Abstract
Use of the mycopathogen Beauveria bassiana (strain GHA), marketed as BotaniGard® ES, was evaluated as a plant protection strategy against the spider mite Tetranychus urticae Koch, which is considered one of the most economically important and cosmopolitan pests of many crops. Tetranychus urticae were treated with four concentrations of conidia (1 × 105, 1 × 106, 1 × 107, or 1 × 108 conidia/ml), and virulence was assessed on mites held at four relative humidity levels (35, 55, 75, and 95 ± 2 % RH) at 25 ± 1 °C. At 1 × 108 spores/ml, the LT50 value was 9.7 h at 95 % RH, which was significantly lower than values for other RH levels. At 1 × 107 spores/ml, the LT50 value was 43.8 h at 95 % RH, which was significantly different from values at 55 and 35 % RH. The efficacy of B. bassiana product was also verified on mites infesting potted bean plants with a concentration of 1 × 108 spores/ml. In double spray treatment where applications were made 2 × on days 5 and 10 after mite infestation, the nymphal and adult population of T. urticae were reduced to zero on days 20 and 15, respectively. With a single spray on day 5, the nymphal population was also greatly reduced, but increased rapidly after day 20. Single and double sprays with B. bassiana reduced leaf damage as measured by image analysis by 33 and 94 % compared to no treatment, respectively. These results suggest that 1 × 108 spores/ml was the most effective dose and that two applications, at a 5-day interval, provided control of T. urticae in our laboratory assay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetranychus urticae Koch (Acari: Tetranychidae) is one of the most polyphagous herbivorous arthropods, feeding on more than 1,100 plant species in over 140 plant families (Grbić et al. 2011; Migeon and Dorkeld 2014). With global warming, the detrimental effects of spider mites on agriculture will increase due to their rapid developmental rate at higher temperatures (Ullah et al. 2012). Chemical pesticides is the most commonly used control tactic for T. urticae, but this species rapidly and commonly develops pesticide resistance (Van Leeuwen et al. 2010). Many biological aspects of the spider mite, including its rapid development, high fecundity, and arrhenotokous reproduction seem to facilitate the rapid evolution of pesticide resistance, in addition to direct changes in the sensitivity of the target site due to point mutations or sequestration/metabolism of the pesticide (Van Leeuwen et al. 2010). Therefore, alternative IPM strategies such as biological control should be developed and applied to supplement the chemical acaricides that are currently being used.
The entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin is a ubiquitous pathogen of many pest arthropods such as aphids, leafhoppers, and whiteflies (Faria and Wraight 2001; Feng et al. 2004; Hatting et al. 2004; Pu et al. 2005) whose repeated fungal application has been found to control certain arthropod pests (Groden et al. 2002). In recent years, interest in using mycoinsecticides, including B. bassiana, for the control of mites has increased (Alves et al. 2002, 2005; Shi and Feng 2006, 2009; Seiedy et al. 2010). The efficacy of entomopathogenic fungi is affected by several abiotic factors, such as temperature, humidity, and solar radiation (Benz 1987; Inglis et al. 2001; Alves et al. 2005; Seiedy et al. 2010), host population level, and the presence of antagonists (James et al. 2003; Toledo et al. 2011). Successful use of mycoinsecticides depends particularly on ambient relative humidity (RH) conditions and concentration of conidia applied (Ferron et al. 1991; Alves et al. 2005; Devi and Rao 2006). To optimize control of spider mite by a particular B. bassiana strain, preliminary evaluation is necessary to determine the effect of both conidial concentration and relative humidity. However, no reports have revealed the interactive effect of conidial concentration of B. bassiana and level of humidity on infection levels in T. urticae except Shi et al. (2008) who tested the ovicidal effect of B. bassiana under various temperature and humidity regimes. In this study, we evaluated the acaricidal effect of a commercial formulation of B. bassiana (BotaniGard® ES) on T. urticae under four conidial concentrations and four RH conditions. We (1) determined the most effective concentration and RH for maximizing the virulence of B. bassiana on T. urticae and (2) confirmed the efficacy of the optimal conidial concentration on potted bean plants.
