Abstract
Climate change is one of the most important factors affecting the phenology, distribution, composition and diversity of organisms. In agricultural systems many pests and natural enemies are arthropods. As poikilotherm organisms, their body temperature is highly dependent on environmental conditions. Because higher trophic levels typically have lower tolerance to high temperatures than lower trophic levels, trends towards increasing local or regional temperatures may affect the strength of predator/prey interactions and disrupt pest control. Furthermore, increasing temperatures may create climate corridors that could facilitate the invasion and establishment of invasive species originating from warmer areas. In this study we examined the effect of environmental conditions on the dynamics of an agro-ecosystem community located in southern Spain, using field data on predator/prey dynamics and climate gathered during four consecutive years. The study system was composed of an ever-green tree species (avocado), an exotic tetranychid mite, and two native species of phytoseiid mites found in association with this new pest. We also present a climatological analysis of the temperature trend in the area of study during the last 28 years, as evidence of temperature warming occurring in the area. We found that the range of temperatures with positive per capita growth rates was much wider in prey than in predators, and that relative humidity contributed to explain the growth rate variation in predators, but not in prey. Predator and prey differences in thermal performance curves could explain why natural enemies did not respond numerically to the pest when environmental conditions were harsh.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is increasing evidence that ecosystems are being affected by local or regional warming on all continents (IPCC 2007; Beaumont et al. 2009; Hole et al. 2009). Recent studies have shown that climate change is altering the phenology, distribution, composition and diversity of plant (Morecroft and Keith 2009), bird (Fiedler 2009), mammal (Humphries 2009) and insect (Pelini et al. 2009) communities, as well as of pelagic and plankton marine (Edwards 2009) and intertidal ecosystems (Mieszkowska 2009), and coral reefs (Attrill 2009). It is also acknowledged that climate change may become a threat for the expansion and maintenance of crops managed with environmentally friendly techniques, such as biological and conservation pest control. This is because pesticide use may increase with warming due to the formation of climate corridors that may facilitate the spread and establishment of exotic pest species. Also, it has been stated recently that global change is influencing virtually every type of interactions between species (Tylianakis et al. 2008, and references therein). As a result, levels of herbivory have increased, likely because higher trophic levels are more sensitive to warming than lower trophic levels (Cagnolo et al. 2002; Voigt et al. 2003; Preisser and Strong 2004); Last, global warming introduces new uncertainties to the future of agriculture in general, because models of climate change predict an increase of the frequency and intensity of extreme events, such as hurricanes, floods, heat waves or severe drought (Lavalle et al. 2009).
It is predicted that climate warming will vary between regions. According to the analysis of the Intergovernmental Panel for Climate Change, IPCC (2007), summers will tend to be warmer in southern Europe, with an increase in the intensity, duration and number of periods with extreme high temperatures. Within southern Europe, the Mediterranean region is amongst the areas considered highly susceptible to climate change, because it is an area of transition between the Euro-Siberian or temperate and the Saharo-Sindic climatic regions (Benito-Garzon et al. 2008).
In agricultural systems many pests and natural enemies are arthropods. As ectotherm organisms, their body temperature is highly dependent on the ambient temperature. Many of the key processes of arthropods, such as metabolism and development, and factors that determine the intensity of trophic interactions, such as predation rate, movement or speed, are mediated by environmental conditions (Beveridge et al. 2010). It is expected, therefore, that changes in local or regional environmental conditions, especially increases of temperature and periods of extreme heat and drought, may profoundly affect pest control. Indeed, trophic cascades could be dampened and biological control jeopardized (Araújo and Luoto 2007; Hegland et al. 2009), if natural enemy efficiency or life-history is negatively affected by environmental warming. Furthermore, disruption of biological pest control may occur more frequently in agricultural systems affected by exotic invasive pests originating from warmer areas, because they are expected to be more adapted to warming than native natural enemies.
In this study we aimed at identifying whether environmental conditions influenced species abundance and dynamics of an agro-ecosystem community located in southern Spain, using field data on predator/prey dynamics and climate gathered during four consecutive years. We attempted to identify whether different responses to summer environmental conditions between predators and the pest could be detected at the population level in the field. We also present a climatological analysis of the temperature trend over the last 28 years in the area of study as evidence of temperature warming occurring in the area.
