constant temperatures were calculated by the methods of Andrewartha and Birch (1954). The intrinsic rate of increase (r m ) was estimated by nonlinear regression according to the function: \(\sum\nolimits_{x = 0}^{\infty } {l_{x} m_{x} e^{{ - r_{m} x}} = 1}\) (Mou et al. 2015), where r m is the intrinsic rate of increase, x is female age in days, l x is the fraction of females surviving to age x (age-specific survival rate), and m x is the expected number of daughters produced per female alive at age x (age-specific fecundity rate), obtained by multiplying the number of eggs by the age-specific sex ratio, which is defined as the proportion of females in the progeny (Roy et al. 2003).
Net reproductive rate (R 0 ) is given by R0 = Σl x m x ; mean generation time (T, days) by T = Σxl x m x /R 0 ; finite rate of increase (λ) by λ = erm; and doubling time (DT) by DT = ln 2/r m (Mou et al. 2015).
Data analysis
One-way ANOVA was used to detect the effect of temperature on developmental time of immature stages, durations of pre-oviposition, oviposition and post-oviposition, longevity and fecundity. Means were compared using Tukey’s honestly significant difference (HSD) test. Besides, the equations describing the relationship between temperature and response variables were determined. These data were presented as mean ± SE. The survival rates and sex ratios of the progeny were compared using χ2 tests. Analyses were conducted using SPSS v.13.0 (SPSS, 1989–2004).
Results
Effects of temperature on development of immature stages
Over the range of temperatures tested, T. cinnabarinus fed on cassava leaves successfully developed from egg to adult, but development of immature stages was influenced by temperature (Table 1, Figure S1A–E).
The developmental rate of all immature stages increased linearly with temperature (Table 2). The correlation coefficient associated with all developmental stages were very high (R2 > 0.95), suggesting that the linear model accurately described the effect of temperature on developmental rate of T. cinnabarinus. The lower threshold temperatures for egg, larva and protonymph varied from (mean ± SE =) 10.92 ± 1.21 to 11.07 ± 2.34 °C (Table 2), and the thermal constant for completion of egg, larva and protonymph ranged from 24.33 ± 0.87 to 53.87 ± 1.174 degree-days (Table 2). The lower developmental threshold temperature (11.07 °C) for deutonymphs was highest and its thermal constant (24.33 degree-days) was the lowest compared to all other stages. The thermal constant was 142.38 degree-days for the whole generation.
Effects of temperature on the longevity and fecundity of female adult
As temperature increased, the longevity of T. cinnabarinus females decreased with the shortest female longevity (17.81 days at 32 °C) being less than one-third of the longest (54.19 days at 16 °C) (Table 3, Figure S1I). Although females laid eggs at all temperatures examined, the duration of the pre-oviposition period varied with temperature (Table 3, Figure S1F). This period decreased with increasing temperature up to 32 °C, after which it increased. The post-oviposition period had a maximum (3.45 days) at 24 °C and a minimum (0.92 days) at 32 °C, but showed no clear trend with temperature. The oviposition period at 32 °C was distinctly shorter compared with the other four temperatures. Females had a significantly longer preovipostion period at 16 °C than at other temperatures. The oviposition period was significantly shorter at 32 °C (Table 3, Figure S1G), whereas it was similar at other temperatures. The post-oviposition period varied from 0.95 ± 0.21 to 3.46 ± 0.80 days and was also influenced by temperature (Table 3, Figure S1H). The total number of eggs and daily egg production per female were highest in 28 °C, and were 81.54 and 4.73, respectively. The total number of eggs laid per female was significantly lower at 16 °C than at other temperatures (Table 3).
The ratio of oviposition period to longevity increased with temperature up to 28 °C. They were 34.8, 51.3, 52.4, 62.1 and 47.2%, respectively, from 16 to 32 °C. At the optimal temperature, 28 °C, T. cinnabarinus has a longer oviposition phase and a faster rate of population increase than at any other of the experimental temperatures (Table 3).
As shown in Table 3, sex ratio varied little with temperature, ranging from 73.8 to 76.6% females with no significant difference among them (Table 3).
