Abstract
A novel methanol assimilating yeast species Komagataella kurtzmanii is described using the type strain VKPM Y-727 (=KBP Y-2878 = UCD-FST 76-20 = Starmer #75-208.2 = CBS 12817 = NRRL Y-63667) isolated by W.T. Starmer from a fir flux in the Catalina Mountains, Southern AZ, USA. The new species is registered in MycoBank under MB 803919. The species was differentiated by divergence in gene sequences for D1/D2 LSU rRNA, ITS1-5.8S-ITS2, RNA polymerase subunit I, translation elongation factor-1α and mitochondrial small subunit rRNA. K. kurtzmanii differs from its phenotypically similar sibling species Komagataella pastoris, Komagataella pseudopastoris, Komagataella phaffii, Komagataella populi and Komagataella ulmi by absence of growth at 35 °C and inability to assimilate trehalose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heterogeneous genus Pichia Hansen has been frequently revised (Kurtzman 1984, 1998, 2011; Kurtzman et al. 2008). In particular, Yamada et al. (1995) described a new monotypic genus Komagataella, into which the methanol assimilating yeast Pichia pastoris has been transferred. The latter species is of great industrial importance, viz. it is widely used in applied genetic engineering and biotechnology (Wegner 1983; Cregg and Madden 1988; Cregg et al. 1993, 2012; Macauley-Patrick et al. 2005; Chen et al. 2012). After discovery of the European species Komagataella pseudopastoris (Dlauchy et al. 2003), the genus Komagataella has been generally accepted (Kurtzman 2005, 2011). Moreover, a new sibling species of K. pastoris was described in North America: Komagataella phaffii (Kurtzman 2005). It was shown that biotechnologically important strains P. pastoris represent both K. pastoris and K. phaffii, and the latter species was used in the commercialized Invitrogen Expression Kit (Kurtzman 2009). Quite recently, two more Komagataella species have been described using single strains: Komagataella populi and Komagataella ulmi (Kurtzman 2012). Both are from North America. In the course of molecular genetic study of methanol assimilating yeasts identified phenotypically as K. pastoris, previously undescribed Komagataella species was found. Based on multigene sequence comparisons, a novel species Komagataella kurtzmanii is proposed.
Materials and methods
Yeast strains, culture conditions and phenotypic characterization
The strains used and their origins are listed in Table 1. Phenotypic characterization of strain VKPM Y-727, representing a novel species of the genus Komagataella, was carried out according to Yarrow (1998) and Kurtzman et al. (2011). Strains of K. pastoris VKPM Y-3262 and K. phaffii VKPM Y-3489 were used as reference testers. Yeast cells were grown at 25 °C on the YPD complete medium (2 % glucose, 1 % peptone, 1 % yeast extract and 2 % agar). Sporulation and zygote formation were induced at 25 °C on different acetate agar media used in genetics and taxonomy of yeasts: (1) 1 % CH3COONa, 0.5 % KCl and 2 % agar; (2) 0.82 % CH3COONa, 0.18 % KCl, 0.25 % yeast extract, 0.1 % glucose and 1.5 % agar (McClary et al. 1959); (3) 0.5 % CH3COONa, 1 % KCl, 1 % glucose and 2 % agar (Chen et al. 2012). Also, ME medium was used (5 % malt extract and 2 % agar).
DNA extraction and sequencing
Genomic DNA was extracted from yeast cells by using Genomic DNA Purification Kit (Fermentas, Lithuania). The genes for D1/D2 26S rRNA, translation elongation factor-1α (EF-1α), RNA polymerase II (subunit RPB1), mitochondrial small subunit (Mt SSU) rRNA and ITS1-5.8S-ITS2 were amplified and sequenced using the oligonucleotide primers (Kurtzman and Robnett 1998, 2003; Kurtzman 2009). Amplification reactions were performed in a volume of 30 μl containing 100 ng of genomic DNA, Taq polymerase (0.05 U, Syntol, Moscow), and the primers (50 pmol each). A thermal cycler Tertsik (Russia) was programmed for 30 cycles of 45 s at 94 °C, 30 s at 52 °C and 2 min at 72 °C. Amplification products were separated by electrophoresis in 1 % agarose gels and detected by staining with ethidium bromide. For sequencing, the amplified products were purified using the GeneClean Purification Kit (Bio101, USA) according to the manufacturer’s instructions. Direct sequencing of both strands of the genes analyzed was performed using Beckman-Coulter automated DNA sequencer (USA). Sequences obtained were analysed with the SeqMan package (DNAStar Inc., Madison, WI, USA). An alignment was done visually using the program BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The MEGA 5 package (Tamura et al. 2011) was applied to generate a distance tree using the neighbour-joining algorithm with Kimura 2-parameter correction. Alignment gaps were deleted pairwise. Type cultures of Ogataea glucozyma NRRL YB-2185 and P. membranifaciens NRRL Y-2026 were used as outgroup species. A total of 1,000 bootstrap replicates were used for analysis. The nucleotide sequences determined in this study have been deposited with GenBank (Table 1). The reference sequences used in the phylogenetic analysis were retrieved from GenBank under the accession numbers indicated in Table 1.