Materials and methods
The agro-ecosystem
Avocado (Persea americana Mill.; Lauraceae) is an ever-green tree species originating from Mesoamerica and introduced in southern Spain during the sixteenth century by the Spaniards. Since then it has become an economically important crop in the coastal areas of Malaga and Granada, in the region of Andalusia, where, except for almond and olive trees, it is the non-citrus fruit tree that occupies most of the cultivated area (MARM 2011). One of the most eminent characteristics of the avocado cultivation in Andalusia was its excellent phytosanitary status. Indeed, avocado trees in Spain had been exempted from important pests, probably because all the avocado tissues (leaves, seeds, roots and fruits) contain specialized oil cells which accumulate alkaloids and terpenes (Armstrong 1964; Platt-Aloia et al. 1983; Platt and Thompson 1992) with insecticidal, antifeedant, and growth inhibitory activities (Rodriguez-Soana et al. 1997, 1998; Rodriguez-Soana and Trumble 2000). However, this situation has recently been endangered because of the arrival of an exotic pest, the persea mite, Oligonychus perseae Tuttle, Baker and Abatiello, a tetranychid species which originated from Mesoamerica.
The persea mite was detected for the first time in commercial avocado crops of the province of Malaga in 2004. It is also described as avocado pest in California (USA), Canary Islands, Costa Rica, Israel, Portugal, Valencia (Vela et al. 2007), and Florida (USA) (Welbourn 2007). This pest builds dense silken nests at the underside of avocado leaves, along the main nerves. Inside the nests adults feed and reproduce, and juveniles undergo development. Feeding causes necrotic spots that can affect up to 90 % of the leaf area (Aponte and McMurtry 1997). Among other functions, nests protect mites against attack from some species of natural enemies, and against adverse environmental conditions (Montserrat et al. 2008; Mori et al. 1999).
Two species of phytoseiid mites have been found in association with this invasive pest in the Andalusian coastal avocado orchards (González-Fernández et al. 2009). Euseius stipulatus (Athias-Henriot) is an omnivorous species, not available commercially, that attacks animal prey and also forages on pollen (Bouras and Papadoulis 2005; Ferragut et al. 1987). Neoseiulus californicus (McGregor) is a specialist predator of tetranychid mites that is commercially available. Previous studies showed that both species actively hunt and prey on the persea mite, E. stipulatus mainly attacking the individuals wandering outside nests, whereas N. californicus uses its forelegs to rip the silken walls of the nests to attack the individuals inside the nests (González-Fernández et al. 2009; Montserrat et al. 2008).
The mite predator/prey community dynamics in avocados
During four consecutive years (2006 through 2009), the abundance of phytoseiid mites and the persea mites was recorded during spring and summer, starting at the end of March until the end of September. Two-season samplings were done because yearly population dynamics of phytoseiid mites in avocado trees typically show two population peaks, the first in spring and the second in summer. In spring, when the persea mite is still rare, the omnivore E. stipulatus is by far the most abundant species. It has recently been suggested that its numerical response is associated with a non-prey food source, pollen (mainly from olive), which is deposited from the atmosphere on the surface of the avocado leaves (González-Fernández et al. 2009). In summer, the two species of phytoseiids co-occur and their populations respond numerically to the abundance of the persea mite (González-Fernández et al. 2009).
Predator and prey populations were monitored in a 1.8 ha avocado orchard (cv ‘Hass’) of ca. 70 avocado trees located at the IHSM La Mayora (Málaga). Ten leaves per tree, covering the whole perimeter of the canopy to account for within-tree abundance variability, at ca. 150 cm height were sampled from 10 to 20 avocado trees every 2 weeks. At each sampling date (a) the number of occupied nests (with mobile stages and/or eggs inside) on the upper margin of the second left vein of the underside of the leaf (UML2, hereafter), and (b) the number of mobile stages of phytoseiid mites on the leaf, was recorded in situ using a field magnifying glass (Ruper 8x). The number of occupied nests on the UML2 is a good estimate of the total number of individuals (mobiles and eggs) per leaf (González-Fernández et al. 2009).
Because phytoseiids in spring probably respond numerically to the abundance of pollen, data of pollen concentration in the atmosphere were obtained from an aerobiology station belonging to the Spanish Aerobiology Network (REA, Red Española de Aerobiología), from 2006 to 2008. This station, which is located 10 km in a straight line from the study area, uses a Hirst type volumetric pollen trap. Data recorded in this station are provided as the number of pollen grains per cubic metre of air and day.