Materials and methods
Mites
Tetranychus urticae, obtained from Dongbu Farm Ceres Company, Nonsan, Korea, were reared on leaf discs (ca. 16 cm2) of common bean, Phaseolus vulgaris L., in a growth chamber (DS-11BPL, Dasol Scientific, Suwon, Korea) in Andong National University, for more than 1 year. Bean leaf discs were placed on water-saturated polyurethane mats in plastic Petri dishes (90 mm diameter, 20 mm depth) at 25 ± 1 °C, 60–70 % RH, and a photoperiod of 16:8 h L:D. To obtain fixed-age females for the bioassay, quiescent deutonymphs were collected from the mite culture and isolated on fresh leaf discs. Newly emerged females were used for the experiments 3–5 days later.
Fungal pathogen and preparation of conidial suspension
The entomopathogenic fungus tested was BotaniGard® ES (B. bassiana, GHA strain, Arysta LifeScience, Tokyo, Japan) obtained commercially. For the bioassay, subcultures were grown on Sabouraud Dextrose Agar (SDA) in Petri dishes and maintained in the dark at the ambient temperature (25 ± 1 °C) for 10–14 days. Conidia were harvested from surface cultures by scraping and were then suspended in 10 ml of sterile distilled water containing 0.05 % Triton X-100 using universal bottles containing glass beads. Conidial suspensions were vortexed for 5 min, and spore concentrations were determined using a haemocytometer (Neubauer-improved haemocytometer, Lauda-Königshofen, Germany). The viability of conidia was determined before the bioassay by spread-plating 0.1 ml of conidial suspension titrated to 1 × 104 conidia ml−1 on SDA plates. Plates were incubated at 25 ± 1 °C, and the percentage germination was determined after 24 h from 100-spore counts by placing a sterile microscope coverslip on each plate under a microscope (Nikon, Eclipse E200, Japan). Each plate was replicated 4×. Conidia germination >90 % was observed in all tests. Suspensions were prepared at concentrations of 1 × 105, 1 × 106, 1 × 107, and 1 × 108 conidia ml−1. The spore suspensions were used just after preparation.
Leaf disc assay
LT10, LT50, and LT90 values for adult females of T. urticae were assessed after application of B. bassiana. Leaf discs (ca. 16 cm2) were prepared using cotyledonous leaves of common bean, which were then individually placed on wet cotton pads in a Petri dish (90 mm diameter). Three to 5 day-old mated females of T. urticae were placed on a new bean leaf disc (ca. 4 × 4 cm2) and incubated for 24 h. There were four replicates for each treatment, and 15 adult mites were used in each replicate. Dead or injured individuals were then removed, and the four concentrations of B. bassiana conidial suspension (1 ml/cm2) were sprayed onto the mite-infested discs using a hand sprayer. In order to calibrate the sprayer, five consecutive sprays were performed on Petri dishes in triplicate, and the deposits were quantified before the bioassay—no significant differences were obtained (data not shown). For a control, just distilled water was sprayed on a subset of leaf discs. After air drying, the mite-infested discs were held under four RH regimes (35, 55, 75, and 95 ± 2 %), all at 25 ± 1 °C and a 16:8 h L:D photoperiod in an incubator until mite death. The four RH regimes were achieved by dissolving MgCl2, Mg(NO3)2·6H2O, NaCl, and K2SO4 in distilled water in desiccators (140 mm diameter, Scienceware®, Wayne, New Jersey, USA) to prepare 35, 55, 75, and 95 ± 2 % RH, respectively (Rockland 1960). Temperature and RH were measured using a data logger (U10-001; Onset Computer Corporation, Cape Cod, MA, USA). Mites that did not move their appendages when touched with a fine brush were regarded as dead, and mortality was recorded at 8-h intervals. The dead mites were transferred autoclaved Petri dishes with a moist filter paper lining to allow the growth of fungus on the surface of the cadavers. Mortality caused by B. bassiana was confirmed by microscopic observation of spores on the surface of the mites.
