Abstract
Critical to most studies in molecular microbial ecology is the application of DNA/RNA extraction methods which can reveal the true level of population biodiversity present in samples from the community under investigation. Activated sludge communities have been studied extensively using molecular methods, but rarely have the nucleic acid isolation methods applied been assessed for their ability to achieve this. This study compares eight published RNA and DNA extraction protocols and one commercially available DNA isolation kit for their capacity to provide high quality nucleic acids that reflect the community composition. Each method was assessed on the basis of nucleic acid yield, purity and integrity, and the ability to provide PCR amplifiable RNA and DNA from known marker populations that varied in their resistance to nucleic acid extraction. Only three consistently provided DNA from each of the marker populations known to be present in the samples from fluorescence in situ hybridisation analysis. The failure of the other methods emphasises the need to validate all DNA/RNA extraction protocols. It is recommended that several validated extraction methods be used and the extracts pooled to further minimise any risk of bias.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crucial to the majority of culture independent investigations of microbial communities, including those in activated sludge systems, is the isolation of high quality nucleic acids. However, due to sample variation (e.g. in the sample matrix, cell surface layer composition of different bacteria, etc.), the use of an inappropriate DNA/RNA extraction method may provide a highly biased view of the abundance of the populations present (von Wintzingerode et al. 1997). Only a relatively small number of published studies have assessed nucleic acid isolation protocols for their suitability with activated sludge samples (Bourrain et al. 1999; Yu and Mohn 1999; Orsini and Romano-Spica 2001; Gabor et al. 2003; Purohit et al. 2003; Lemarchand et al. 2005; Roh et al. 2006; Guobin et al. 2008; McIlroy et al. 2008a). Unfortunately, the majority of studies have adapted protocols intended for habitats like soil without providing justification for their selection. Activated sludge is different to the environments against which most popular extraction methods were developed, in that the biomass is typically organised as cellular aggregates or flocs (Nielsen 2002). These flocs can be quite robust and resistant to extraction methods (Watanabe et al. 1998; Bourrain et al. 1999; Yu and Mohn 1999; McIlroy et al. 2008a). Which DNA extraction methods are best suited for such samples has not been comprehensively assessed.
The approaches used to extract nucleic acids from environmental samples incorporate chemical (see Zhou et al. 1996), enzymatic (Porteous et al. 1994), mechanical (Ogram et al. 1987), heat (Picard et al. 1992), and freeze–thaw (Tsai and Olson 1991) treatments, often in combination. Mechanical lysis is popular as it provides high nucleic acid yields and can facilitate the disruption of aggregated biomass (Moré et al. 1994; Watanabe et al. 1998; Bourrain et al. 1999; Frostegård et al. 1999; Yu and Mohn 1999; Bürgmann et al. 2001; Stach et al. 2001; Lakay et al. 2007; Guobin et al. 2008; McIlroy et al. 2008a). While most protocols are dedicated to DNA isolation, those able to extract RNA and DNA simultaneously provide the opportunity to obtain additional valuable information on the metabolic activities of populations in the community (von Wintzingerode et al. 1997; Hurt et al. 2001).
In this study we have compared eight different published methods for their ability to extract high quality nucleic acids from several activated sludge samples. These were chosen for their demonstrated ability to extract both RNA and DNA, and their suitability for high throughput processing at minimal cost. The effectiveness of each method with activated sludge was compared using 16S rRNA group-specific PCR of selected microbial populations which have been either well-represented, under-represented or absent from previous 16S rRNA gene clone libraries (see Meyer et al. 2006; Ahn et al. 2007). Fluorescence in situ hybridization (FISH) was employed to assess the ability of each method to recover nucleic acids from the selected marker populations independently of the extraction process and to allow a rational basis for method selection.
Materials and methods
Sampling
Activated sludge biomass samples were obtained from three sources. Source LS1 was a laboratory-scale enhanced biological phosphorus removal (EBPR) sequencing batch reactor (SBR) that was continuously aerated and fed with clarified effluent from a non-EBPR treatment plant located in Melton, Victoria, Australia. Operational conditions of this reactor are given in Ahn et al. (2007). Samples were taken at the end of the cycle and stored at −80°C. Biomass samples used for FISH analyses were removed at the same time, washed twice in 1× phosphate buffered saline (PBS) and fixed in 4% (w/v) paraformaldehyde and 1× PBS at 4°C for 3 h, before being stored in 50% ethanol and 1× PBS at 20°C until further use.