Effect of environmental conditions on predator/prey abundance
Climate data used in the analyses were obtained from an agro-climate station that belongs to the Government of Andalusia. This station was chosen because it is located close to the area of study (ca. 15 km linear distance), and like the avocado orchard positioned close to the sea. The station provided daily records on maximum, minimum and average temperature and relative humidity at a height of ca. 2 m above the ground.
The per capita growth rates between two successive samplings (r t,t+τ ) were calculated for both predators and prey as
were τ is the time interval (days) between the first and the second sampling event and N t is the populations size at time t. For each value of r t,t+τ concurrent values of temperature and humidity were obtained averaging the daily records of temperature and relative humidity during the interval (t,t + τ). Data were divided into two sets. The first set included all data gathered during the four springs, when pollen in the atmosphere was present and the persea mite was rare. The second set included all data gathered during the four summer seasons, when pollen in the atmosphere was absent and the persea mite was present.
The variation of the interval per capita growth rate of predators during spring, and of predators and prey during summer, was analysed by non-linear regression models (Proc NLR, SPSS). The predictor variables were: the first and second order terms of (a) the interval average temperature (T t,t+τ ), and (b) the interval average relative humidity (RH t,t+τ ). First and second order terms were included because temperature and relative humidity usually have non-linear effects on life history of arthropods; (c) the interval average number of grains of pollen/m3 of air (Pol t,t+τ ), when modelling the per capita growth rate of predators in spring; (d) the interval average of the abundance of prey (Prey t,t+τ ), when modelling the per capita growth rate of predators in summer; (e) the interval average of the abundance of predators (Pred t,t+τ ), when modelling the per capita growth rate of prey in summer. First, predictor variables were analysed alone to test how much of the variation in r t,t+τ they could explain. Next, we analysed the whole model (i.e. first and second order term of T t,t+τ and RH t,t+τ as well as the interaction between T t,t+τ and RH t,t+τ , and either Pol t,t+τ , Prey t,t+τ , or Pred t,t+τ ) with a backwards stepwise procedure. This method starts with all the variables present, and then it determines, at each step, how ‘insignificant’ the contribution is of a variable in the regression to be removed (threshold at P = 0.05). Subsequently, results were used to identify and model the estimated relationship between the interval per capita growth rate, r t,t+τ , as function of the significant predictor variables.
Warming trend in the area of study
Our experimental system included an invasive pest species that is native from Mesoamerica, where the climate is warmer than in South-Eastern Spain. Also, the area of our study is located in the Mediterranean region, an area classified as highly susceptible to warming and where climate change models predict increasing temperature trends in spring and summer (IPCC 2007). We analyzed whether such a trend existed in the area using monthly temperature data recorded from 1980 to 2008 in a climate station located in the IHSM La Mayora. Data were stored as monthly average, maximum and minimum temperatures. For the analysis, records of temperature were transformed into ‘seasonal temperatures’, averaging the records registered during 3 months. Seasons were defined using the standard meteorological definition, this being: Winter = December + January + February; Spring = March + April + May; Summer = June + July + August; and Autumn = September + October + November. Variables used in the analyses were the average, maximum, and minimum seasonal temperature. Linear regressions were performed, with temperature records as the dependent and time as the independent variables. Because four simultaneous tests were conducted per temperature data set, statistical significance was set at α/4 = 0.0125 (Bonferroni method; Sokal and Rohlf 1995).
Results
Predator/prey mite community dynamics
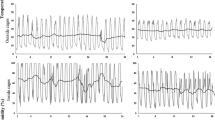
The annual population dynamics of the persea mite showed an exponential population increase at the beginning of summer followed by a steep decline at the end of summer (Fig. 1c, white dots). The 4-year population dynamics of the predatory mites showed two different patterns. In spring during all 4 years of the study, the phytoseiid populations showed a positive correlation with the abundance of pollen in the atmosphere (Fig. 1b, black dots and green stars, respectively). In summer there was a second phytoseiid population peak, corresponding with a numerical response to the abundance of the persea mite in 2006 and 2007, but not in 2008 and 2009 (Fig. 1c, black and white dots, respectively). The inspection of the daily records of temperature and relative humidity during the sampling periods revealed that summers in 2008 and 2009 had, relative to those in 2006 and 2007, prolonged periods with high temperatures combined with low relative humidity (Fig. 1a, vertical distance between red and blue dots).
