Abstract
Societal processes of rural change and globalization may change homegardens and their contribution to the conservation of agrobiodiversity, particularly of species occurring naturally in regional vegetation. The best way to determine if this occurs is through longitudinal studies. We conducted such a study, inventorying tree species in a sample of 38 homegardens in 2009, 2012 and 2015. The homegardens were located in the subregions of mountain slopes, fluvial plains and coastal plains in the tropical lowlands of Tabasco, Mexico. We analysed changes in species richness by geographic origin, species richness and species composition in each inventory. We identified 169 tree species in the three inventories, of which 74.6% were native or neotropical and 25.4% introduced. Of the 140 species recorded in 2009, 88% remained in 2015, whereas 12% had been replaced and nine additional species had arrived. Mean species richness increased between 2009 and 2015 (P = 0.03) and between 2012 and 2015 (P = 0.001). Increases resulted from increased mean neotropical (P = 0.01) and introduced (P = 0.01) species richness, and constant native species richness. Differences in species composition between the three subregions in 2009 persisted in 2012 and 2015 (P < 0.001 in all years). These results show how the highly dynamic character of homegardens combines with the renewal and persistence of their agrobiodiversity, and underpins the continued relevance of homegarden for agrobiodiversity conservation and livelihoods in tropical lowlands amidst rural change and globalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homegardens are agroecosystems where families promote the coexistence of useful plants and animals with the naturally occurring biota (Vandermeer et al. 1998). They are located around rural homesteads, and managed based on family labour in order to obtain products for household consumption, selling on local markets and exchange (Fernandes and Nair 1986).
Aggregated regional species richness of tropical homegardens is generally large, but variable due to variation of natural and social conditions (Eyzaguirre and Watson 2002; Kumar and Nair 2004). Variability in species composition between individual homegardens, in areas with similar geographical conditions, is motivated by families’ preferences and socio-economic strategies. Additional variation in species composition responds to diverging soil characteristics, climate and vegetation types—with which species are exchanged (Moreno-Black et al. 1996; Dawson et al. 2013)—and cultural, social and economic differences between subregions (Vandermeer et al. 1998; Altieri 2000; Scales and Marsden 2008; Bernholt et al. 2009; Das and Das 2015; Poot-Pool et al. 2015).
Variation in species composition and within-species diversity of individual homegardens are at the basis of the exchange of plants through social networks. This exchange contributes to the continuity of robust and resilient homegarden networks that continuously renew regional agrobiodiversity pools (Trinh et al. 2003; Ban and Coomes 2004; Buchmann 2009; Dawson et al. 2013). Exchange is one of the mechanisms that allows homegardens to play a major role in in situ conservation of landraces of commonly cultivated, frequently exotic species, as well as in circa situm conservation of native species. These species are increasingly scarce in the productive landscape, but are cultivated or tolerated in homegardens (Moreno-Black et al. 1996; Fu et al. 2003; Trinh et al. 2003; Aguilar-Støen et al. 2008; Buchmann 2009; Dawson et al. 2013).
Several studies refer to homogenisation and decrease of homegarden agrobiodiversity, linked to the intensification of agricultural production, proximity to urban areas and the introduction of species with high commercial value (Dewey 1981; Michon and Mary 1994; Nair 2006; Peyre et al. 2006; Wiersum 2006; Scales and Marsden 2008; Abebe et al. 2009; Bernholt et al. 2009; Abebe et al. 2013). Changes in species composition and richness in homegardens are indeed generalized, as families continuously renew and adapt their livelihood strategies and preferences in the context of the rapid social, economic and cultural transformation of rural areas (Ban and Coomes 2004; Zimmerer 2007; Buchmann 2009; Hecht 2010). However, this does not necessarily imply a general reduction of agrobiodiversity, but rather its renewal in the intrinsic process of adaptation of homegardens to changing conditions. This explains why, besides losses, increases or stability of agrobiodiversity have been reported (Kehlenbeck and Maass 2006; Kehlenbeck et al. 2007; Buchmann 2009; Poot-Pool et al. 2015).