Sources FS1 and FS2 were two full-scale EBPR waste water treatment plants. Both were Modified University of Cape Town (MUCT) configured systems located at Castlemaine and Kyneton (both in Victoria, Australia), respectively. Samples (100 ml) were taken from their aerobic tanks and, while continuously mixed, 200 μl aliquots were immediately dispensed into 1.5 ml microcentrifuge tubes using wide bore pipette tips. These were transported on dry ice to the laboratory for storage at −80°C.
Nucleic acid extraction
A 200 μl aliquot of biomass (8–14 mg wet weight) was pelleted by centrifugation at 6,800g for 5 min at 4°C. Nucleic acids were extracted using the methods of Corgié et al. (2006) (abbreviated to CR), Costa et al. (2004) (CS), Griffiths et al. (2000) (GR), McIlroy et al. (2008a) (MI), McVeigh et al. (1996) (MV), Orsini and Romano-Spica (2001; OR; using their RNA protocol, with the microwave set to 800 W), Tillett and Neilan (2000) (TN), and Yu and Mohn (1999) (YM). For a brief comparison of the principle differences between the cell lysis steps of each method, see Table 1. All protocols were modified to ensure that the non-lysis steps were kept as uniform as possible. The method of McVeigh et al. (1996) was further adapted to exclude the initial wash and to separate the phenol and chloroform-isoamyl alcohol steps. On occasions, an additional precipitation step was required to remove excess salt from the final DNA pellet for the MV protocol.
Following their extraction, the nucleic acids were precipitated by the addition of 0.6 volumes of 2-propanol, incubated on ice for 15 min, and pelleted at 20,800g for 15 min at 4°C. Pellets were then washed twice with 1 ml of 70% (w/v) ethanol, air dried, and re-suspended in 50 μl TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0). All bead beating steps used 0.6 g of 0.1 mm diameter glass beads (Daintree Scientific, St Helens, TAS, Australia), and samples were homogenized in a mini bead beater (Biospec, Bartsville, OK, USA) running at its maximum speed. All buffers and reagents used in steps where RNA degradation might occur were either treated with DEPC or made up with DEPC treated water.
DNA was also extracted with the UltraClean Soil DNA Isolation Kit™ following manufacturer’s instructions for maximum yield (MO BIO Laboratories, Carlsbad, CA, USA; abbreviated to MO).
SYBR Gold™ staining of biomass
After lysis, pelleted biomass debris were washed three times in 1× PBS and fixed in paraformaldehyde as described above for FISH biomass preparation. Samples were applied to Vectabond™ (Vector Laboratories, Burlingame, CA, USA) coated slides, stained with SYBR Gold™ nucleic acid stain (Invitrogen, Carlsbad, CA, USA) for 10 min, and rinsed briefly with distilled water. Slides were mounted in VectaShield (Vector Laboratories, Burlingame, CA, USA) and examined with an Eclipse 800 epifluorescence microscope (Nikon, Lidcome, NSW, Australia).
Nucleic acid gel electrophoresis
Before DNA gel electrophoresis, samples were treated with 10 ng Ribonuclease A (Sigma–Aldrich, St. Louis, MO, USA) at room temperature for 30 min (if applicable). Agarose gels (1% w/v) were prepared in 1× TAE buffer and electrophoresed at 90 V in 1× TAE buffer and post-stained in 1× TAE buffer containing 0.1% ethidium bromide. Prior to RNA electrophoresis and reverse transcriptase (RT) PCR, samples were treated with 40 U Recombinant RNasin® Ribonuclease Inhibitor (Promega, Madison, WI, USA), 1× Buffer RDD (QIAGEN, Hilden, NRW, Germany), 5 U DNase I (QIAGEN, Hilden, NRW, Germany) at room temperature for 30 min. DNase I was inactivated at 75°C for 10 min. The method for RNA gel electrophoresis was performed according to the method of Goda and Minton (1995).