a Daily average temperature (red dots) and relative humidity (blue dots) registered from early spring to late summer, during four consecutive years. b Population dynamics of phytoseiid mites (black dots) and grains of pollen in the atmosphere (green stars), during the same period as in a. c Population dynamics of the phytoseiid mites (black dots) and the persea mite (white dots), during the same period as in a. Vertical lines delimit the time period with presence of Oligonychus perseae in the avocado trees
Effect of environmental conditions on predator/prey abundance
When predictor variables were tested alone, only the interval average number of grains of pollen/m3 of air significantly explained the variation of the per capita growth rate of predators in spring (Table 1). When all the variables were included in the model, the abundance of pollen and the interaction between temperature and relative humidity were significant.
With regard to the summer data set, when predictor variables were evaluated alone only the first and second order terms of temperature had an effect on the per capita growth rate of both predator and prey (Tables 2 and 3). Figure 2 depicts the predicted interval per capita growth rate of predator and prey as function of the interval average temperature obtained from the parameter estimates of the regression analysis. Results show that the range of temperatures with positive growth rates was wider in prey than in predators (19.7–29.9 and 20.7–26.9 °C, respectively). When whole models were analysed, the first and second order terms of temperature significantly explained the variation in the interval per capita growth rate of prey and predators (Tables 2 and 3), and the interaction between T t,t+τ and RH t,t+τ contributed to the variation of r t,t+τ in predators (Table 3). Figure 3a, b depict the predicted interval per capita growth rate of predators as function of the average interval temperature and relative humidity obtained from the parameter estimates of the regression analysis. Results show that medium temperatures (T t,t+τ ≈ 24 °C) led to positive growth rates over a wide range of relative humidities (RH t,t+τ > 50 %). However, higher and lower temperatures led to positive growth rates only with increasing RH t,t+τ . Interval average temperature values below and above 20 and 27.5 °C, respectively, resulted in negative predator growth rates independently of the relative humidity (Fig. 3a, b).
a Three-dimensional and b 2-dimensional illustration of the predicted interval per capita growth rate (r t,t+τ ) of predators as function of the interval average temperature (T t,t+τ ) and the interval average relative humidity (RH t,t+τ ). Values were obtained from the parameter estimates of the regression analysis. Green (in print: light grey) and red (dark grey) areas stand for positive and negative values of r t,t+τ , respectively
Warming trend in the area of study
Table 4 shows the results of the linear regressions carried out on climate data of each of the four seasons (winter, spring, summer and autumn) and each of the three variables (average, maximum and minimum seasonal temperature) considered, from 1980 to 2008. Results suggest that over the last 28 years temperatures of summer and spring showed an increasing trend (Fig. 4). Temperatures of winter and autumn showed no trends (Fig. 2). These results are in agreement with the predictions of the IPPC (2007).
Discussion
Can environmental conditions jeopardize herbivore population control?
Our results indicate that environmental conditions during summer, in particular high temperatures, had a negative effect on both predators and prey. However, the range of temperatures with positive interval growth rates was much wider in prey than in predators (Fig. 2). Furthermore, predator positive growth rates at temperatures below or above ca. 24 °C were only possible when relative humidity was high enough (Fig. 3a, b). Temperature and relative humidity affect life-history parameters of E. stipulatus and N. californicus. Ferragut et al. (1987) showed that female survival and total reproduction of E. stipulatus decreased drastically between 25 and 32 °C, and egg-hatching did not occur when relative humidity was below 60 %. The authors also showed that females that emerged and mated at 32 °C laid no eggs (Ferragut et al. 1987), what could be attributed to oocytes, testes and sperm being injured by heat shock (Krebs and Loeschke 1994; Scott et al. 1997; Wang et al. 2009a, b). Walzer et al. (2007) found that egg-hatching and larval and protonymph survival of N. californicus decreased dramatically with decreasing relative humidity, and Nguyen and Amano (2009, 2010) found that the duration of copulation and total reproduction were drastically reduced with temperatures above 30 °C.