Results
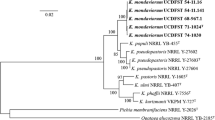
The D1/D2 nucleotide sequence obtained for strain VKPM Y-727 differs by 4–6 nucleotide substitutions from the corresponding sequences of the five known Komagataella species (Table 2). To elucidate a taxonomic status of VKPM Y-727 we conducted multigene sequence analysis. ITS1-5.8S-ITS2, EF-1α and RPB1 sequences of strain VKPM Y-727 differ markedly from those of K. pastoris, K. phaffii, K. ulmi, K. populi and K. pseudopastoris (Table 2). On the other hand, VKPM Y-727 and K. ulmi NRRL YB-407 showed identical Mt SSU sequences, which differed by a single nucleotide substitution from K. phaffii NRRL Y-7576. In terms of pairwise multigene sequence similarity the closest relative to the proposed new Komagataella species is K. phaffii. Based on the multigene sequence comparisons a phylogenetic tree was depicted (Fig. 1). Three pairs of the most closely related species can be distinguished with high bootstrap support: K. pastoris/K. ulmi, K. kurtzmanii/K. phaffii and K. pseudopastoris/K.populi. The data suggest that strain VKPM Y-727 represents a novel species of the genus Komagataella.
Neighbour-joining tree showing phylogenetic relationship between the sibling species of the genus Komagataella based on combined sequences for D1/D2 and ITS1-5.8S-ITS2 rDNA, translation elongation factor-1α, RNA polymerase II (subunit RPB1), and mitochondrial SSU rRNA. Bootsrap values from 1,000 replications are shown. Bar, 20 estimated base substitutions per 1,000 nucleotide positions. T type culture
Description of Komagataella kurtzmanii G.I. Naumov, E.S. Naumova, O.V. Tyurin and D.G. Kozlov sp. nov.
Growth on 5 % malt extract (ME) agar
After 3 days at 25 °C, cells divide by multilateral budding and are spherical (2–7 μm) to ovoid (2–7 × 3–6 μm), occur singly and in pairs (Fig. 2a). Colony growth is white, butyrous and with a smooth semi-glistening surface.
Komagataella kurtzmanii sp. nov. VKPM Y-727. a Budding cells at 25 °C on 5 % ME agar at 3 days. b Intact asci with four hat-shaped ascospores (the arrows indicate two asci) and c zygotes, deliquescent asci (the arrows indicate two asci and a zygote) of 4 days incubation at 25 °C on acetate medium. Bar, 5 μm
Dalmau plate culture on morphology agar
After 7 days at 25 °C, growth under the coverglass formed neither hyphae nor pseudohyphae.
Formation of ascospores
Ascosporulation occurs on 5 % ME agar and on different acetate media after 4 days at 25 °C. The best sporulation is observed on the medium containing 0.5 % sodium acetate, 1 % potassium chloride, 1 % glucose and 2 % agar. Asci may be unconjugated or show conjugation between a cell and its bud or between independent cells. One to four hat-shaped ascospores are formed in each ascus and they are soon liberated (Fig. 2b, c). In view of conjugation between cells and their buds, the species appears to be homothallic.
Etymology
Komagataella kurtzmanii (kurtz.man’i.i. N.L. gen. masc. n. Kurtzmanii referring to Cletus P. Kurtzman, for his great contributions to yeast taxonomy, in particular studying the genus Komagatella.).