Age-specific survival and fecundity rate
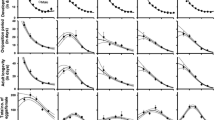
The rates of age-specific survival (l x ) and fecundity (m x ) of T. cinnabarinus were greatly influenced by temperature (Fig. 1). Age-specific survival rate (l x ) started to decline earlier as temperature increased from 16 to 32 °C (Fig. 1, dotted lines). The first death of an adult female occurred on day 12 at 32 °C, which was the earliest among all temperatures examined (15, 19, 23 and 32 days from 28 to 16 °C, respectively).
Age-specific fecundity rate (m x ) peaked earlier as temperature increased from 16 to 32 °C. Among the five temperatures examined, the first oviposition occurred on day 27, 15, 11, 9 and 7, respectively.
Daily egg production peaked on day 50 (1.2 eggs/day), 50 (4.0 eggs/day), 18 (5.7 eggs/day), 12 (6.4 eggs/day) and 12 (2.3 eggs/day) as temperature increased from 16 to 32 °C. Daily egg productions per female at 24 and 28 °C were similar, but significantly higher than at the other three temperatures. Therefore, the egg production per female had the same trend. Though daily egg production per female was higher at 32 than at 20 °C, the total egg production per female was less at 32 than at 20 °C due to the shorter oviposition period at the former temperature.
Demographic parameters
The intrinsic rate of increase (r m ) and the finite rate of increase (λ) reached the maximum value at 32 °C (Table 4). The value of r m varied from 0.07 to 0.25 day−1, and λ value ranged from 1.07 to 1.28 day−1. Net reproductive rate (R 0 ) was highest at 28 °C and lowest at 16 °C. Population doubling time was as long as 11.01 days at 16 °C, but only 2.77 days at 32 °C. Generation time decreased with increasing temperature, ranging from 43.17 to 13.91. DT and T were less than one-third of the longest.
Discussion
Although many researchers have reported life tables for T. cinnabarinus, few studies have focused on this species fed on cassava. In the present paper, T. cinnabarinus, fed on cassava leaves, successfully completed development over a temperature range from 16 to 32 °C, with temperature having a significant effect on the development of various immature stages. Population growth rates largely determine the pest status of spider mites and temperature strongly affects population growth (Roy et al. 2003; Gotoh et al. 2010). Therefore, knowing the temperature requirements of the different stages of mite pests can be used to forecast their potential distribution and abundance (Maula and Khan 2016). Because temperature so markedly affects developmental time as demonstrated by the present study (from 28 day at 16 °C to 6.7 days at 32 °C), our results indicate that the intensity of monitoring programs aimed at detecting and managing this pest will vary greatly depending on seasonal temperatures.
Development of T. cinnabarinus has been studied by many authors. Wu et al. (1988) and Gao et al. (2012) investigated its development at five temperatures, 15, 20, 25, 30 and 35 °C. There was a clear negative relationship between temperature and developmental time of T. cinnabarinus with each immature stage being shortened with increasing temperature. Wu et al. (1988) reported that T. cinnabarinus fed on leaves of broad bean required 6.0 ± 1.18 to 26.8 ± 2.83 days for complete juvenile development at temperatures ranging from 35 to 15 °C and 15:9 (L:D) photoperiod. Similarly, Gao et al. (2012) reported that the immature stages of T. cinnabarinus lasted from 6.1 ± 0.45 to 34.4 ± 1.66 days depending on temperature. Results of the present study are similar in that higher temperature can drastically shorten the developmental time of T. cinnabarinus.
The lower thermal threshold for development was 11.29 °C and the thermal constant was 157.07 degree-days in T. cinnabarinus fed on bean leaves (Gao et al. 2012). When fed on mulberry leaves, they were 8.90 °C and 159.20 degree-days for a whole generation (Tang et al. 1994). Lower thermal threshold temperatures of 8.2, 13.4 and 8.4 °C have been reported for the susceptible, the abamectin-resistant, and the fenpropathrin-resistant strain of T. cinnabarinus, respectively, fed on cowpea leaves (He et al. 2005). These findings indicate that even though different populations of T. cinnabarinus share similar thermal constants, lower thermal threshold for development may vary markedly.