The effects of extreme environmental conditions in life-history parameters of the two species could explain why predatory mites did not respond numerically to the presence of the persea mite in 2008 and 2009. On the one hand, in the avocado growing areas of southern Spain temperatures above 30 °C are common in summer. On the other hand, the summers of 2008 and 2009 were characterized by prolonged periods of high temperatures combined with the so-called ‘terral’ winds. This is a very dry and hot northern wind that is characteristic of the area of study during summers, due to a foehn effect caused by the mountains in the northern part of the province. Periods of high temperatures combined with low air relative humidity seem to have been more frequent and prolonged in 2008 and 2009 than in 2006 and 2007 (Fig. 1a, vertical separation between blue and red dots). Longer periods of exposure to such extreme environmental conditions may have affected individuals to an extent that translated into observable effects at the population level in the field. Indeed, our results predict that high temperatures combined with low relative humidities will lead to negative growth rates in predators. Climate data used in our analyses were taken from sensors positioned at ca. 2 m above the ground, thereby providing data of the macroclimate. Mites, however, respond to the microclimate of leaves, which is likely to be less extreme than macroclimate. Nevertheless, macroclimate can be used as a proxy for the microclimate because they are positively correlated (Zhang et al. 2002).
Negative effects of extreme environmental conditions on life-history parameters have been described in many species of arthropod natural enemies that are being used as biological control agents (McMurtry and Scriven 1965; Zhang and Kong 1985; Ferragut et al. 1987; Bakker et al. 1993; Croft et al. 1993; Castagnoli and Simoni 1994; Broufas and Koveos 2001; DeCourcy-Williams et al. 2004; Ferrero et al. 2007, 2010; Hance et al. 2007; Walzer et al. 2007; Momen and Abdel-Khalek 2008, 2007; Vasconcelos et al. 2008; Zhang et al. 2008). Furthermore, thermal performance curves of natural enemies and their prey typically differ, with natural enemies having lower tolerance to higher temperature than prey (Roy et al. 2003; Hance et al. 2007; Stavrinides et al. 2010a, b; results here). Climate change predicts not only increases of local and regional temperatures, a trend that is observed in our area, but also increases in the frequency and duration of extreme environmental conditions. Therefore, one would expect thermal and relative humidity performance curves of natural enemies and prey to further diverge under climate change scenarios. Such divergence may be accentuated when pest species originate from warmer areas, because they are expected to be better adapted to overall higher temperatures. This is probably the case for the pest species in our study, which builds dense silken nests that likely protect them from harsh environmental conditions, as it has been shown in another related species, Schizotetranychus longus (Mori et al. 1999). This is certainly a scenario that should be taken into account for the future of biological control. Indeed, extreme environmental conditions can allow pests to escape control by predators, and may be a direct cause of biological control disruption in some agricultural systems (Roy et al. 2003; Stavrinides et al. 2010a, b).
Future directions
Several authors have stated that species interactions should be incorporated into climate-change models to better predict the impact of climate change on natural and agro-ecosystem communities (Wilmers et al. 2007; Tylianakis et al. 2008; Gilman et al. 2010). Certainly, that would be an appropriate approach, because behavioural features of individuals are directly related to environmental conditions, and species interactions are commonly climate-dependent (Beveridge et al. 2010; Gilman et al. 2010). A first step to improve predictions should be to determine how environmental conditions alter the interactions among the species present in a given agricultural community. Environmental conditions can alter foraging behaviour or dispersal capabilities (Skirvin and Fenlon 2003a, b), and affect competitive abilities and predatory capabilities of species (Tylianakis et al. 2008). Knowledge on which interactions between species of the agricultural community are most sensitive to changes in environmental conditions would help to increase the probability of success of biological control strategies. On the one hand, it would provide information on which species of natural enemies are likely to persist during periods of adverse environmental conditions. Subsequently, pest control strategies could focus on methods aimed at improving their establishment, or at artificially increasing their populations. On the other hand, knowledge on the thermal and relative humidity performance curves of natural enemies, relative to these of their prey, could help to optimize predator use. Indeed, we could know when the establishment and the persistence of released natural enemies is likely to fail, and the environmental values against which measures to soften local environmental conditions should be applied.
In this study we showed that local environmental conditions can influence the abundance and population dynamics of pests and their natural enemies. Therefore, they can be a direct cause of biological control disruption. Because climate change models predict an increase of the frequency and intensity of extreme events, such as heat waves, it becomes necessary (a) to understand the relationship between the effect of warming on higher trophic levels and herbivore population outbreaks; (b) to intensify preventive methods, especially those involving control of imported plant material; and (c) to encourage scientific collaboration with research teams of countries where potential future pests are from, in order to develop and establish proper control.