Changes in composition in individual homegardens may balance each other or result in a net increase or reduction of species richness in homegarden networks, i.e. the homegardens that are interconnected through the social and economic processes of exchange of plants, propagules and products. The outcome influences homegardens’ contribution to in situ and circa situm conservation of agrobiodiversity (Kehlenbeck et al. 2007; Dawson et al. 2013; Mohri et al. 2013), which is important from two viewpoints (Buchmann 2009). One is that maintained species richness indicates that homegardens are indeed sustainable systems from the viewpoint of biodiversity conservation (Torquebiau 1992; Nair 2001), particularly if it concerns native species (Dawson et al. 2013). The other considers that agrobiodiversity conveys robustness and resilience to local livelihoods, particularly in the context of current climate change, though the relationship between species richness and social-ecological functionality requires further exploration (Ordonez et al. 2014).
Current knowledge about homegarden agrobiodiversity change has been inferred mainly from differences, observed in one-time inventories, between groups of homegardens that differ in some condition thought to be related to change. Some studies compare homegardens among owner groups of different cultural background or economic activity, distance to cities or market orientation (Michon and Mary 1994; Wezel and Ohl 2005; Abdoellah et al. 2006; Peyre et al. 2006; Abebe et al. 2009; Kabir and Webb 2009; Akinnifesi et al. 2010; Mohri et al. 2013; Wiehle et al. 2014; Das and Das 2015). Though findings based on these methods frequently point to a reduction of agrobiodiversity, also increases have been documented (e.g., Wiehle et al. 2014). Sunwar et al. (2006) reconstructed change over a 10–15-year period based on one-time interviews. They found that about 20 species had been lost, but did not consider the introduction of species over the same period. To determine change and its direction unequivocally, baseline studies and repeated measurements are highly desirable (Nair 2001; Wiersum 2006; Bernholt et al. 2009; Ordonez et al. 2014; Wiehle et al. 2014; Kehlenbeck and Maass 2006).
Here we present information of tree species inventories that we conducted three times—in 2009, 2012 and 2015—in the same sample of homegardens in the post-deforestation conditions of the tropical lowlands of Tabasco, México. We determined total, native, neotropical and introduced tree species richness and abundance in tropical homegardens in three subregions with particular characteristics of geomorphology and productive landscape (mountain slopes, fluvial plains and coastal plains) and on the aggregate regional scale. We hypothesise that species richness is maintained on both the subregional and regional scale, as a result of both gains and losses of species that are an intrinsic part of the dynamics of homegardens. Checking this hypothesis is relevant for the appropriate design of strategies that aim to conserve and enhance agrobiodiversity.
Methodology
Study area
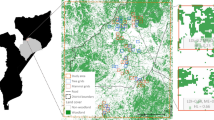
We carried out this research in the lowlands in the state of Tabasco, southeast Mexico (Fig. 1). According to the WorldClim database (http://www.worldclim.org/, Hijmans et al. 2005) mean annual temperature ranges from 25.5 °C in the mountain slopes area, with altitudes up to 400 meters above sea level, and 26.5 °C in the coastal plains. Mean annual precipitation is 2679 mm in the mountain slopes area and 1904 mm in the coastal plains. The original vegetation was typical of tropical lowlands, composed mainly of medium and high evergreen rainforests and periodically flooded forests and hydrophilic vegetation. Today, forest cover is a tiny fraction of what it was in the 1960s due to deforestation, induced by development policies that encouraged agricultural modernisation and ended in extensive animal husbandry (Tudela 1989).

(adapted from INEGI 2014)
Distribution of the sample of 38 homegardens inventoried in 2009, 2012 and 2015 in coastal plains, fluvial plains and mountain slopes subregions in Tabasco, Mexico
Subregional land-use mosaics vary strongly, associated with geomorphological factors (West et al. 1987). The mountain slopes area, bordering with the Chiapas mountain ranges, presents fragments of the original rainforests, pasturelands and small fields with subsistence crops. The fluvial plains land use mosaic shows remnants of riparian vegetation amidst fields under mechanized cultivation of annual crops, cacao plantations and pasturelands. In the coastal plains, pasturelands with isolated “macuilis” (Tabebuia rosea DC) trees combine with coconut plantations (Cocos nucifera L.), lagoons and mangrove forests. The botanical composition of homegardens varies according to the landscape mosaics embedding them (Van der Wal et al. 2014; Alcudia-Aguilar et al. 2017).