Potted bean plant assay
This experiment with B. bassiana was conducted with a concentration of 1 × 108 conidia ml−1 prepared by diluting Botaniguard with distilled water (1 ml 1.6 × 1010 conidia ml−1 Botaniguard + 159 ml distilled water) at 20–25 °C and an RH of 33.5–51.0 %. Twenty-five adult gravid females of T. urticae were released onto each kidney bean plant which was 2 weeks old from the time of seeding. Tanglefoot (The Tanglefoot Company, Grand Rapids, MI, USA) was applied at the base of each plant to prevent the mites from moving down the stem and into the soil. The mite-infested plants were divided into three treatment groups (double sprays, single spray, and control), and each treatment was conducted with six plants. After 5 days, an initial count of nymphs and adult spider mites was made the day before the first spray. Mite-infested leaves of kidney bean were sprayed using a hand sprayer. All leaves in each plant were examined for the presence of nymphs and adults using a hand magnifier and microscope. After the first spray (1 ml/cm2), mite densities (the number of nymphs and adults per plant) were monitored every 5 days using the same sampling method. In the ‘double spray’ treatment, the second spray was conducted 5 days after the first. In the control treatment, kidney bean plants were sprayed once with distilled water only. The efficacy of B. bassiana application was evaluated based on the counts of live nymphs and adult spider mites under microscope.
Measurement of leaf area damaged by mites
Spider mites live and feed inside silk webs on the underside of leaves (Aponte and McMurtry 1997). Feeding by mites induces necrosis of leaf tissue below silk mats, resulting in a pattern of small (ca. 1–5 mm2) white spots located primarily along leaf veins of kidney bean. In order to study the effect of application frequency of B. bassiana on leaf damage, we quantified mite feeding damage using image analysis software (SigmaScan Pro, SPSS, Chicago, IL, USA) by measuring the area of these white spots. Damaged area was corrected for control leaves where neither mite nor fungus was applied using the following formula:
Statistical analyses
The percentage of dead mites was corrected using Abbott’s (1925) formula. The LT10, LT50, and LT90 were determined by Probit analysis using POLO-Plus (LeOra software 1987). The correction of overlapping confidence intervals of the LT10, LT50, and LT90 was used to establish whether or not lines were significantly different at the 5 % level (Robertson et al. 2007). Before analysis, the values were ln-transformed (number of nymphs, number of adults) or arcsine transformed (leaf area damage) to normalize the data. For the potted plant results, a repeated measure ANOVA was used to compare the temporal variation in T. urticae (nymphs and adults) density among treatments and the sampling dates (SAS Institute 2000). Sigma Plot 8.0 was used for graphical representation of leaf damage differences using box-plots.
Results
Observation of fungal infection
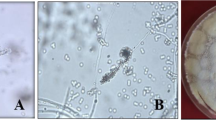
Spider mite death caused by mycosis began 3 days after fungal application in the leaf disc assay. Infected females became sluggish, darker, and slightly swollen before death. The mycotic cadavars showed inconspicuous fungal out-growth on the bean leaves at 95 and 75 ± 2 % RH (Fig. 1a–d). In high RH conditions, most dead mites became well mycotized within 3 days of being transferred into SDA media (Fig. 1e). Dead mites in control did not produce any mycotic cadavars on SDA media (Fig. 1f). In addition, typical mycelia and conidia of B. bassiana grew on the mycotic cadavers, indicating the potential for transmission to other individuals.
Laboratory bioassay
Adult mite mortality in response to the various combinations of conidial concentration and RH is shown in Table 1 and Fig. 2. Morality greatly increased with the conidial concentration of B. bassiana, being highest at a concentration of 1 × 108 and a RH of 95 ± 2 %. At concentrations of 1 × 108 and 1 × 107 conidia/ml, the LT50-values at 95 ± 2 % RH were 9.7 and 43.8 h, respectively. These periods were significantly shorter than those for lower RHs (Table 1). At lower concentrations of 1 × 106 and 1 × 105 conidia/ml, the LT50 did not differ significantly among the examined RH regimes (Table 1). The effect of RH was found to be significant only in the treatments with higher conidial concentrations (Table 1; Fig. 2).