References
Aponte O, McMurtry JR (1997) Damage on ‘Hass’ avocado leaves, webbing and nesting behaviour of Oligonychus perseae (Acari: Tetranichidae). Exp Appl Acarol 21:265–272
Araújo MB, Luoto M (2007) The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr 16:743–753
Armstrong WW (1964) Distribution of oil cells in Persea. Master Thesis, University of California, Riverside, p 40
Attrill MJ (2009) Changes in coral reef ecosystems as an indicator of climate and global change. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
Bakker FM, Klein ME, Mesa NC, Braun AR (1993) Saturation deficit tolerance spectra of phytophagous mites and their phytoseiid predators on cassava. Exp Appl Acarol 17:97–113
Beaumont LJ, Hughes L, Pitman AJ (2008) Why is the choice of future climate scenarios for species distribution modelling important? Ecol Lett 11:1135–1146
Beaumont LJ, Gallagher RV, Thuiller W, Downey PO, Leishman MR, Hughes L (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15:409–420
Benito-Garzon M, Sanchez de Dios R, Sainz-Ollero H (2008) Effects of climate change on the distribution of Iberian tree species. Appl Veg Sci 11:169–178
Beveridge OS, Humphries S, Petchey OL (2010) The interacting effects of temperature and food chain length on trophic abundance and ecosystem function. J Anim Ecol 79:693–700
Bouras SL, Papadoulis GT (2005) Influence of selected fruit tree pollen on life history of Euseius stipulatus (Acari: Phytoseiidae). Exp Appl Acarol 36:1–14
Broufas GD, Koveos DS (2001) Development, survival and reproduction of Euseius finlandicus (Acari, Phytoseiidae) at different constant temperatures. Exp Appl Acarol 26:441–460
Cagnolo L, Molina SI, Valladares GR (2002) Diversity and guild structure of insect assemblages under grazing and exclusion regimes in a montane grassland from Central Argentina. Biodivers Conserv 11:407–420
Castagnoli M, Simoni S (1994) The effect of different constant humidities on eggs and larvae of Amblyseius californicus (McGregor) (Acarina: Phytoseiidae). Redia 77:349–359
Croft BA, Messing RH, Dunley JE, Strong WB (1993) Effects of humidity on eggs and immatures of Neoseiulus fallacis, Amblyseius andersoni, Metaseiulus occidentalis and Typhlodromus pyri (Phytoseiidae): implications for biological control on apple, caneberry, strawberry and hop. Exp Appl Acarol 17:451–459
DeCourcy-Williams ME, Kravar-Garde L, Fenlon JS, Sunderland KD (2004) Phytoseiid mites in protected crops: the effect of humidity and food availability on egg hatch and adult life span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 32:1–13
Edwards M (2009) Sea life (pelagic and planktonic ecosystems) as an indicator of climate and global change. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
Ferragut F, García-Mari F, Costa-Comelles J, Laborda R (1987) Influence of food and temperature on development and oviposition of Euseius stipulatus and Typhlodromus phialatus (Acari : Phytoseiidae). Exp Appl Acarol 3:317–329
Ferrero M, de Moraes GJ, Kreiter S, Tixier M-S, Knapp M (2007) Life tables of Phytoseiulus longipes feeding on Tetranychus evansi at four temperatures (Acari: Phytoseiidae, Tetranychidae). Exp Appl Acarol 41:45–53
Ferrero M, Gigot C, Tixier MS, Van Houten YM, Kreiter S (2010) Egg hatching response to a range of air humidities for six species of predatory mites. Entomol Exp Appl 135:237–244
Fiedler W (2009) Bird ecology as an indicator of climate and global change. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331
González-Fernández JJ, de la Peña F, Hormaza JI, Boyero JR, Vela JM, Wong E, Trigo MM, Montserrat M (2009) Alternative food improves the combined effect of an omnivore and a predator on biological pest control. A case study in avocado orchards. Bull Entomol Res 99(5):433–444
Hance T, van Baaren J, Vernon P, Boivin G (2007) Impact of extreme temperatures on parasitoids in a climate change perspective. Annu Rev Entomol 52:107–126
Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland O (2009) How does climate warming affect plant-pollinator interactions? Ecol Lett 12:189–195