DNA and RNA levels in crude extracts were quantified separately after capturing an image of each agarose gel. Adobe Photoshop 7 (Adobe, San Jose, CA, USA) was used to measure the integrated fluorescence intensity volume of each sample lane. Nucleic acid concentrations were calculated after a comparison of the fluorescence intensity volumes of a three point standard curve prepared from DNA/RNA standards of known concentrations. Duplicate readings were obtained from duplicate extractions for each method, and for each point of the standard curve. The error represents the range of values obtained. It should be noted that values given do not indicate the integrity of the nucleic acid, and may be overestimated for sheared samples.
Spectrophotometry
Absorption levels of extracts were obtained using a ND-1000 NanoDrop Spectrophotometer.
Restriction digest inhibition assessment
Restriction enzyme digestion reactions were performed with 2.5 μl of RNAse treated crude DNA extract from each extraction, 60 ng (approx) of uncut pGEM-T plasmid (Promega, Madison, WI, USA), 10 U of Eco RI and 1× Eco RI buffer (Roche Diagnostics, Castle Hill, NSW, Australia), and were incubated at 37°C for 30 min. Successful DNA digestion was assessed using agarose electrophoresis.
Oligonucleotide probe and primers
Oligonucleotide probes and primers used are shown in Table 2. Primers were supplied by GeneWorks, Adelaide, SA. Probes were purchased from Sigma-Genosys (Castle Hill, NSW, Australia).
RT-PCR
RT-PCR reactions were performed in 200 μl thin-walled PCR tubes, with a reaction volume of 25 μl, on an iCycler IQ™ Multicolor Real-Time Detection System (BIO-RAD, Hercules, CA, USA). RNA was combined with 0.5 μg primer 27F per μg RNA and incubated at 70°C for 5 min, and then incubated on ice for 5 min. Reactions contained 1× AMV reverse transcriptase reaction buffer (Promega, Madison, WI), 40 U Recombinant RNasin® ribonuclease inhibitor, 1 mM dNTP mix and 40 U AMV reverse transcriptase (Promega, Madison, WI, USA), and were made up to volume with DEPC treated distilled water. Reactions were heated at 42°C for 1 h, and then screened by PCR for members of the domain Bacteria with the primers 1396F and 1492R. For all primer sequences see Table 2.
PCR
PCR reactions were performed in 200 μl thin-walled PCR tubes, using an iCycler IQ™ Multicolor Real-Time Detection System (BIO-RAD, Hercules, CA, USA). Reactions contained 1× GoTaq® Green Master Mix (Promega, Madison, WI, USA), 200 nM primers, 3 μl of a 1:10 or a 1:100 dilution of nucleic acid extract and distilled water to a final volume of 25 μl. Reactions were heated to 95°C for 5 min followed by 40 cycles of 95°C for 1 min, annealing at the specified temperature for 30 s, and extension at 72°C for 30 s, before a final extension at 72°C for 10 min. A no-template negative control was included for every PCR reaction.
The PCR reactions targeting the 16S rRNA genes of Dechloromonas-related organisms used primers Dech454F and BTW0663R at an annealing temperature of 62.8°C. Those targeting 16S rRNA genes of cluster II Defluviicoccus-related populations used primers DF988F and 1492R, at an annealing temperature of 56.5°C, while those targeting the 16S rRNA genes of Rhodocyclus-related populations were performed using primers PAO462F and PAO651R, at an annealing temperature of 62.8°C. To ensure that negative results were not due to insufficient DNA the amount added to each PCR reaction was normalised down for all samples giving an initial positive result with the DF988F and 1492R primers to 13 and 3 ng for the LS1 and FS1 samples, respectively. These DNA concentrations were chosen to be equivalent to those in the sample with the lowest nucleic acid yield. PCR products were detected by agarose gel electrophoresis using 5 μl of the PCR reaction mix.