Potted bioassay
Initial population levels of adult spider mites were 17.2, 17.0, and 18.0 per plant before the assay which were subjected to the treatments of double, single, or no fungal spray, respectively (F 2,15 = 0.12, P = 0.88) (Fig. 3). Adult spider mites increased rapidly in the control treatment, reaching 436 adults per plant 30 days after inoculation (Fig. 3). In the single spray treatment, mites decreased after the application of B. bassiana on day 5 until day 15, after which they rebounded, reaching 73.0 per plant on day 35. In the double spray treatment, adult mite numbers reached zero per plant on day 15 (F 2,15 = 59.62, P < 0.001). Repeated measures ANOVA showed significant differences in the adult population among treatments or sample days from day 15 to 35 (on day 15: F 2,105 = 635.13; on day 20: F 2,105 = 2,138.05; on day 25: F 2,105 = 1,939.64; on day 30: F 2,105 = 2,986.06; on day 35: F 2,105 = 1,661.49, all P < 0.001). The time × treatment interaction was also significant in adult of T. urticae (F 7,105 = 373.32, P < 0.001).
In the control, nymphal density increased rapidly, reaching about 956 per plant after 30 days of inoculation, and infested leaves became white and wilted. But in fungal application treatments, nymphal density was greatly reduced on days 10, 15, and 20 after inoculation (on day 10: F 2,15 = 84.88; on day 15: F 2,15 = 117.56; on day 20: F 2,15 = 237.34, all P < 0.001). In the single spray treatment, nymphs started to rebound on day 25 and increased rapidly for the rest of the study period. However, in the double spray treatment, nymphs decreased to zero by day 20 (Fig. 3). Repeated measures ANOVA revealed that the reduction in the total number of nymphs was maintained in the rest of the study period (on day 25: F 2,15 = 1,854.39; on day 30: F 2,15 = 3,437.72; on day 35: F 2,15 = 4,735.78, all P < 0.001). The time × treatment interaction was significant in nymph of T. urticae (F 7,105 = 234.77, P < 0.001).
Leaf area damage
Piercing and sucking leaf cell contents by T. urticae resulted in the loss of chlorophyll and reduced photosynthetic area, leaving the leaf covered with white spots. Analysis of plant damage 35 days after T. urticae inoculation showed significant differences among the treatments (F 2,27 = 207.196, P < 0.001). The control leaves showed the largest area of leaves damaged (42.8 %), followed by the single fungal spray (28.7 %) and then the double spray treatment (2.6 %). Leaf damage in the single and double spray treatments was reduced by 33 and 94 % compared to the control, respectively (Fig. 4).
Discussion
The efficacy of B. bassiana against T. urticae was evaluated both in leaf discs and in potted plants of Ph. vulgaris. The leaf disc assay suggested a threshold concentration of B. bassiana of 1 × 108 conidia/ml to be most effective for the management of T. urticae. A number of entomopathogenic fungi, including B. bassiana, have been evaluated for the control of spider mites with varying efficacy. Chandler et al. (2005) tested B. bassiana Naturalis-L at a rate of 1 × 108 conidia/ml against T. urticae, and found it reduced mite populations by 97 % in a tomato greenhouse. However, B. bassiana 432.99 and Naturalis-L isolates applied at a rate of 1 × 108 conidia/ml caused lower rates of mortality of T. urticae in the laboratory (46.2–72.2 % and 52.1–95.2 %, respectively). Andreeva and Shternshis (1995) and Tamai et al. (1999) reported B. bassiana isolate 447 to be ineffective against T. urticae in a laboratory assay, causing only 51.7 % mortality, even when applied at 1 × 109 conidia/ml at 70 ± 5 % RH (Tamai et al. 1999). Other isolates of B. bassiana also caused only low mortality (16–33 %) against T. urticae when used at the rate of 1 × 107 conidia/ml by 6 days post-inoculation (Chandler et al. 2005). Several factors may be responsible for this variation in the efficacy of B. bassiana against spider mites, including isolate identity, dose, experimental conditions including temperature and humidity, host species, interval of application, and plant variety. Variable enzymatic and DNA characteristics among isolates of B. bassiana may also be involved in this fungus’ differential pathogenicity and virulence to various arthropods (Almeida et al. 1997; Moino et al. 1998).