Fermentation and growth reactions
Glucose is fermented. Galactose, sucrose, maltose, lactose, raffinose and trehalose are not fermented. Assimilation of carbon compounds: glucose, l-rhamnose, methanol, ethanol, glycerol, dl-lactate and succinate. No growth occurs on galactose, l-sorbose, sucrose, maltose, cellobiose, trehalose, lactose, melibiose, raffinose, melezitose, inulin, soluble starch, d-xylose, l-arabinose, d-arabinose, d-ribose, d-glucosamine, N-acetyl-d-glucosamine, erythritol, ribitol, xylitol, l-arabinitol, galactitol, d-mannitol, d-glucitol, methyl α-d-glucoside, salicin, d-gluconate, d-glucoronate, d-galacturonate, 2-keto-d-gluconate, 5-keto-d-gluconate, citrate, inositol and d-glucono-1,5-lacton.
Assimilation of nitrogen compounds: ethylamine and l-lysine. No growth occurs on potassium nitrate, nitrite, d-glucosamine and imidazole.
Growth at 35 °C is negative. Growth on vitamin-free medium is negative. Growth on YM agar with 10 % sodium chloride is negative. Growth in 50 % glucose/yeast extract (0.5 %) is negative. Growth on 1 % acid acetic medium is negative. Growth in the presence of 0.1 % cycloheximide is positive.
Type
VKPM Y-727 (=KBP Y-2878 = UCD-FST 76-20 = Starmer #75-208.2), the type strain, is preserved as a lyophilized preparation in the All-Russian Collection of Industrial Microorganisms, Moscow, Russia, also as CBS 12817, with the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, and as NRRL Y-63667, with the ARS Culture Collection, National Center for Agricultural Utilization Research, Peoria, Illinois, USA. MycoBank accession number = 803,919. Strain was isolated by W.T. Starmer from a fir flux in the Catalina Mountains, Southern AZ, USA.
The nucleotide sequences of the of 26S rRNA (D1/D2 domain), translation elongation factor-1α (EF-1α), RNA polymerase II (subunit RPB1), Mt SSU rRNA and ITS-region (ITS1-5.8S-ITS2) are deposited in GenBank under the following accession numbers: KC715720, KC715721, KC715722, KC715723 and KC771256, respectively.
Discussion
Based on the results of multigene sequence comparisons and taxonomic phenotypic analysis, a novel member of the genus Komagataella is formally described, K. kurtzmanii sp. nov. The genus Komagataella represents a phylogenetically distinct clade currently containing six sibling species: K. kurtzmanii, K. pastoris, P. phaffii, K. populi, K. pseudopastoris and K. ulmi (Kurtzman 2012; present study). According to our preliminary data, the Komagataella species may have the same mating type system. In particular, after mating of auxotrophic mutants of K. kurtzmanii and K. phaffii on acetate medium, prototrophic hybrids can be selected on minimal medium. The genus Komagataella apparently meets the concept of a genetic genus in fungi, which suggests that member species possess a common mating type system allowing them to be crossed in any combination (Naumov 1979, 1988). Due to postzygotic isolation, the resulting interspecies hybrids are sterile, having non-viable ascospores. Note that the genetic genera form well separated clusters in phylogenetic trees based on sequence data of rRNA genes (Kurtzman and Robnett 1998). Initially proposed for some Saccharomyces yeasts and mycelium fungus Neurospora, the concept of genetic genus was successfully applied to many polytypic ascomycetous genera, viz. Saccharomyces (Naumov 1987a, 1996; Naumov et al. 2000, 2010), Williopsis (Naumov 1987b), Arthroascus (Naumov et al. 2006), Kluyveromyces (syn. Zygofabospora) (Naumov 1986; Naumov and Naumova 2002), Zygowilliopsis (Naumov et al. 2009), Galactomyces (Naumova et al. 2001) and Ogataea (Hansenula) polymorpha complex (Naumov et al. 1997).
The six species currently assigned to the genus Komagataella are phenotypically very similar (Table 3). The proposed new species K. kurtzmanii differs from the others by absence of growth at 35 °C and inability to assimilate trehalose, d-glucitol, d-mannitol. Taking into account a high variability of physiological properties in yeasts (Kudrjawzev 1960; Scheda and Yarrow 1966) and the fact that K. kurtzmanii, K. populi and K. ulmi are described based on single strains, it seems difficult to separate all six Komagataella species from one another solely by conventional physiological tests. Thus, multigene sequence comparisons must be used for reliable identification of Komagataella species.