Hole DG, Willis SG, Pain DJ, Fishpool LD, Butchart SHM, Collingham YC, Rahbek C, Huntley B (2009) Projected impacts of climate change on a continent-wide protected area network. Ecol Lett 12(5):420–431
Humphries MM (2009) Mammal ecology as an indicator of climate change. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
IPCC (2007) Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Parry KL, Canziani OF, Palutikif JP, vann der Linden PJ, Hanson CE (eds) Cambridge University Press, Cambridge
Krebs RA, Loeschke L (1994) Effects of exposure to short-term heat stress on fitness components in Drosophila melanogaster. J Evol Biol 7:39–49
Lavalle C, Micale F, Houston TD, Camia A, Hiederer R, Lazar C, Conte C, Amatulli G, Genovese G (2009) Climate change in Europe. 3. Impact on agriculture and forestry. A review. Agron Sustain Dev 29:433–446
MARM (2011) Anuario de Estadística del Ministerio de de Medio Ambiente y Medio Rural y Marino. Available via dialog http://www.mapa.es/es/estadistica/pags/anuario/2009/indice.asp
McMurtry JA, Scriven GT (1965) Studies on predator-prey interactions between Amblyseius hibisci and Oligonychus punicae (Acarina: Phytoseiidae, Tetranichidae) under greenhouse conditions. Ann Entomol Soc Am 59:793–800
Mieszkowska N (2009) Intertidal indicators of climate and global change. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
Momen FM, Abdel-Khalek A (2008) Effect of the tomato rust mite Aculops lycopersici (Acari: Eriophyidae) on the development and reproduction of three predatory phytoseiid mites. Int J Trop Inst Sci 28:53–57
Montserrat M, de la Peña F, Hormaza JI, González-Fernández JJ (2008) How do Neoseiulus californicus (Acari: Phytoseiidae) females penetrate densely webbed spider mite nests? Exp Appl Acarol 44:101–106
Morecroft MD, Keith SA (2009) Plant ecology as an indicator of climate and global change. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
Mori K, Saito Y, Sakagami T (1999) Efects of the nest web and female attendance on survival of young in a subsocial spider mite, Schizotetranychus longus (Acari: Tetranychidae). Exp Appl Acarol 23:411–418
Nguyen TPT, Amano H (2009) Mating duration and egg production of the predaceous mite Neoseiulus californicus (Acari: Phytoseiidae) vary with temperature. J Asia Pac Entomol 12:297–299
Nguyen TPT, Amano H (2010) Temperature at immature and adult stages differentially affects mating duration and egg production of Neoseiulus californicus females mated once (Acari: Phytoseiidae). J Asia-Pac Entomol 13(2010):65–68
Pelini SL, Prior KM, Parker DJ, Dzurisin JDK, Lindroth RL, Hellmann JJ (2009) Climate change and temporal and spatial mismatches in insect communities. In: Letcher T (ed) Climate change: observed impacts on planet Earth. Elsevier B.V, Amsterdam
Platt KA, Thompson WW (1992) Idioblast oil cells of avocado: distribution, isolation, ultraestructure, histiochemistry, and biochemistry. Int J Plant Sci 153:301–310
Platt-Aloia KA, Oross JW, Thompson WW (1983) Ultraestructure and development of oil cells in mesocarp of avocado fruit. Bot Gaz 144:49–55
Preisser EL, Strong DR (2004) Climate affects predator control of an herbivore outbreak. Am Nat 163(5):754–762
Rodriguez-Soana C, Trumble JT (2000) Secretory avocado idioblast oil cells: evidence of their defensive role against non-adapted insect herbivore. Entomologia Experimentalis et Applicatta 94:183–194
Rodriguez-Soana C, Millar JG, Trumble JT (1997) Growth inhibitory, insecticidal, and feeding deterrent effects of (12Z,15Z)-1-acetoxy-2-hydroxy-4-oxo-heneicosa-12,15,-dienne, a compound from avocado fruit, to Spodoptera exigua. J Chem Ecol 23:1819–1831
Rodriguez-Soana C, Millar JG, Maynard DF, Trumble JT (1998) Novel antifeedant and insecticidal compounds from avocado idioblast cell oil. J Chem Ecol 24:867–889
Roy M, Brodeur J, Cloutier C (2003) Effect of temperature on intrinsic rates of natural increase (rm) of a coccinellid and its spider mite prey. Biocontrol 48:57–72
Scott M, Berrigan D, Hoffmann AA (1997) Costs and benefits of acclimation to elevated temperature in Trichogramma carverae. Entomol Exp Appl 85:211–219