FISH
FISH and Quantitative FISH (qFISH) were performed as detailed by Daims et al. (2005) using the formamide concentrations recommended in the original papers describing each probe. The samples were examined with a Leica TCS SP2 (model DMRE2) confocal scanning laser microscope (Leica, North Ryde, NSW, Australia) and the bio-volume values calculated using the DAIME software (Daims et al. 2006) on at least 40 fields of view taken at 630× magnification. The non-EUB probe was included as a negative control to assess any non-specific binding (Wallner et al. 1993). FISH probe sequences used are listed in Table 2.
SDS–polyacrylamide gel electrophoresis
SDS–polyacrylamide gel electrophoresis (PAGE) was according to the method of Laemmli (1970). The equivalent of 5 μl of total nucleic acid extract was loaded and gels were electrophoresed at 200 V 1× running buffer (pH 8.3) and stained with Coomassie blue R-250.
Results and discussion
Nucleic acid yields
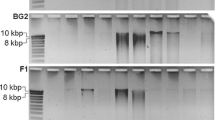
The final nucleic acid yields from each extraction method were quantified by gel electrophoresis (Fig. 1). In general, higher DNA yields were obtained with methods incorporating bead beating as the mechanical lysis step than from those employing a milder vortexing step in the presence of beads (i.e. MO), or relying totally on chemical lysis (i.e. TN and OR). These findings agree with others from similar studies (Moré et al. 1994; Watanabe et al. 1998; Bourrain et al. 1999; Frostegård et al. 1999; Yu and Mohn 1999; Bürgmann et al. 2001; Stach et al. 2001; Lakay et al. 2007; Guobin et al. 2008; McIlroy et al. 2008a). Of the methods where bead beating was incorporated, lowest DNA yields were obtained with the YM extraction method despite it having the longest beating time (Fig. 1). This may have resulted from the presence of SDS in the lysis buffer contributing to the formation of a stable foam that reduced the effectiveness of the bead beating process. A similar problem was observed in the development of the MI method, which was overcome by the inclusion of a silicone antifoam in the lysis buffer (McIlroy et al. 2008a).
Nucleic acid purity
The purity of the nucleic acids obtained with each method was assessed spectrophotometrically (Table 3). It is important to note that purification steps were standardised for each of these methods, mainly to allow comparisons of the efficiency of the cell lysis step to be made, but also to remove superfluous steps from the original protocols. The purity of DNA extracted with the CR, GR, MV and OR methods was higher than that generated with the other extraction protocols. However, all nine methods yielded DNA sufficiently free of inhibitors to allow PCR and restriction enzyme digestion (Table 3, data not shown). Similarly, the RNA obtained was suitable for use in RT-PCR (Table 3). The only exception was the CS extract from FS1, where an unidentified inhibitor of DNase I prevented removal of genomic DNA during RNA purification (data not shown). All extracts appeared to be free of high levels of proteins as none of the extracts gave visible bands on SDS–PAGE protein gels (data not shown).
Nucleic acid integrity
In addition to total yield, the physical integrity of the nucleic acid is a crucial factor in selecting an extraction method as excessive shearing can limit the ability to amplify large gene regions. Furthermore, DNA shearing may lead to the generation of PCR artefacts such as chimeras (Liesack et al. 1991).
Using the LS1 biomass, all non-bead beating methods produced high molecular weight DNA (Fig. 2) and extracts with 23S:16S rRNA ratios closer to the expected value (Table 3). The bead beating methods all resulted in some DNA shearing (Fig. 2). Of these bead beating protocols the DNA integrity was best from the CS and YM protocols, while the other methods showed higher levels of shear damage (Fig. 2). It should be noted that even methods with higher levels of shear damage did not prevent the successful later use of the DNA in PCR, as shown in Table 3.