Homegarden selection
We observed homegardens on three nested scales: (1) individual homegardens, with agrobiodiversity reflecting environmental conditions as well as household’s economic situation and preferences (Abdoellah et al. 2006; Poot-Pool et al. 2012; Ordonez et al. 2014); (2) the set of homegardens in subregions where a particular combination of geomorphology, soil, vegetation and geography leads to a typical aggregate agrobiodiversity (Fernandes and Nair 1986; Kumar et al. 1994; Ordonez et al. 2014); and (3) all sampled homegardens, which together reflect aggregate regional agrobiodiversity. In 2009, we selected a sample of villages that were representative of the subregions of coastal plains, fluvial plains and mountain slopes in Tabasco (van der Wal and Bongers 2013). Within these villages, we selected actively managed homegardens, with owners willing to accept species inventories on each occasion, resulting in a final sample of 38 homegardens (Fig. 1).
Change monitoring
We inventoried trees, woody shrubs and tree-like perennial herbs (bananas, Musa spp.)—from now on simply “trees”- in homegardens for the first time in 2009, and repeated inventories in the same homegardens in 2012 and 2015. In the first inventory, we identified all trees with a DBH greater than 2 cm and indicated tree positions on an in situ map of each homegarden. The successive inventory updates enabled us to determine changes in tree species richness and abundance. Common and scientific names were determined at the sample site based on species identification guides and research team knowledge. In case of uncertainty, we took samples for later identification by botanists. We determined the biogeographical origin of species consulting the Tropicos and WCSP databases (Tropicos.org 2015; WCSP 2015). We distinguished between native species, with distribution limited to southeast Mexico, Belize and Guatemala; neotropical species, widely recorded in tropical America; and species introduced from outside America. This allowed determining whether recorded changes affected species richness and abundance of the three groups.
Data analysis
We used individual based rarefaction to compare species richness between subregions. To identify regional differences in species composition, we performed non-parametric multivariate analysis (PERMANOVA) on abundance data with the Bray-Curtis distance measure, applying Hotelling’s pairwise post hoc tests to determine differences between pairs. To identify changes in number of trees and species’ richness, after testing for normality of the data sets, we used ANOVA and post hoc Tukey tests. If data were not normally distributed, we used the Friedman χ 2 test, applying sequential Bonferroni corrected significances in the Pairwise Wilcoxon Rank Sum Test. We performed analyses on the total sample (38 homegardens) and on each subregional group: homegardens in coastal plains (8), fluvial plains (17) and mountain slopes (13). For each group, we studied the changes of the native, neotropical and introduced species richness. All analysis were performed in PAST (Hammer et al. 2001).
Results
Taxa and number of trees in homegardens in 2009, 2012 and 2015
We identified 169 species in the three inventories, belonging to 121 genera and 46 families, with the total species richness in each year steadily increasing from 140 to 149 species over the 6-year study interval (Table 1). The Fabaceae family contributed 24 species, followed by Rutaceae with ten species, Arecaceae and Malvaceae and with nine species, and Moraceae and Sapindaceae with eight and seven species. The most abundant genera were Citrus (eight species), Musa, Annona and Inga (five species), and Ficus (four species). Electronic Supplementary Material 1 provides a detailed list of the recorded species, with family, biogeographical origin and occurrence in each sampling year. The most frequent species, registered in more than 75% of the homegardens, were Annona muricata L., Citrus limon (L.) Osbeck, Citrus sinensis (L.) Osbeck, Mangifera indica L., Cocos nucifera L. and Tabebuia rosea DC. Forty-eight species (28.4%) were native to southeast Mexico, Belize and Guatemala; 78 (46.2%) neotropical and 43 (25.4%) introduced.
Change in numbers of trees and species, and species turnover in Tabasco (regional scale)
Over the 6-year study interval, the number of trees increased or decreased in 19 homegardens, and species richness increased in 24 and decreased in 11. Changes varied slightly over the three-year intervals. In the 09–12 interval, the number of trees increased in 17 and decreased in 21 homegardens, and species richness increased in 16 and decreased in 19. In the 12–15 interval, the number of trees increased in 25 homegardens and decreased in 13, whereas species richness increased in 25 and decreased in 10 (see Electronic Supplementary Material 2).
On average, homegardens had more than 22 species and 85 trees in the three sampling years, both variables showing wide ranges (Table 2). There were no significant differences in the mean number of trees per homegarden between the inventory years on the regional scale [χ 2(2) = 2.71, P = 0.26], and neither were there in the number of trees by biogeographical origin of species (P = 0.06, 0.32 and 0.36 for introduced, native and neotropical species). When we considered only woody introduced species—eliminating Musa spp. from analysis—there were significantly less introduced trees in 2012 (20.2) as compared to 2009 (23.8) and 2015 (24.1) [χ 2(2) = 14.18, P < 0.001; and P < 0.005 in the post hoc tests] (Table 2).