The intrinsic rate of increase (r m ) is a key demographic parameter useful for predicting the population growth potential of an animal under given environmental conditions (Andrewartha and Birch 1954). Variation in development time of T. cinnabarinus at similar temperatures has been attributed to factors such as diet (host), photoperiod and relative humidity. Wu et al. (1988) found that photoperiod and temperature could affect r m value of T. cinnabarinus population. As temperature increased from 20 to 35 °C, r m values were increased from 0.134 to 0.343 day−1 at a photoperiod of 15L:9D, as well as from 0.172 to 0.284 day−1 at a photoperiod of 9L:15D. In the present study, the r m value was also increased with temperature, similar to the results of Northcraft and Waston (1987). Likewise, the r m value of T. cinnabarinus populations fed on M. alba increased from 0.077 to 0.333 day−1 as temperature increased from 15 to 28 °C (Wang et al. 2008). However, high temperature may also depress population growth. For instance, the research of Gao et al. (2012) demonstrated that r m values for T. cinnabarinus showed a humped pattern as temperature increased from 15 to 35 °C. The r m values in the present paper were slightly lower than reported by previous researchers. This difference might be due to different hosts, rearing methods and/or other environmental conditions such as relative humidity (Xia et al. 2012). More eggs were deposited at 24 °C and 38% RH than at any other combination of temperatures and relative humidity, when T. cinnabarinus was reared on bean (Hazan et al. 1974).
The host plant may influence the population dynamics of T. cinnabarinus. Liu and Wu (1988) found that the development of T. cinnabarinus varied among six hosts at 29.5 ± 0.5 °C, with r m values on cotton, corn, green bean, sesame, watermelon and potato of 0.218, 0.096, 0.306, 0.122, 0.231 and 0.311 day−1, respectively. The r m values of T. cinnabarinus fed on eggplant leaves were 0.087 (15L:9D) and 0.066 day−1 (9L:15D) at 25 °C (Cu et al. 1994). Likewise, r m values for T. cinnabarinus varied between cultivars of closely related host species, with values on Xinong6071 (Morus bombycis, 2x), Hetianbaisang (M. alba, 3x), Xinyizhilai (M. alba, 2x) and Dashi (Morus atropurpurea, 3x) being 0.419, 0.371, 0.362 and 0.353 day−1, respectively, at 28 ± 1 °C (Wu et al. 2006), and 0.231 day−1 on Fengchisang (Morus sp.) at 26 ± 1 °C (Tao et al. 2005). With three varieties of muskmelon, C. melo, the values for r m and R 0 of T. cinnabarinus were 0.22 day−1 and 29 for Galia, 0.20 day−1 and 45 for Cantaloupe and 0.19 day−1 and 32 for Honeydew, respectively (Peralta and Tello 2011). Fed on leaves of carnation, Dianthus caryophyllus, the r m values was 0.183 day−1 (Tello et al. 2009). The r m values of T. cinnabarinus fed on cassava were higher at 32 °C than many of the values obtained when using the above-mentioned host plants, indicating that cassava leaves are more suitable for its population increasing.
In the present study, mean generation time (T) and doubling time (DT) decreased with increasing temperature. The same phenomenon has been reported in several other studies (Gao et al. 2012; He et al. 2005; Northcraft and Waston 1987; Tang et al. 1994; Wang et al. 2008) indicating that high temperatures play a key role in accelerating population growth of T. cinnabarinus. In field investigations, the peak populations of T. cinnabarinus coincided with the high temperatures experienced during August (Peng et al. 2014). On the other hand, net reproductive rate (R 0 ) increased with temperature up to 28 °C, decreasing notably thereafter. Wang et al. (2008) made similar observations with respect to R 0 when the temperature increased from 15 to 28 °C. However, a distinct, non-linear relationship was observed when rearing temperature increased from 15 to 35 °C, with R 0 values increasing followed by a steep decline at temperatures above 25 °C (32.97, 49.04, 99.64, 66.86 and 15.56) (Gao et al. 2012). These results indicate that higher temperatures may depress T. cinnabarinus populations, a fact that likely explains the observation of a second population peak occurring in November which was higher than the one during summer (Peng et al. 2014). The pattern may reflect that summer temperatures reached or exceeded 35 °C. At this temperature, r m and R 0 decline to 0.28 day−1 and 15.56, respectively (Gao et al. 2012).