Our results clearly indicated that infection of T. urticae by B. bassiana was highly dependent on conidial concentration and, to a lesser extent with an exception at 95 % RH of 1 × 108 spores/ml. A conidial concentration lower than 1 × 108 conidia/ml caused similar mortality irrespective of RH. Due to the small size and cryptic habitat of mites, there might be less possibility of contact for concentrations below this threshold. When invaded by fewer conidia, the insect immune system may be successful in suppressing them through phagocytosis, melanization, or encapsulation responses. Thus, no infection would be apparent if the conidial dose were lower than such a threshold (Devi and Rao 2006). Several studies with other entomopathogenic fungi showed that a certain minimum pathogen load is often required for successful infection, such as 107 conidia/ml of B. bassiana against Mylabris pustulata (Devi and Rao 2006), 1.6 × 108 conidia/m2 of M. anisopliae against Anopheles gambiae s s and Culex quinquefasciatus (Scholte et al. 2003), and 1 × 106 conidia/ml of M. anisopliae isolate Qu-M984 against Pseudococcus viburni (Pereira et al. 2011). Furthermore, higher concentrations of B. bassiana have been known to infect Lygus hesperus successfully even at low humidity (Dunn and Mechalas 1963). Ambient humidity has also been found to have little impact on B. bassiana (Moore 1973; Ferron 1977). Ramoska (1984) reported that B. bassiana isolate RS 792 was infective against the chinch bug, Blissus leucopterus (Say), at relative humidities of 30–100 %. No significant effect of relative humidities of 12–100 % was found on grasshopper mortality related to B. bassiana isolate Bd GK 2016 (Marcandier and Khachatourians1987).
To simulate the potential effectiveness of B. bassiana under semi-field conditions, a bioassay was also conducted on potted bean plants with two treatments of application frequency. Double sprays of formulated B. bassiana at 1 × 108 conidia/ml at 5 day intervals successfully suppressed T. urticae infesting bean plants. The 5 day interval between the two applications was determined by the duration of the egg stage of the two-spotted spider mite is about 4 days at 25 °C (Kavousi et al. 2009). Thus, nymphs hatched from the surviving eggs could be further infected from the second spray, although the ovicidal effect of B. bassiana has also been demonstrated (Shi et al. 2008). In a similar study by Gatarayiha et al. (2011), 2 × applications of B. bassiana R444 (1.6 × 1012 conidia/ha) at 1 or 2-week intervals showed better control of T. urticae than applications at 3 or 4-week intervals in eggplant. Nevertheless, in the same study, even repeated applications with the higher dose of B. bassiana did not cause higher mortality, which was only 40.7–56.3 % at 49 days after the initial spray. Feeding damage on the bean leaves in this study was also reduced by 94 %, while Gatarayiha et al. (2011) found only a 60–66 % reduction in eggplant damage from the repeated application of B. bassiana. It was interesting to see that B. bassiana reduced the T. urticae population well even under low RH condition (33.5–51.0 %) in the laboratory, although it took 72 h to cause 96 % adult mortality, similar to that shown in this study’s leaf disc assay at 55 ± 2 % RH. This is probably due to the fact that the microhabitat on the leaf surface retains moisture (Ferron 1977, Shipp et al. 2003) and thus a higher RH than ambient conditions (Willmer 1986).
In conclusion, B. bassiana in concentrations of 1 × 108 spores/ml caused the highest mortality of T. urticae, with the infection rate reaching 100 % within 88 h, irrespective of RH. In addition, double sprays of B. bassiana (1 × 108 spores/ml) at 5-day intervals successfully suppressed T. urticae populations on potted bean plants. Incorporating entomopathogenic fungi into integrated mite management programs could reduce the dependence on synthetic acaricides and increase the levels of control, especially in the early season. However, field application of B. bassiana needs to be evaluated.