Mean species richness varied between years [χ 2(2) = 8.41, P = 0.01]. It increased significantly over the six-year interval (P = 0.03) and between 2012 and 2015 (P = 0.001), whereas there was no change between 2009 and 2012 (P = 0.52). Analysis by biographic origin of species showed significant differences in neotropical species richness between years [χ 2(2) = 9.15, P = 0.01], with more species in 2015 than in 2009 (P = 0.02) and 2012 (P = 0.01). Also introduced species richness varied between years (F = 5.63, P = 0.01), with more introduced species in 2015 than in 2009 (P = 0.03) and 2012 (P = 0.01). There were no differences between years in mean native species richness (F = 1.65, P = 0.2) (Table 2).
Hundred and seventeen species persisted in the three inventories, of which 27.4% were native, 42.6% neotropical and 30.0% introduced. Between 2009 and 2012, eight species disappeared from the sample of 38 homegardens and 13 new species appeared. Between 2012 and 2015, five of the disappeared species came back, 16 new species appeared and 14 species disappeared. Over the whole period from 2009 to 2015, 26 species—equivalent to 17.4% of all species in 2015—came in as new species. Of these, 2 were introduced, 8 native and 16 neotropical. Over the same period, 17 species disappeared: 4 introduced, 5 native and 8 neotropical species (Fig. 2).
Subregional agrobiodiversity and its change
The species richness cannot readily be compared between the subregions, due to the different numbers of sampled homegardens and trees in coastal plains (8 homegardens and 1561 trees), fluvial plains (17 and 2430) and mountain slopes (13 and 1828). Our rough data show the presence of a total 93 species in coastal plains, 117 in fluvial plains and 119 in mountain slopes. Individual- based rarefaction, based on random samples of the same number of trees (1500) for each subregion, shows however that subregional species richness is highest in the mountain slopes (115 species), followed by fluvial plains (105) and coastal plains (92).
PERMANOVA showed significant differences in composition between subregions in 2009 (F = 2.014, P < 0.001) and between all pairs with P < 0.02. The differences persisted in the 2012 inventory, both between subregions (F = 1.925, P = 0.001) and between all pairs (P < 0.01), The 2015 inventory data also showed significant differences between subregions (F = 2.237, P < 0.001), with P < 0.01 in all post hoc tests.
Homegardens on the coastal plains showed significant differences in average species richness between years for all species and for introduced species separately (Table 3). However, when we only looked at the introduced woody species (eliminating Musa counts from observations), the observed differences between years were blurred (F = 2.58, P = 0.11) in the coastal plains, whereas they maintained in the whole Tabasco sample (F = 4.434, P = 0.02) with differences between 2015 and 2012 (P = 0.02). There were also no significant differences between years in the average species richness for each origin nor for all species in fluvial plains and mountain slopes subregions. Also the average number of trees per homegarden did not show differences between years in the three subregions. Differences were neither observed when data were analysed separately by geographical origen of the species that trees belonged to.
Discussion
Homegardens in Tabasco collectively boast a remarkable reservoir of agrobiodiversity. The observed aggregate species richness of 140, 145 and 149 in the three inventories, and 169 species over the observed time-interval, situates Tabasco in the high range of tree species richness in tropical areas (Kumar and Nair 2004). This is partly due to the combining of subregions with distinct geomorphological, geographical and cultural conditions, where each subregion contributes species that do not occur in the other subregions (β-diversity) (Alcudia-Aguilar et al. 2017) (Fig. 2). Inventories in three subregions with distinct natural and social conditions in India showed a similar regional tree species richness of 161 species (Das and Das 2015).
Species composition varied significantly between the coastal plains, fluvial plains and mountain slopes subregions in Tabasco. This is in agreement with findings indicating that soil and climatic conditions, the subregional productive landscape and cultural aspects influence species composition (Hodgkin 2001; Das and Das 2015; Alcudia-Aguilar et al. 2017). An example are the species employed in fencing. Bursera simaruba and Pachira aquatica prevail in the coastal plains; Erythtrina americana in the fluvial plains; and Gliricidia sepium and Eugenia rubella in the mountain slopes. P. aquatica in the coastal plains is adapted to the conditions of tropical wetlands. The prevalence of E. americana in the fluvial plains subregion relates with its wide use as a shade species in cacao (Theobroma cacao) plantations. G. sepium is frequently used for fencing in the pasturelands in the mountain slopes subregion. Subregional homegarden agrobiodiversity reflects both the natural conditions and the prevailing productive strategies, resulting in different species assemblages across contexts (Altieri 2000; Alcudia-Aguilar et al. 2017).