Several studies have reported a relatively stable offspring sex-ratio for T. cinnabarinus, although this ratio may vary with temperature (Wang et al. 2008). Excluding pajaro and sweet charlie cultivars, sex ratios of T. cinnabarinus on six strawberry cultivars were similar (Kazak and Kibritci, 2008). Offspring sex-ratio did not vary significantly with temperature between two strains of the carmine spider mite (70% at 25 °C and 75% at 30 °C). Sex ratio was also similar for the strain studied on apples (73% at 25 °C and 77% at 30 °C) (Sahraoui and Grissa 2006). Similar sex ratios were observed in the present study, indicating that temperature has no significant effect on the sex ratio of T. cinnabarinus. The most important arthropod pest affecting cassava is the cassava green mite, M. tanajoa (Poubom et al. 2005). The highest r m value of this pest was 0.28 at 31 °C (Yaninek et al. 1989), which was similar to that with T. cinnabarinus in the present paper. The studies of T. cinnabarinus on cassava indicate that it has strong potential to be a serious pest of cassava, especially if natural enemies are lacking. For instance, in the Zhanzhou area of Hainan province, T. cinnabarinus increased very fast and resulted in serious damage from March to June every year, indicating that it deserves serious attention as a potential pest (Fu et al. 1994). In a previous study, the same kind of damage was found in the cassava fields of Jiangxi province (Peng et al. 2014).
More than 46 mite species were recorded from cassava fields in Mozambique, 46% belonging to the Phytoseiidae. In Malawi, more than 52 species were recorded from cassava fields, 62% of which were phytoseiids. Mites of this family were the most abundant predators in many countries (Zannou et al. 2005). Neoseiulus barkeri is the dominant phytoseiid and was produced on an industrial scale in Jiangxi province (Xia et al. 2012). We found that predation rate and a/T h values (a for the attack rate coefficients, T h for the handling time) of female of N. barkeri on T. cinnabarinus feeding on cassava leaves increased with the rising temperature, with the former peaking at 28 °C and the latter being significantly higher at 28 and 32 °C (Song et al. 2012). The potential for N. barkeri to serve as a potential biological control agent against T. cinnabarinus in cassava should be evaluated in the field.
References
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Auger P, Migeon A, Ueckermann EA, Tiedt L, Navarro MN (2013) Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): review and new data. Acarologia 53(4):383–415
Baeyens J, Kang Q, Appels L, Dewil R, Lv Y, Tan T (2015) Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci 47:60–88
Bellotti AC (2008) Cassava pests and their management. In: Capinera JL (ed) Encyclopedia of entomology, 2nd edn. Springer, Dordrecht
Biswas GC (2004) Some biological aspects of carmine spider mite, Tetranychus cinnabarinus Boisd. (Acari: Tetranychidae) infesting egg-plant from Rajshahi [Bangladesh]. J Biol Sci (Pakistan)
Bredeson JV, Lyons JB, Prochnik SE, Wu GA, Ha CM, Edsinger-Gonzales E, Grimwood J, Schmutz J, Rabbi IY, Egesi C, Nauluvula P, Lebot V, Ndunguru J, Mkamilo G, Bart RS, Setter TL, Gleadow RM, Kulakow P, Ferguson ME, Rounsley S, Rokhsar DS (2016) Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat Biotechnol 34(5):562–570
Campbell A, Franzer BD, Gilbert N, Gutierrez AP, McKauer M (1974) Temperature requirements of some aphids and their parasites. J Appl Ecol 11:431–438
Chen W, Wu F, Zhang J (2016) Potential production of non-food biofuels in China. Renew Energy 85:939–944