References
Abbott SW (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Almeida JEM, Alves SB, Pereira RM (1997) Selection of Beauveria spp. isolates for control of the termite Heterotermes tenuis (Hagen, 1858). J Appl Entomol 121:539–543
Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J Invert Pathol 81:70–77
Alves SB, Tamai MA, Rossi LS, Castiglioni E (2005) Beauveria bassiana pathogencity to the citrus rust mite Phyllocoptruta olivora. Exp Appl Acarol 37:117–122
Andreeva IV, Shternshis MV (1995) Micro biological formulations against web mites in greenhouses. Zaschitarastenii Moskva 11:41–42
Aponte O, Mcmurtry JA (1997) Damage on ‘Hass’ avocado leaves, webbing and nesting behaviour of Oligonychus perseae (Acari: Tetranychidae). Exp Appl Acarol 21:265–272
Benz G (1987) Environment. In: Fuxa JR, Tanada Y (eds) Epizootiology of insect diseases. John Wiley and Sons, New York, pp 177–214
Chandler D, Davidson G, Jakobson RJ (2005) Laboratory and glasshouse evaluation of entomopathogenic fungi against the twospotted spider mite, Tetranychus urticae (Acari: Tetranychidae), on tomato, Lycopersicon esculentum. Biocontrol Sci Technol 15:37–54
Devi KU, Rao CUM (2006) Allee effect in the infection dynamics of the entomopathogenic fungus Beauveria bassiana (Bals) Vuill. on the beetle, Mylabris pustulata. Mycopathologia 161:385–394
Dunn PH, Mechalas BJ (1963) The potential of Beauveria bassiana (Balsamo Vuill.) as a microbial insecticide. J Invert Pathol 5:451–459
Faria M, Wraight SP (2001) Biological control of Bemisia tabaci with fungi. Crop Prot 20:767–778
Feng MG, Chen B, Ying SH (2004) Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci Tech 14:531–544
Ferron P (1977) Influence of relative humidity on the development of fungal infection caused by Beauveria bassiana (Fungi imperfecti, Moniliales) in imagines of Acathoscelides obtectus (Col.: Bruchidae). Entomophaga 22:393–396
Ferron P, Fargues J, Riba G (1991) Fungi as microbial insecticides against pests. In: Arora DK, Ajello L, Mukerji KG (eds) Handbook of applied mycology, humans, animals and insects, vol 2. Marcel Dekker Inc., New York, pp 665–705
Gatarayiha MC, Laing MD, Miller RM (2011) Field evaluation of Beauveria bassiana efficacy for the control of Tetranychus urticae Koch (Acari: Tetranychidae). J Appl Entomol 135:582–592
Grbić M, Van Leeuwen T et al (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492
Groden E, Wraight SP, Drummond FA (2002) Microbial control of Colorado potato beetle in potatoes in rain-fed potato agroecosystems in the Northeastern US. Proceedings, international colloquium on invertebrate pathology and microbial control, Foz do Iguacu, Brazil 8, pp 265–269
Hatting JL, Wraight SP, Miller RM (2004) Efficacy of Beauveria bassiana (Hyphomycetes) for control of Russian wheat aphid (Homoptera: Aphididae) on resistant wheat under field conditions. Biocontrol Sci Technol 14:459–473
Inglis DG, Goettel SM, Butt MT, Strasser H (2001) Use of Hyphomycetes fungi for managing insect pests. In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents. CABI Publishing, Wallingford, pp 23–27
James RR, Buckner JS, Freeman TP (2003) Cuticular lipids and silverleaf whitefly stage affect conidial germination of Beauveria bassiana and Paecilomyces fumosoroseus. J Invert Pathol 84:67–74
Kavousi A, Chi H, Talebi K, Bandani A, Ashouri A, Naveh VH (2009) Demographic traits of Tetranychus urticae (Acari:Tetranychidae) on leaf discs and whole leaves. J Econ Entomol 102:595–601
LeOra Software (1987) POLO-PC. A user’s guide to probit or logit analysis. LeOra Software Inc., Berkeley 22
Marcandier S, Khachatourians GG (1987) Susceptibility of migratory grasshopper, Melanoplus sanguinipes (Fab.) (Orthoptera: Acrididae), to Beauveria bassiana (Bals.) Vuillemin (Hyphomycetes): influence of relative humidity. Can Entomol 119:901–907