Skirvin DJ, Fenlon JS (2003a) Of mites and movement: the effects of plant connectedness and temperature on movement of Phytoseiulus persimilis. Biol Control 27:242–250
Skirvin DJ, Fenlon JS (2003b) The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 31:37–49
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. 3rd edn. WH Freeman and Co., New York, p 887, ISBN: 0-7167-2411-1
Stavrinides MC, Lara JR, Mills NJ (2010a) Comparative influence of temperature on development and biological control of two common vineyard pests (Acari: Tetranychidae). Biol Contr 55:126–131
Stavrinides MC, Daane KM, Lampinen BD, Mills NJ (2010b) Plant water stress, leaf temperature, and spider mite (Acari: Tetranychidae) outbreaks in California Vineyards. Environ Entomol 39(4):1232–1241
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11(12):1351–1363
Vasconcelos GJN, de Moraes GJ, Júnior ID, Knapp M (2008) Life history of the predatory mite Phytoseiulus fragariae on Tetranychus evansi and Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae) at five temperatures. Exp Appl Acarol 44:27–36
Vela JM, González-Fernández J, Wong E, Montserrat M, Farré JM, Boyero JR (2007) El ácaro del aguacate (Oligonychus perseae): Estado actual del problema e investigación en Andalucía. Agrícola Vergel 306:301–308
Voigt W, Perner J, Davis AJ, Eggers T, Schumacher J, Bahrmann R, Fabian B, Heinrich W, Kohler G, Lichter D, Marstaller R, Sander FW (2003) Trophic levels are differentially sensitive to climate. Ecology 84(9):2444–2453
Walzer A, Castagnoli M, Simoni S, Liguori M, Palevsky E, Schausberger P (2007) Intraspecific variation in humidity susceptibility of the predatory mite Neoseiulus californicus: survival, development and reproduction. Biol Control 41:42–52
Wang XG, Johnson MW, Daane KM, Opp S (2009a) Combined effects of heat stress and food supply on flight performance of olive fruit fly (Diptera: Tephritidae). Ann Entomol Soc Am 102:727–734
Wang XG, Johnson MW, Daane KM, Nadel H (2009b) High summer temperatures affect the survival and reproduction of olive fruit fly (Diptera: Tephritidae). Environ Entomol 38(5):1496–1504
Welbourn WC (2007) Triology 46(4): 6. Available via dialog, http://www.freshfromflorida.com/pi/enpp/triology/archive/4604.pdf
Wilmers CC, Post E, Hastings A (2007) The anatomy of predator-prey dynamics in a changing climate. J Anim Ecol 76:1037–1044
Zhang N, Kong J (1985) Responses of Amblyseius fallacies Garman to various relative humidity regimes. Chin J Biol Control 1:6–9
Zhang Y, Jewett TJ, Shipp JL (2002) A dynamic model to estimate in-canopy and leaf-surface microclimate of greenhouse cucumber crops. Trans ASAE 45:179–192
Zhang Y, Wang L, Wu K, Wyckhuys KAG, Heimpel GE (2008) Flight performance of the soybean aphid, Aphis glycines (Homoptera: Aphididae) under different temperature and humidity regimens. Environ Entomol 37:301–306
Acknowledgments
The authors are indebted to Prof. Dr. Gøsta Nachman for his statistical advice and comments on an early version which significantly improved the manuscript. J.A. Jacas and J.I. Hormaza are thanked for their valuable comments on the manuscript. This work was financed by la Junta de Andalucía (Proyecto de Excelencia P08-AGR-3694), the Spanish Ministry of Science and Innovation (AGL2011-30538-C03-03), and the Spanish National Research Council (CSIC, project PIE200940I121). Field surveys would not have been possible without logistic help from J. González Fernández, J.M. Hermoso and E. Guirado. J.M. Sanchez Pulido is thanked for providing data on temperature records in the area of study. M. M. Trigo is thanked for providing data of pollen in the atmosphere. The animals used for the research of this publication are no test animals in the legal sense.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montserrat, M., Sahún, R.M. & Guzmán, C. Can climate change jeopardize predator control of invasive herbivore species? A case study in avocado agro-ecosystems in Spain. Exp Appl Acarol 59, 27–42 (2013). https://doi.org/10.1007/s10493-012-9560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9560-y