Cu CD, Li H, Hong B, He DH, Xia XL, Ren XY (1994) Establishment and analysis of life table for experimental population of Tetranychus cinnabarinus in Ningxia. J Ningxia Agric Coll 15(2):26–31
Fu YG, Lin YM, Zhang JH (1994) Characteristics of laboratory population of Tetranychus cinnabarinus (Boiduval). Chin J Trop Crops 15(1):103–107
Gao P, Zhou YS, Zhao YW (2012) Effect of temperature on the population growth of Tetranychus cinnabarinus in laboratory. North Hortic 11:147–149
Gotoh T, Sugimoto N, Pallini A, Knapp M, Hernandez-Suarez E, Ferragut F, Ho CC, Migeon A, Navajas M, Nachman G (2010) Reproductive performance of seven strains of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) at five temperatures. Exp Appl Acarol 52:239–259
Hazan A, Gerson U, Tahori AS (1974) Life history and life tables of the carmine spider mite. Acarol 15(3):414–440
He L, Zhao ZM, Cao XF, Deng XP, Wang JJ (2005) Effect of temperature on development and fecundity of resistant Tetranychus cinnabarinus (Boiduval). Acta Entomol Sinica 48(2):203–207
Howeler R, Lutaladio N, Thomas G (2013) Save and Grow: Cassava—a guide to sustainable production intensification. FAO-UN, Rome
Kazak C, Kibritci C (2008) Population parameters of Tetranychus cinnabarinus Boisduval (Prostigmata: Tetranychidae) on eight strawberry cultivars. Turk J Agric For 32(1):19–27
Liu XC, Wu KM (1988) The influences of different host plant to development and reproduction of carmine spider mite Tetranychus cinnabarinus. Acta Agric Boreali-Sinica 3(4):86–91
Luo YJ, Yang ZG, Xie DY, Ding W, Da AS, Ni J, Chai JP, Huang P, Jiang XJ, Li SX (2014) Molecular cloning and expression of glutathione S-transferases involved in propargite resistance of the carmine spider mite, Tetranychus cinnabarinus (Boisduval). Pestic Biochem Physiol 114:44–51
Maula F, Khan IA (2016) Effect of temperature variation on the developmental stages of Tetranychus urticae Koch and Panonychus ulmi Koch (Tetranychidae: Acarina) under laboratory conditions in Swat valley of Khyber Pakhtunkhwa, Pakistan. J Entomol Zool Stud 4(1):279–283
McMurtry JA, Scriven GT (1964) Studies on the feeding, reproduction and development of Amblyseius hibisci (Acarina: Phytoseiidae) on various food substances. Ann Entomol Soc Am 57:649–655
Mou DF, Lee CC, Smith CL, Chi H (2015) Using viable eggs to accurately determine the demographic and predation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J Appl Entomol 139(8):579–591
Northcraft PD, Watson TF (1987) Developmental biology of Tetranychus cinnabarinus (Boisduval) under three temperature regimes. Southwest Entomol 12(1):45–50
Pan WQ, Li HY, Yan WH, Li ZG, Wei YC, Huang J (2011) Outbreak regularity of cassava Tetranychus cinnabarinus in Wuming county. Chinese J Trop Agric 31(8):34–38
Peng ZR, Song SX, Zhong L, Song JH, Wang J, Xia B (2014) Population dynamic and spatial distribution type of Tetranychus cinnabarinus damaged Manihot esculenta Crantz. J Nanchang Univ (Nat Sci) 38(5):488–491
Peralta CO, Tello MV (2011) Life tables of Tetranychus cinnabarinus (Acari: Tetranychidae) on three varieties of muskmelon, Cucumis melo. Rev Colomb Entomol 37(1):21–26
Poubom CFN, Awah ET, Tchuanyo M, Tengoua F (2005) Farmers’ perceptions of cassava pests and indigenous control methods in Cameroon. Int J Pest Manag 51:157–164
Riahi E, Shishehbor P, Nemati AR, Saeidi Z (2013) Temperature effects on development and life table parameters of Tetranychus urticae (Acari: Tetranychidae). J Agric Sci Technol 15(4):661–672
Roy M, Brodeur J, Cloutier C (2003) Temperature and sex allocation in a spider mite. Oecologia 135:322–326
Sahraoui H, Grissa KL (2006) Demographic traits of two phytophagous mites (Tetranychus cinnabarinus and Aculops lycopersici) and biological control on tomato. In: X international symposium on the processing tomato, vol 758, pp 81–88