Highest species richness was found on mountain slopes and lowest in coastal plains. The higher species richness in the mountain slopes subregion may be related to the presence of large forest fragments. These are a source of genetic material that households introduce or tolerate in their homegardens (Moreno-Black et al. 1996; Das and Das 2015; Alcudia-Aguilar et al. 2017). Difficult or expensive access to urban centers and low income enhance diversity in the mountain slopes homegardens as a means to meet daily household needs (Kehlenbeck and Maass 2006; Poot-Pool et al. 2015). Lower species richness in coastal homegardens may relate to soil salinity and flooding (van der Wal et al. 2014), favouring abundances of the few species that tolerate these conditions. On the other hand, salinity and flooding may also enhance the introduction of new species to see if they adapt. This may be one explanation of the increased introduced species richness in the coastal plains over the evaluation period (Table 3).
Trends towards the homogenisation of tropical homegardens have been documented on a subregional level (Michon and Mary 1994; Peyre et al. 2006). Though factors such as market orientation of production could blur differences between subregions, such an outcome has not been documented up to date. On the contrary, Das and Das (2015) report how differences in species composition, associated with cultural differences, prevail up to date in India. We found that the differences in species composition between subregions in the 2009 inventory persisted in 2012 and 2015. This shows that there is no process of homogenization taking place in Tabasco, at least not over the evaluation period, and that besides species richness β-diversity is preserved.
At the same time, our research shows how dynamic homegardens in tropical lowlands are. Changes of agrobiodiversity occurred in almost all homegardens, but there was not a uniform decrease or increase. In the 2009–2012 interval species richness decreased in most homegardens, whereas it increased in most of them in the 2012–2015 interval. The changes in the individual homegardens resulted in as much as 17.4% of new species in the aggregate regional sample of homegardens in 2015 and the loss of 12.1% of the species present at the onset of change monitoring in 2009. Loss and renewal resulted in a net increase of total, neotropical and introduced species richness, as well as the maintaining the native components. The percentage of introduced species even decreased slightly from 28.2% in 2009 to 26.4% in 2012 and 25.7% in 2015. Homegarden tree species composition in the Tabasco tropical lowlands does not change towards the introduced component, as has been described for other conditions (Niinemets and Peñuelas 2008). On the contrary, our results indicate that the proportion of native and neotropical species) increased over the evaluation period.
The preservation in homegarden networks in Tabasco of the species that are common in the regional vegetation types underpins their continued relevance for conservation (Vandermeer et al. 1998; Eyzaguirre and Watson 2002; Scales and Marsden 2008; Dawson et al. 2013; Ordonez et al. 2014). This confirms findings of other regional studies. Even in an urban environment in Northeastern Brasil, 60% of the plant species were native to the country (Akinnifesi et al. 2010). Similar percentages occurred in Vietnam (Trinh et al. 2003) and Sudan (Wiehle et al. 2014). In Bangladesh, 59% of the species in homegardens were native of the Indian subcontinent (Kabir and Webb 2008), and in Northeast India as much as 86% (Das and Das 2015). This may reflect a general response to the consequences of deforestation and the reduced availability of useful species. In Thailand, families actively transplanted native species from the forest to homegardens to maintain access to them (Moreno-Black et al. 1996). Also in Soutwest China families enhanced the conservation of useful native species in homegardens to avoid losing them due to deforestation (Fu et al. 2003). A global pattern thus emerges, wherein homegardens—as a part of a continuum of resource areas—increasingly take up the conservation of regional species among their functions, thus countering biodiversity loss from other resource areas.
Agrobiodiversity dynamics in homegardens in the Tabasco tropical lowlands reflect how rural families adapt their livelihoods in the context of globalization and evolving new ruralities (Hecht 2010). Adaptation involves both the diversification of income sources in rural areas beyond agriculture, and the continuous updating of homegardens’ agrobiodiversity (Mohri et al. 2013), readily planting or tolerating useful species, as well as introducing new species considered worthy to try. Exchange of plants through social networks is essential in the updating process (Ban and Coomes 2004; Buchmann 2009; Zimmerer 2007). This process involves at the same time a high turnover of species, the conservation of the useful species that are common in regional vegetation types, as well as species introductions. Together these practices allow families to keep combining economical, ecological, social and cultural homegarden functions as they respond to the changing livelihood context (Buchmann 2009). They are at the core of homegardens’ contribution to agrobiodiversity conservation and livelihoods.