Effects of homogenisation on biomass integrity
Nucleic acid stained biomass was macro and microscopically examined before and after each extraction process to assess the overall extent of cell lysis. The MO, OR and YM methods contained visible macroscopic flocs after the cell lysis step (Table 3). This problem has been reported with other activated sludge samples, suggesting that harsh mechanical lysis may be required when working with such biomass (Watanabe et al. 1998; Bourrain et al. 1999; Yu and Mohn 1999; McIlroy et al. 2008a). Even methods incorporating mechanical lysis still yielded suspensions containing small numbers of microscopic flocs and small cell aggregates following the lysis stage (examples of post extraction biomass are shown in (Fig. 3)). It may be that complete cell lysis is unattainable for nucleic acid extraction from activated sludge samples without unacceptable mechanical shear damage to the nucleic acids, and a compromise between the two is required. A similar problem has been reported for soil samples where some cells appeared to escape lysis (Moré et al. 1994; Zhou et al. 1996).
Assessing cell lysis efficiency using observed target populations
Although comparing DNA yields provides an approximate estimate of relative cell lysis efficiency, yield alone can not be assumed to correlate necessarily with, or reflect accurately the biodiversity of individual populations in a sample (Stach et al. 2001; Gabor et al. 2003; Luna et al. 2006). As intact biomass always remained after the lysis step, the representative nature of the DNA and RNA in the extract was estimated using PCR to target selected marker populations, whose presence in the sample was assessed by qFISH (Table 3).
FISH was selected over community profiling methods like denaturing gradient gel electrophoresis (DGGE; Duarte et al. 1998; Krsek and Wellington 1999; Griffiths et al. 2000; Maarit Niemi et al. 2001; Gabor et al. 2003; de Lipthay et al. 2004; Fortin et al. 2004; Desai and Madamwar 2006; Dong et al. 2006; Yang et al. 2007; Guobin et al. 2008; Mitchell and Takacs-Vesbach 2008) as the population abundance data provided is independent of the nucleic acid extraction process. This allows the presence or absence of any marker populations in a sample to be quantified directly, and thus avoids the need to use broad marker groups, or to make untested assumptions as to which populations might be present, as has been necessary in other community based studies (Kuske et al. 1998; Yeates et al. 1998; Bürgmann et al. 2001; Gabor et al. 2003; Roh et al. 2006).
Three key marker populations were used to assess nucleic extraction efficiency. These were Dechloromonas-, Defluviicoccus- and Accumulibacter-related groups. Earlier studies had shown that Defluviicoccus- and Accumulibacter-related populations were conspicuously absent, or severely underrepresented in clone libraries generated from DNA extracted from several activated sludge communities, despite FISH data suggested they were among the numerically dominant populations (Meyer et al. 2006; Ahn et al. 2007; McIlroy et al. 2008b). These two groups were therefore selected as marker populations representing populations in activated sludge that appeared to be more difficult to extract nucleic acids from. The Accumulibacter-related organisms were present in all three activated sludge communities examined in this study contributing 1.4–11.8% of the total biovolume as determined by qFISH. Similarly, the Defluviicoccus-related organisms were found in all biomass samples (except FS2), where they contributed between 2.9 and 4.3% of the total cell biovolume (Table 3). The PCR data showed that only the CS, MI and MV extraction methods were able to extract DNA consistently from the Defluviicoccus-related cells in each of the two sludge communities where their presence was confirmed by FISH analysis (Table 3). Defluviiccoccus-related populations were never detected in DNA extracted with the OR, TN or MO protocols. These results may explain why these dominating populations were not observed in a 16S rRNA gene clone library generated from DNA extracted using the MO method from an EBPR SBR community similar to that of the LS1 (Ahn et al. 2007).
Dechloromonas-related organisms were seen frequently in the same 16S rRNA gene library (Ahn et al. 2007) suggesting that they may be a suitable marker group for comparatively easily extracted populations. The qFISH data showed these were a dominating population in the LS1 community at ~8.9% of the total cell biovolume (Table 3). All the methods examined in the current study could extract PCR-detectable levels of DNA from Dechloromonas-related organisms (Table 3). Tracking DNA from readily lysed organisms indicates if DNA released early in the cell lysis step is subsequently damaged beyond detection (e.g. if lysis process is too harsh), or lost through binding to the sample matrix (Frostegård et al. 1999).