Migeon A, Dorkeld F (2014) Spider mites web: a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 01 July 2014
Moino A Jr, Alves SB, Pereira RM (1998) Efficacy of Beauveria bassiana (Balsamo) Vuillemin isolates for control of stored-grain pests. J Appl Entomol 122:301–305
Moore GE (1973) Pathogenicity of three entomogenous fungi to southern pine beetle at various temperatures and humidities. Environ Entomol 2:54–57
Pereira A, Casals P, Salazar AM, Gerding M (2011) Virulence and pre-lethal reproductive effects of Metarhizium anisopliae var. anisopliae on Pseudococcus viburni (Hemiptera: Pseudococcidae). Chilean J Agric Res 71:554–559
Pu XY, Feng MG, Shi CH (2005) Impact of three application methods on the field efficacy of a Beauveria bassiana- based mycoinsecticide against the false-eye leafhopper, Empoasca vitis (Homoptera: Cicadellidae) in tea canopy. Crop Prot 24:167–175
Ramoska WA (1984) The influence of relative humidity on Beauveria bassiana infectivity and replication in the chinch bug, Blissus leucopterus. J Invert Pathol 43:389–394
Robertson JL, Russell RM, Preisler HK, Savin E (2007) Bioassays with arthropods, 2nd edn. CRC Press, Boca Raton
Rockland LB (1960) Relative humidity variations with temperature of saturated salt solutions. Analyt Chem 32:1375–1376
SAS Institute (2000) SAS user’s guide: statistics, version 9.2. SAS Institute, Cary
Scholte E-J, Njiru BN, Smallegange RC, Takken W, Knols BGJ (2003) Infection of adult malaria (Anopheles gambiae s.s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malar J 2:29
Seiedy M, Saboori A, Allahyari H, Talaei-Hassanloui R, Tork M (2010) Laboratory investigation on the virulence of two isolates of the entomopathogenic fungus Beauveria bassiana against the two spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 36:527–532
Shi WB, Feng MG (2006) Field efficacy of application of Beauveria bassiana formulation and low rate of pyribaden for sustainable control of citrus red mite Panonychus citri (Acari: Tetranychidae) in orchards. Biol Control 39:210–217
Shi WB, Feng MG (2009) Effects of fungal infection on reproductive potential and survival time of Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 48:229–237
Shi WB, Feng MG, Liu SS (2008) Sprays of emulsifiable Beauveria bassiana formulation are ovicidal towards Tetranychus urticae (Acari:Tetranychidae) at various regimes of temperature and humidity. Exp Appl Acarol 46:247–257
Shipp JL, Zhang Y, Hunt DWA, Ferguson G (2003) Influence of humidity and greenhouse microclimate on the efficacy of Beauveria bassiana (Balsamo) for control of greenhouse arthropod pests. Environ Entomol 32:1154–1163
Tamai MA, Alves NB, Neves PS (1999) Pathogenicity of Beauveria bassiana (Bals.) Vuill. against Tetranychus urticae Koch. Sci Agric 56:285–288
Toledo AV, Alippi AM, Remes Lenicov AMM (2011) Growth inhibition of Beauveria bassiana by bacteria isolated from the cuticular surface of the corn leafhopper, Dalbulus maidis and the planthopper, Delphacodes kuscheli, two important vectors of maize pathogens. J Insect Sci 11:29
Ullah MS, Haque MA, Nachman G, Gotoh T (2012) Temperature-dependent development and reproductive traits of Tetranychus macfarlanei (Acari: Tetranychidae). Exp Appl Acarol 56:327–344
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572
Willmer P (1986) Microclimatic effects on insects at the plant surface. In: Juniper B, Southwood R (eds) Insects and the plant surface. Edward Arnold, London, pp 65–80
Acknowledgments
Mohammad Shaef Ullah was supported by the BK21 plus program of Ministry of Education, Science, and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ullah, M.S., Lim, U.T. Laboratory bioassay of Beauveria bassiana against Tetranychus urticae (Acari: Tetranychidae) on leaf discs and potted bean plants. Exp Appl Acarol 65, 307–318 (2015). https://doi.org/10.1007/s10493-014-9871-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9871-2