References
Abdoellah OS, Hadikusumah HY, Takeuchi K, Okubo S, Parikesit (2006) Commercialization of homegardens in an Indonesian village: vegetation composition and functional changes. Agrofor Syst 68:1–13. doi:10.1007/s10457-005-7475-x
Abebe T, Wiersum KF, Bongers F (2009) Spatial and temporal variation in crop diversity in agroforestry homegardens of southern Ethiopia. Agrofor Syst 78:309–322. doi:10.1007/s10457-009-9246-6
Abebe T, Sterck FJ, Wiersum KF, Bongers F (2013) Diversity, composition and density of trees and shrubs in agroforestry homegardens in Southern Ethiopia. Agrofor Syst 87:1283–1293. doi:10.1007/s10457-013-9637-6
Aguilar-Støen M, Moe SR, Camargo-Ricalde SL (2008) Home gardens sustain crop diversity and improve farm resilience in Candelaria Loxicha, Oaxaca, Mexico. Hum Ecol 37:55–77. doi:10.1007/s10745-008-9197-y
Akinnifesi FK, Sileshi GW, Ajayi OC, Akinnifesi AI, de Moura EG, Linhares JFP, Rodrigues I (2010) Biodiversity of the urban homegardens of So Luis city, Northeastern Brazil. Urban Ecosyst 13:129–146
Alcudia-Aguilar A, van der Wal H, Suárez-Sánchez J, Martínez-Zurimendi P, Castillo-Uzcanga MM (2017) Home garden agrobiodiversity in cultural landscapes in the tropical lowlands of Tabasco, México. Agrofor Syst. doi:10.1007/s10457-017-0078-5
Altieri MA (2000) Multifunctional dimensions of ecologically-based agriculture in Latin America. Int J Sustain Dev World Ecol 7:62–75
Ban N, Coomes OT (2004) Home gardens in Amazonian Peru: diversity and exchange of planting material. Geogr Rev 94:348–367
Bernholt H, Kehlenbeck K, Gebauer J, Buerkert A (2009) Plant species richness and diversity in urban and peri-urban gardens of Niamey, Niger. Agrofor Syst 77:159–179. doi:10.1007/s10457-009-9236-8
Buchmann C (2009) Cuban home gardens and their role in social-ecological resilience. Hum Ecol 37:705–721. doi:10.1007/s10745-009-9283-9
Das T, Das AK (2015) Conservation of plant diversity in rural homegardens with cultural and geographical variation in three districts of Barak Valley, Northeast India1. Econ Bot 69:57–71. doi:10.1007/s12231-015-9299-6
Dawson IK, Guariguata MR, Loo J, Weber JC, Lengkeek A, Bush D, Cornelius J, Guarino L, Kindt R, Orwa C, Russell J, Jamnadass R (2013) What is the relevance of smallholders’ agroforestry systems for conserving tropical tree species and genetic diversity in circa situm, in situ and ex situ settings? Rev Biodivers Conserv 22:301–324. doi:10.1007/s10531-012-0429-5
Dewey KG (1981) Nutritional consequences of the transformation from subsistence to commercial agriculture in Tabasco. Mex Hum Ecol 9:151–187. doi:10.1007/bf00889132
Eyzaguirre PB, Watson J (2002) Homegardens and agrobiodiversity: an overview across regions. In: Watson JW, Eyzaguirre PB (eds) Home gardens and in situ conservation of plant genetic resources in farming systems. IPGRI, Witzenhausen, pp 10–13
Fernandes ECM, Nair PKR (1986) An evaluation of the structure and function of tropical homegardens. Agric Syst 21:279–310. doi:10.1016/0308-521x(86)90104-6
Fu Y, Guo H, Chen A, Cui J, Padoch C (2003) Relocating plants from swidden fallows to gardens in Southwestern China. Econ Bot 57:389–402. doi:10.1663/0013-0001(2003)057[0389:rpfsft]2.0.co;2
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hecht S (2010) The new rurality: globalization, peasants and the paradoxes of landscapes. Land Use Policy 27:161–169. doi:10.1016/j.landusepol.2009.08.010
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hodgkin T (2001) Home gardens and the maintenance of genetic resources. In: Watson JW, Eyzaguirre PB (eds) Home gardens and in situ conservation of plant genetic resources in farming systems. International Plant Genetic Resources Institute, Rome, pp 14–18