Shen GM, Song CG, Aoyang QY, Xiao YH, Zhang YJ, Pan Y, He L (2016) Transgenic cotton expressing CYP392A4 double-stranded RNA decreases the reproductive ability of Tetranychus cinnabarinus. Insect Sci 24:559–568
Song SX, Liu GH, Xin TR, Zou ZW, Xia B (2012) Predation of Neoseiulus barkeri on Tetranychus cinnabarinus. J Nanchang Univ (Nat Sci) 36(5):486–489
Southwood TRE (1978) Ecological methods, with particular reference to the study of insect populations. Chapman and Hall, London
Tang YX, Long SZ, Qi DM, Zhao H, Zhou KY (1994) Study on the development threshold temperature and effective accumulated temperature of Tetranychus cinnabarinus (Boiduval). Acta Sericol Sinica 20(4):241–242
Tao SQ, Wu FA, Yu MD (2005) Analysis of laboratory population life table of Carmine spider mite (Tetranychus cinnabarinus) infesting Feng chi sang Morus L. Acta Arachnol Sinica 14(1):33–36
Tello MV, Vargas MR, Araya CJ (2009) Life history parameters of Tetranychus cinnabarinus (Acari: Tetranychidae) on leaves of carnation, Dianthus caryophyllus. Rev Colomb Entomol 35(1):47–51
Wang XK, Wu FA, Tao SQ, Wang W, Cheng JL (2008) Effects of temperature on population of Carmine spider mite (Tetranychus cinnabarinus) fed with White mulberry (Morus alba). Acta Ecol Sinica 28(6):2645–2653
Wu QH, Zhong J, Xu YM (1988) Combined effects of temperature and light on the experimental population of the carmine mite, Tetranychus cinnabarinus (Acarina: Tetranychidae). Acta Ecol Sinica 8(1):66–77
Wu FA, Zhou JX, Yu MD, Wang QL, Xu L, Lu C, Jing CJ (2006) Statistical inference on the intrinsic rate of increase of the carmine spider mite, Tetranychus cinnabarinus on different mulberry cultivars (Morus L.) under laboratory conditions. Acta Entomol Sinica 49(2):287–294
Xia B, Zou ZW, Li PX, Lin P (2012) Effect of temperature on development and reproduction of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Aleuroglyphus ovatus. Exp Appl Acarol 56:33–41
Yaninek JS, Gutierrez AP, Herren HR (1989) Dynamics of Mononychellus tanajoa (Acari: Tetranychidae) in Africa: experimental evidence of temperature and host plant effects on population growth rate. Environ Entomol 18(4):633–640
Zannou ID, Hanna R, de Moraes GJ, Kreiter S, Phiri G, Jone A (2005) Mites of cassava (Manihot esculenta Crantz) habitats in Southern. Int J Acarol 31(2):149–164
Acknowledgements
The research was funded by the National Foundation of Nature Science of Jiangxi Province (Grant Nos. 20151BAB204016, 20161BBF60066, 20161ACB20003 and 20172BCB22004), the National Natural Science Foundation of China (grant nos. 31460553 and 31760621) and Foundation from the Administration of Science and Technology in Nanchang City (Grant No. 2013HZCG008), Fund from Education Department of Jiangxi Province (Grant No. KJLD14014). We would like to thank Douglas S. Richmond (Purdue University, USA) for his support and comments on our writing.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

10493_2018_241_MOESM1_ESM.jpg
Figure S1 The relationship between temperatures and development periods of Tetranychus cinnabarinus (A) egg; (B)larva; (C) protonymph; (D) deutonymph; (E) immature; (F) preoviposition; (G) oviposition; (H) post-oviposition; (I) longevity. (JPEG 431 kb)
Rights and permissions
About this article
Cite this article
Zou, Z., Xi, J., Liu, G. et al. Effect of temperature on development and reproduction of the carmine spider mite, Tetranychus cinnabarinus (Acari: Tetranychiae), fed on cassava leaves. Exp Appl Acarol 74, 383–394 (2018). https://doi.org/10.1007/s10493-018-0241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0241-3