Of the five molecular markers used in this study, the D1/D2, ITS1-5.8S-ITS2, EF-1α and RPB1 gave congruent resolutions for species differentiation. In the case of the Mt SSU sequence, it is impossible to distinguish K. phaffii, K. ulmi and K. kurtzmanii. Earlier, it was noticed that species phylogeny based on nuclear and mitochondrial gene sequences can be quite different in fungi (Wu et al. 2008; Schoch et al. 2012). The ITS region is recently recommended as a universal DNA barcode marker for species discrimination in fungi (Schoch et al. 2012). This region of rRNA is characterized by a significant interspecies divergence and a low level of intraspecies polymorphism. Indeed, all six species of Komagataella can be clearly differentiated by the ITS-sequences, which differ by 13–35 nucleotides and numerous indels. On the other hand, no intaspecific variation was observed for the ITS-sequences in K. pastoris and K. phaffii (Kurtzman 2009).
At present only single strains are known for K. populi, K. ulmi and K. kurtzmanii, probably because the complex composition of the genus Komagataella has been established recently (Kurtzman 2005, 2011, 2012). ITS sequence comparisons of natural isolates and strains maintained in different yeast collections may result in finding new Komagataella species, as well as additional strains of the known species. Taking into account great industrial importance of K. pastoris and K. phaffii, their sibling species may serve as a new gene pool for basic and applied studies.
References
Chen MT, Lin S, Shandil I, Andrews D, Stadheim TA, Choi BK (2012) Generation of diploid Pichia pastoris strains by mating and their application for recombinant protein production. Microb Cell Factories 11:91
Cregg JM, Madden KR (1988) Development of the methylotrophic yeast, Pichia pastoris, as a host system for the production of foreign proteins. Dev Ind Microbiol 29:33–41
Cregg JM, Vedvick TS, Raschke WC (1993) Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology 11:905–910
Cregg JM, Latham J, Litton M, Schatzman R, Tolstorukov II (2012) Methods of synthesizing heteromultimeric polypeptides in yeast using a haploid mating strategy. US Patent 8.268.582
Dlauchy D, Tornai-Lehoczki J, Fülöp L, Péter G (2003) Pichia (Komagataella) pseudopastoris sp. nov., a new yeast species from Hungary. Antonie Van Leeuwenhoek 83:327–332
Kudrjawzev WI (1960) Die systematic der Hefen. Academic Verlag, Berlin
Kurtzman CP (1984) Synonymy of the yeast genera Hansenula and Pichia demonstrated through comparisons of deoxyribonucleic acid relatedness. Antonie Van Leeuwenhoek 50:209–217
Kurtzman CP (1998) Pichia E.C. Hansen emend. Kurtzman. In: Kurtzman CP, Fell JW (eds) The yeasts, a taxonomic study, 4th edn. Elsevier, Amsterdam, pp 273–352
Kurtzman CP (2005) Description of Komagataella phaffii sp. nov. and the transfer of Pichia pseudopastoris to the methylotrophic yeast genus Komagataella. Int J Syst Evol Microbiol 55:973–976
Kurtzman CP (2009) Biotechnological strains of Komagataella (Pichia) pastoris are Komagataella phaffii as determined from multigene sequence analysis. J Ind Microbiol Biotechnol 36:1435–1438
Kurtzman CP (2011) Komagataella Y. Yamada, Matsuda, Maeda, Mikata (1995). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier Science B.V, Amsterdam, pp 491–495
Kurtzman CP (2012) Komagataella populi sp. nov. and Komagataella ulmi sp. nov., two new methanol assimilating yeasts from exudates of deciduous trees. Antonie Van Leeuwenhoek 101:859–868
Kurtzman CP, Robnett CJ (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 73:331–371
Kurtzman CP, Robnett CJ (2003) Phylogenetic relationships among yeasts of the “Saccharomyces complex” determined from multigene sequence analyses. FEMS Yeast Res 3:417–432
Kurtzman CP, Robnett CJ, Basehoar-Powers E (2008) Relationships among species of Pichia, Issatchenkia and Williopsis determined from multigene phylogenetic analysis and the proposal of Barnettozyma gen. nov., Lindnera gen. nov. and Wickerhamomyces gen. nov. FEMS Yeast Res 8:939–954