INEGI (2014) Instituto Nacional de Estadística y Geografía. Marco Geoestadístico 2014 version 6.2. http://www.inegi.org.mx/geo/contenidos/geoestadistica/m_g_0.aspx. Accessed Sep 2015
Kabir ME, Webb EL (2008) Floristics and structure of southwestern Bangladesh homegardens. Int J Biodivers Sci Manag 4:54–64
Kabir ME, Webb EL (2009) Household and homegarden characteristics in southwestern Bangladesh. Agrofor Syst 75:129–145. doi:10.1007/s10457-008-9142-5
Kehlenbeck K, Maass BL (2006) Are tropical homegardens sustainable? Some evidence from Central Sulawesi, Indonesia. In: Kumar BM, Nair PKR (eds) Tropical homegardens. A time-tested example of sustainable agroforestry, vol 3. Advances in agroforestry. Springer, Dordrecht, pp 25–41
Kehlenbeck K, Arifin H, Maass B (2007) Plant diversity in homegardens in a socio-economic and agro-ecological context. In: Tscharntke T, Leuschner C, Zeller M, Guhardja E, Bidin A (eds) Stability of tropical rainforest margins. Linking ecological, economic and social constraints of land use and conservation. Springer, Berlin, pp 297–319
Kumar BM, Nair PKR (2004) The enigma of tropical homegardens. Agrofor Syst 61–62:135–152. doi:10.1023/B:AGFO.0000028995.13227.ca
Kumar BM, George SJ, Chinnamani S (1994) Diversity, structure and standing stock of wood in the homegardens of Kerala in peninsular India. Agrofor Syst 25:243–262
Michon G, Mary F (1994) Conversion of traditional village gardens and new economic strategies of rural households in the area of Bogor, Indonesia. Agrofor Syst 25:31–58. doi:10.1007/bf00705705
Mohri H, Lahoti S, Saito O, Mahalingam A, Gunatilleke N, Irham TH, Hitinayake G, Takeuchi K, Herath S (2013) Assessment of ecosystem services in homegarden systems in Indonesia, Sri Lanka, and Vietnam. Ecosyst Serv 5:124–136. doi:10.1016/j.ecoser.2013.07.006
Moreno-Black G, Somnasang P, Thamathawan S (1996) Cultivating continuity and creating change: women’s home garden practices in northeastern Thailand. Agric Hum Values 13:3–11. doi:10.1007/bf01538222
Nair PKR (2001) Do tropical homegardens elude science, or is it the other way around? Agrofor Syst 53:239–245. doi:10.1023/a:1013388806993
Nair PKR (2006) Whither homegardens? In: Kumar BM, Nair PKR (eds) Tropical homegardens. A timetested example of sustainable agroforestry, vol 3. Advances in agroforestry. Springer, Dordrecht, pp 355–370
Niinemets Ü, Peñuelas F (2008) Gardening and urban landscaping: significant players in global change. Trends Plant Sci 13(2):60–65. doi:10.1016/j.tplants.2007.11.009
Ordonez JC, Luedeling E, Kindt R, Tata HL, Harja D, Jamnadass R, van Noordwijk M (2014) Constraints and opportunities for tree diversity management along the forest transition curve to achieve multifunctional agriculture. Curr Opin Environ Sustain 6:54–60. doi:10.1016/j.cosust.2013.10.009
Peyre A, Guidal A, Wiersum KF, Bongers F (2006) Dynamics of homegarden structure and function in Kerala, India. Agrofor Syst 66:101–115. doi:10.1007/s10457-005-2919-x
Poot-Pool WS, van der Wal H, Flores-Guido S, Pat-Fernández JM, Esparza-Olguín L (2012) Economic stratification differentiates home gardens in the Maya village of Pomuch. Mex Econ Bot 66:264–275. doi:10.1007/s12231-012-9206-3
Poot-Pool WS, van der Wal H, Flores-Guido S, Pat-Fernández JM, Esparza-Olguín L (2015) Home Garden Agrobiodiversity Differentiates Along a Rural—Peri–Urban Gradient in Campeche. Méx Econ Bot 69:203–217. doi:10.1007/s12231-015-9313-z
Scales BR, Marsden SJ (2008) Biodiversity in small-scale tropical agroforests: a review of species richness and abundance shifts and the factors influencing them. Environ Conserv 35:160–172. doi:10.1017/s0376892908004840