Applicability
The duration of, and health risk associated with each method was also assessed (Table 3), as both factors are important in selecting a protocol suited for high throughput analysis. Time is also critical especially with RNA extractions given the typically short half-life of bacterial mRNA (Kaberdin and Bläsi 2006). All protocols in the study with the exception of the MO method, take ~100 min to complete. Both the CR and MV methods incorporate bead beating with phenol–chloroform, while the TN method requires the use of hot phenol–chloroform, which raises safety concerns given the toxicity of these chemicals.
Conclusions
The results presented here suggest that the best methods for obtaining the most representative nucleic acids from activated sludge bacterial communities are the MI and MV protocols. The MV method generates nucleic acid of higher purity, yield and integrity than the MI method, but uses phenol in the bead beating step. The lower quality of the nucleic acids obtained with the MI method does not appear to prevent their use in downstream molecular applications, and it may be better suited for high throughput processing. Because of variations in floc size, density, and populations found in different activated sludge communities, it is recommended that several different validated methods are used in parallel and the extracted nucleic acids pooled if greater biodiversity is to be captured, as was recommended by Juretschko et al. (2002). The failure of most of the methods applied to consistently isolate DNA from the marker populations in these samples demonstrates the need to validate the nucleic acid isolation protocols used in all molecular ecological studies.
Abbreviations
- EBPR:
-
Enhanced biological phosphorus removal
- FISH:
-
Fluorescence in situ hybridisation
- SBR:
-
Sequencing batch reactor
- PBS:
-
Phosphate buffered saline
- MUCT:
-
Modified University of Cape Town
- PAGE:
-
Polyacrylamide gel electrophoresis
- SDS:
-
Sodium dodecyl sulphate
- CTAB:
-
Cetyl trimethylammonium bromide
- NaTCA:
-
Sodium trichloroacetate
- DGGE:
-
Denaturing gradient gel electrophoresis
References
Ahn J, Schroeder S, Beer M, McIlroy S, Bayly RC, May JW, Vasiliadis G, Seviour RJ (2007) Ecology of the microbial community removing phosphate from wastewater under continuously aerobic conditions in a sequencing batch reactor. Appl Environ Microbiol 73:2257–2270
Amann RI, Binder BJ, Olson RJ, Chisolm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Bourrain M, Achouak W, Urbain V, Heulin T (1999) DNA extraction from activated sludges. Curr Microb 38:315–319
Bürgmann H, Pesaro M, Widmer F, Zeyer J (2001) A strategy for optimizing quality and quantity of DNA extracted from soil. J Microb Methods 45:7–20
Corgié SC, Beguiristain T, Leyval C (2006) Profiling 16S bacterial DNA and RNA: difference between community structure and transcriptional activity in phenanthrene polluted sand in the vicinity of plant roots. Soil Biol Biochem 38:1545–1553
Costa R, Gomes NCM, Milling A, Smalla K (2004) An optmized protocol for simultaneous extraction of DNA and RNA from soils. Brazilian J Microbiol 35:230–234
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microbiol 66:1175–1182
Daims H, Brühl A, Amann R, Schleifer K, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Daims H, Stoecker K, Wagner M (2005) Fluorescence in situ hybridization for the detection of prokaryotes. In: Osborn AM, Smith CJ (eds) Molecular microbial ecology. Taylor & Francis, New York, pp 213–239
Daims H, Lücker S, Wagner M (2006) daime, a novel image analysis program for microbial ecology and biofilm research. Environ Microbiol 8:200–213
de Lipthay JR, Enzinger C, Johnsen K, Aamand J, Sørensen SJ (2004) Impact of DNA extraction method on bacterial community composition measured by denaturing gradient gel electrophoresis. Soil Biol Biochem 36:1607–1614
Desai C, Madamwar D (2006) Extraction of inhibitor-free metagenomic DNA from polluted sediments, compatible with molecular diversity analysis using adsorption and ion-exchange treatments. Bioresour Technol 98:761–768