Kurtzman CP, Fell JW, Boekhout T, Robert V (2011) Methods for isolation, phenotypic characterization and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier Science B.V, Amsterdam, pp 87–110
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22:249–270
McClary DD, Nulty WL, Miller GR (1959) Effect of potassium versus sodium in the sporulation of Saccharomyces. J Bacteriol 78:362–368
Naumov GI (1979) Genetic concept of genus in fungi. Dokl Biol Sci 241(4):345–347
Naumov GI (1986) Genosystematics of yeast genus Zygofabospora Kudriavzev emend. G. Naumov. Mol Genet Microbiol Virol (Moscow) 5:10–14
Naumov GI (1987a) Genetic basis for classification and identification of the ascomycetous yeasts. Stud Mycol 30:469–475
Naumov GI (1987b) Results of the genosystematics of the yeast Williopsis Zender and Zygowilliopsis Kudriavzev. Mol Genet Microbiol Virol (Moscow) 2:1–7
Naumov GI (1988) Genus as a genetic system. In: Aspects of microevolution. Nauka, Moscow, pp 112–113 (in Russian)
Naumov GI (1996) Genetic identification of biological species in the Saccharomyces sensu stricto complex. J Ind Microbiol 17:295–302
Naumov GI, Naumova ES (2002) Five new combinations in the yeast genus Zygofabospora Kudriavzev emend. G. Naumov (pro parte Kluyveromyces) based on genetic data. FEMS Yeast Res 2:39–46
Naumov GI, Naumova ES, Kondratieva VI, Bulat SA, Mironenko NV, Mendonça-Hagler LC, Hagler AN (1997) Genetic and molecular delineation of three sibling species in the Hansenula polymorpha complex. Syst Appl Microbiol 20:50–56
Naumov GI, James SA, Naumova ES, Louis EJ, Roberts IN (2000) Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int J Syst Evol Microbiol 50:193–1942
Naumov GI, Naumova ES, Smith MT, de Hoog GS (2006) Molecular-genetic diversity of the genus Arthroascus: Arthroascus babieviae sp.nov., Arthroascus fermentans var. arxii nov. var. and geographic populations of Arthroascus schoenii. Int J Syst Evol Microbiol 56:1997–2007
Naumov GI, Kondratieva VI, Naumova ES (2009) Taxonomic genetics of Zygowilliopsis yeasts. Russ J Genet 45:1422–1427
Naumov GI, Naumova ES, Masneuf-Pomarède I (2010) Genetic identification of new biological species Saccharomyces arboricolus Wang et Bai. Antonie Van Leeuwenhoek 98:1–7
Naumova ES, Smith MTh, Boekhout T, Hoog GS, Naumov GI (2001) Molecular differentiation of sibling species in the Galactomyces geotrichum complex. Antonie Van Leeuwenhoek 80:263–273
Scheda R, Yarrow D (1966) The instability of physiological properties used as criteria in the taxonomy of yeasts. Arch Mikrobiol 55:209–225
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci (USA) 109:6241–6246
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Wegner EH (1983) Biochemical conversions by yeast fermentation at high-cell densities. US Patent 44114329
Wu Q, James SA, Roberts IN, Moulton V, Huber KT (2008) Exploring contradictory phylogenetic relations in yeasts. FEMS Yeast Res 8:641–650
Yamada Y, Matsuda M, Maeda K, Mikata K (1995) The phylogenetic relationships of methanol-assimilating yeasts based on the partial sequences of 18S and 26S ribosomal RNAs: the proposal of Komagataella gen. nov. (Saccharomycetaceae). Biosci Biotechnol Biochem 59:439–444
Yarrow D (1998) Methods for the isolation, maintenance and identification of yeasts. In: Kurtzman CP, Fell JW (eds) The yeasts, a taxonomic study, 4th edn. Elsevier, Amsterdam, pp 77–100
Acknowledgments
Synthesis of oligonucleotide primers and sequencing of the D1/D2 26S rRNA, translation elongation factor-1α, RNA polymerase II, mitochondrial small subunit rRNA and ITS1-5.8S-ITS2 were performed using the equipment of the Centre for Collective Use of GosNIIgenetika (Moscow).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naumov, G.I., Naumova, E.S., Tyurin, O.V. et al. Komagataella kurtzmanii sp. nov., a new sibling species of Komagataella (Pichia) pastoris based on multigene sequence analysis. Antonie van Leeuwenhoek 104, 339–347 (2013). https://doi.org/10.1007/s10482-013-9956-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-013-9956-7