Sunwar S, Thornström C-G, Subedi A, Bystrom M (2006) Home gardens in western Nepal: opportunities and challenges for on-farm management of agrobiodiversity. Biodivers Conserv 15:4211–4238. doi:10.1007/s10531-005-3576-0
Torquebiau E (1992) Are tropical agroforestry home gardens sustainable? Agric Ecosyst Environ 41:189–207
Trinh LN et al (2003) Agrobiodiversity conservation and development in Vietnamese home gardens. Agric Ecosyst Environ 97:317–344. doi:10.1016/s0167-8809(02)00228-1
Tropicos.org (2015) Missouri Botanical Garden. http://www.tropicos.org
Tudela F (1989) La modernización forzada del trópico: el caso de Tabasco. Proyecto integrado del Golfo, El Colegio de México, Mexico city
van der Wal H, Bongers F (2013) Biosocial and bionumerical diversity of variously sized home gardens in Tabasco. Mex Agrofor Syst 87:93–107. doi:10.1007/s10457-012-9526-4
Van der Wal H, Suárez-Sánchez J, Alcudia-Aguilar A, Cerino-Zabala M, de la Cruz-Arias V, Isidro-Hernández J, Santiago-Montejo PA (2014) Estrategias de supervivencia, funciones de huertos familiares y su adaptación en la cuenca baja del río Grijalva. In: González-Espinoza M, Brunel-Manse C (eds) Montañas, pueblos y agua. Dimensiones y realidades de la cuenca Grijalva. Juan Pablos, San Cristóbal de las Casas, México D.F., pp 406–431
Vandermeer J, van Noordwijk M, Anderson J, Ong C, Perfecto I (1998) Global change and multi-species agroecosystems: concepts and issues. Agric Ecosyst Environ 67:1–22. doi:10.1016/s0167-8809(97)00150-3
WCSP (2015) World checklist of selected plant families. Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/
West RC, Psuty NP, Thom BG (1987) Las Tierras Bajas de Tabasco en el Sureste de México., Segunda edición. Gobierno del Estado de Tabasco, México
Wezel A, Ohl J (2005) Does remoteness from urban centres influence plant diversity in homegardens and swidden fields?: a case study from the Matsiguenka in the Amazonian rain forest of Peru. Agrofor Syst 65:241–251
Wiehle M, Goenster S, Gebauer J, Mohamed SA, Buerkert A, Kehlenbeck K (2014) Effects of transformation processes on plant species richness and diversity in homegardens of the Nuba Mountains. Sudan Agrofor Syst 88:539–562. doi:10.1007/s10457-014-9717-2
Wiersum KF (2006) Diversity and change in homegarden cultivation in Indonesia. In: Kumar BM, Nair PKR (eds) Tropical homegardens: a time-tested example of sustainable agroforestry. Springer, Dordrecht, pp 13–24
Zimmerer KS (2007) Agriculture, livelihoods, and globalization: the analysis of new trajectories (and avoidance of just-so stories) of human-environment change and conservation. Agric Hum Values 24:9–16. doi:10.1007/s10460-006-9028
Acknowledgements
We thank the owners of the sampled homegardens for their participation in this study. Teresita Avilez-López and Pedro Antonio Santiago-Montejo provided invaluable assistance in fieldwork. The Mexican National Council of Science and Technology (CONACYT) financed scholarship 307868 for postgraduate studies of the first author at El Colegio de la Frontera Sur, México, as well as a scholarship for mobility abroad (290936) at Wageningen UR, The Netherlands. Fieldwork was partially financed through the project “Sustainability challenges in the Usumacinta watershed in Tabasco: ecosystems, climate change and social response”, funded by the Government of Tabasco and CONACYT (project number TAB-2012-C-28-194316). Three anonymous reviewers provided well-thought comments that contributed to strengthen the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Serrano-Ysunza, A.A., van der Wal, H., Gallardo-Cruz, J.A. et al. A 6-year longitudinal study on agrobiodiversity change in homegardens in Tabasco, México. Agroforest Syst 92, 1485–1494 (2018). https://doi.org/10.1007/s10457-017-0094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-017-0094-5