Dong D, Yan A, Liu H, Zhang X, Xu Y (2006) Removal of humic substances from soil DNA using aluminium sulfate. J Microb Methods 66:217–222
Duarte GF, Rosado AS, Seldin L, Keijzer-Wolters AC, van Elsas JD (1998) Extraction of ribosomal RNA and genomic DNA from soil for studying the diversity of the indigenous bacterial community. J Microbiol Methods 32:21–29
Fortin N, Beaumier D, Lee K, Greer C (2004) Soil washing improves the recovery of total community DNA from polluted and high organic content sediments. J Microb Methods 56:181–191
Frostegård A, Courtois S, Ramisse V, Clerc S, Bernillon D, Le Gall F, Jeannin P, Nesme X, Simonet P (1999) Quantification of bias related to the extraction of DNA directly from soils. Appl Environ Microbiol 65:5409–5420
Gabor E, de Vries E, Janssen D (2003) Efficient recovery of environmental DNA for expression cloning by indirect extraction methods. FEMS Microbiol Ecol 44:153–163
Goda S, Minton N (1995) A simple procedure for gel electrophoresis and northern blotting of RNA. Nucleic Acids Res 23:3357–3358
Griffiths R, Whiteley A, O’Donnell A, Bailey M (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491
Guobin S, Wenbiao J, EKH LAM, Xinhui X (2008) Purification of total DNA extracted from activated sludge. J Environ Sci 20:80–87
Hurt R, Qiu X, Wu L, Roh Y, Palumbo A, Tiedje J, Zhou J (2001) Simultaneous recovery of RNA and DNA from soils and sediments. Appl Environ Microbiol 67:4495–4503
Juretschko S, Loy A, Lehner A, Wagner M (2002) The microbial community composition of a nitrifying-denitrifying activated sludge from an industrial sewage treatment plant analyzed by the full-cycle rRNA approach. System Appl Microbiol 25:84–99
Kaberdin VR, Bläsi U (2006) Translational initiation and fate of bacterial mRNAs. FEMS Microbiol Rev 30:967–979
Krsek M, Wellington E (1999) Comparison of different methods for the isolation and purification of total community DNA from soil. J Microbiol Methods 39:1–16
Kuske C, Banton K, Adorada D, Stark P, Hill K, Jackson P (1998) Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl Environ Microbiol 64:2463–2472
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lakay F, Botha A, Prior B (2007) Comparative analysis of environmental DNA extraction and purification methods from different humic acid-rich soils. J Appl Microbiol 102:265–273
Lane D (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Modern microbial methods: nucleic acid techniques in bacterial systematics. Wiley, England, pp 115–175
Lemarchand K, Berthiaume F, Maynard C, Harel J, Payment P, Bayardelle P, Masson L, Brousseau R (2005) Optimization of microbial DNA extraction and purification from raw wastewater samples for downstream pathogen detection by microarrays. J Microb Methods 63:115–126
Liesack W, Weyland H, Stackebrandt E (1991) Potential risks of gene amplification by PCR as determined by 16S rDNA analysis of a mixed-culture of strict barophilic bacteria. Microb Ecol 21:191–198
Loy A, Schulz C, Lücker S, Schöpfer-Wendels A, Stoecker K, Baranyi C, Lehner A, Wagner M (2005) 16S rRNA gene-based oligonucleotide microarray for environmental monitoring of the betaproteobacterial order “Rhodocyclales”. Appl Environ Microbiol 71:1373–1386
Luna G, Dell’Anno A, Danovaro R (2006) DNA extraction procedure: a critical issue for bacterial diversity assessment in marine sediments. Environ Microbiol 8:308–320
Maarit Niemi R, Heiskanen I, Wallenius K, Lindström K (2001) Extraction and purification of DNA in rhizosphere soil samples for PCR-DGGE analysis of bacterial consortia. J Microb Methods 45:155–165
McIlroy S, Porter K, Seviour RJ, Tillett D (2008a) Simple and safe method for simultaneous isolation of microbial RNA and DNA from problematic populations. Appl Environ Microbiol 74:6806–6807
McIlroy S, Hoefel D, Schroeder S, Ahn J, Tillett D, Saint C, Seviour R (2008b) FACS enrichment and identification of floc-associated alphaproteobacterial tetrad-forming organisms in an activated sludge community. FEMS Microbiol Lett 285:130–135
McVeigh H, Munro J, Embley T (1996) Molecular evidence for the presence of novel actinomycete lineages in a temperate forest soil. J Ind Microbiol Biotechnol 17:197–204
Meyer RL, Saunders AM, Blackall LL (2006) Putative glycogen-accumulating organisms belonging to the Alphaproteobacteria identified through rRNA-based stable isotope probing. Microbiology 152:419–429
Mitchell KR, Takacs-Vesbach CD (2008) A comparison of methods for total community DNA preservation and extraction from various thermal environments. J Ind Microbiol Biotechnol 35:1139–1147
Moré M, Herrick J, Silva M, Ghiorse W, Madsen E (1994) Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Environ Microbiol 60:1572–1580
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nielsen PH (2002) Activated sludge—the floc. In: Bitton G (ed) Encyclopedia of environmental microbiology, vol 1. Wiley, New York, pp 54–61
Ogram A, Sayler GS, Barkay T (1987) The extraction and purification of microbial DNA from sediments. J Microb Methods 7:57–66
Orsini M, Romano-Spica V (2001) A microwave-based method for nucleic acid isolation from environmental samples. Lett Appl Microbiol 33:17–20
Picard C, Ponsonnet C, Paget E, Nesme X, Simonet P (1992) Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol 58:2717–2722
Porteous L, Armstrong J, Seidler R, Watrud L (1994) An effective method to extract DNA from environmental samples for polymerase chain reaction amplification and DNA fingerprint analysis. Curr Microbiol 29:301–307
Purohit H, Kapley A, Moharikar A, Narde G (2003) A novel approach for extraction of PCR-compatible DNA from activated sludge samples collected from different biological effluent treatment plants. J Microb Methods 52:315–323
Roh C, Villatte F, Kim B, Schmid R (2006) Comparative study of methods for extraction and purification of environmental DNA from soil and sludge samples. Appl Biochem Biotechnol 134:97–112
Stach J, Bathe S, Clapp J, Burns R (2001) PCR-SSCP comparison of 16S rDNA sequence diversity in soil DNA obtained using different isolation and purification methods. FEMS Microbiol Ecol 36:139–151
Suzuki M, Taylor L, DeLong E (2000) Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl Environ Microbiol 66:4605–4614
Tillett D, Neilan BA (2000) Xanthogenate nucleic acid isolation from cultured and environmental Cyanobacteria. J Phycology 36:251–258
Tsai Y, Olson B (1991) Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol 57:1070–1074
von Wintzingerode F, Göbel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Wallner G, Amann R, Beisker W (1993) Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136–143
Watanabe K, Yamamoto S, Hino S, Harayama S (1998) Population dynamics of phenol-degrading bacteria in activated sludge determined by gyrB-targeted quantitative PCR. Appl Environ Microbiol 64:1203–1209
Yang ZH, Xiao Y, Zeng GM, Xu ZY, Liu YS (2007) Comparison of methods for the total community DNA extraction and purification from compost. Appl Microbiol Biotechnol 74:918–925
Yeates C, Gillings M, Davison A, Altavilla N, Veal D (1998) Methods for microbial DNA extraction from soil for PCR amplification. Biol Proced Online 1:40–47
Yu Z, Mohn WW (1999) Killing two birds with one stone: simultaneous extraction of DNA and RNA from activated sludge biomass. Can J Microbiol 45:269–272
Zhou J, Bruns M, Tiedje J (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322
Zilles J, Peccia J, Kim M, Hung C, Noguera D (2002) Involvement of Rhodocyclus-related organisms in phosphorus removal in full-scale wastewater treatment plants. Appl Environ Microbiol 68:2763–2769
Acknowledgments
This work was supported by Australian Research Council (ARC) Discovery (DP0557646DS) and Victorian State Government Smartwater research grants. S. McIlroy received an ARC APA PhD scholarship. The authors thank Dr Bradley Campbell and Dr Johwan Ahn for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McIlroy, S.J., Porter, K., Seviour, R.J. et al. Extracting nucleic acids from activated sludge which reflect community population diversity. Antonie van Leeuwenhoek 96, 593–605 (2009). https://doi.org/10.1007/s10482-009-9374-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-009-9374-z