Home Garden Agrobiodiversity Differentiates Along a Rural—Peri–Urban Gradient in Campeche, México
Home Garden Agrobiodiversity Differentiates Along a Rural—Peri–Urban Gradient in Campeche, México. Agrobiodiversity in tropical home gardens is thought to decline with increasing urbanization, but information in this regard is scarce. We characterized livelihoods and compared attributes of home gardens of rural, semi–rural, and peri–urban families in Campeche, México. We hypothesized a decline of agrobiodiversity of cultivated trees and shrubs, its native component, and the diversity of uses from rural to peri–urban livelihoods, and increases for cultivated herbs. We registered all cultivated species in 12 randomly selected home gardens in each condition, and determined species’ origin and use. Total and average observed species richness of trees and shrubs in rural and semi–rural home gardens was similar and higher than in peri–urban home gardens, but equally sized samples all had similar richness. Tree density and basal area were largest in semi–rural home gardens. Total observed and average species richness of herbs increased along the rural—peri–urban gradient, and richness of equally sized samples of herbs was higher in peri–urban than in rural and semi–rural home gardens. Peri–urban home gardens had the highest richness of equally sized samples of all plants. Differences in richness were associated with a shift in livelihoods, influencing plant uses, and hence species composition along the gradient: peri–urban families combined some fruit trees with a large diversity of ornamental herbs. Rural and semi–rural families maintained tree and shrub species of distinct uses, cultivated less ornamental species and a larger native component than peri–urban home gardens. We conclude that agrobiodiversity does not decline along the rural—peri–urban gradient, but differentiates.
La agrobiodiversidad en huertos familiares diferencía a lo largo de un gradiente rural—peri–urbano en Campeche, México
La agrobiodiversidad en huertos familiares diferencía a lo largo de un gradiente rural—peri–urbano en Campeche, México. Se piensa que la agrobiodiversidad en huertos familiares tropicales disminuye conforme avanza la urbanización, sin embargo, la información al respecto es escasa. Caracterizamos los medios de vida y comparamos atributos de huertos familiares de familias rurales, semi–rurales y peri–urbanos en Campeche, México, hipotetizando una reducción de la agrobiodiversidad de árboles y arbustos cultivados, de su componente nativo, y de la diversidad de usos de medios de vida rurales hacia peri–urbanas, e incrementos con respecto a herbáceas cultivadas. Se registraron todas las especies cultivadas en 12 huertos seleccionados al azar en cada condición, y se determinó el origen y uso de las especies. La riqueza total observada y media de árboles y arbustos en huertos rurales y semi–rurales era similar y mayor que en huertos peri–urbanos, sin embargo, en muestras de igual tamaño de las tres condiciones la riqueza fue similar. La densidad arbórea fue mayor en los huertos semi–rurales. La riqueza total y media de especies herbáceas fue mayor en huertos peri–urbanos que en los rurales y semi–rurales. Huertos peri–urbanos tenían la mayor riqueza de muestras de igual tamaño de todas las plantas. Diferencias en riqueza estaban asociadas con variaciones en los medios de vida, al influir sobre los usos de las plantas, y con ello sobre la composición de especies a lo largo del gradiente: familias peri–urbanas combinaban algunos árboles frutales con una gran diversidad de herbáceas ornamentales. Familias rurales y semi–rurales mantenían especies arbóreas y arbustivas de distintos usos, cultivaban menos especies ornamentales, y un componente nativo más grande que huertos peri–urbanos. Concluimos que la agrobiodiversidad no disminuye a lo largo del gradiente rural—peri–urbano, sino que diferencía.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The largest reservoir of agrobiodiversity is found in peasant production systems and particularly in home gardens (Galluzzi et al. 2010; Watson and Eyzaguirre 2002). Home gardens’ high diversity allows families to produce a wide variety of products (fruit, vegetables, medicines, wood, forage) and services (shade, recreational space, esthetics, wildlife habitat, microclimate, and nutrient recycling) (Cilliers et al. 2012; Kehlenbeck et al. 2007). Produce is used for home consumption, marketing, and/or gifts, depending on families’ livelihood strategies.
It has been reported that agrobiodiversity in peasant production systems, including home gardens, is in decline (Abebe et al. 2013; Chandrashekara and Baiju 2010; Kehlenbeck et al. 2007), involving both species diversity and structural diversity. Increasing commercialization opportunities encourages the cultivation of few marketable crops for bulk production instead of maintaining many species for diverse production, and would induce the removal of high shade trees, thus reducing structural complexity (Abdoellah et al. 2002; Michon and Mary 1994; Rico–Gray et al. 1990). Decline could also be caused by the replacement of products hitherto obtained from plant species by ever more available industrial products (Abebe et al. 2013; Peng and Xuehua 2007; Poot–Pool et al. 2012; Thompson et al. 2003) and reinforced by the loss of agricultural knowledge (Eyzaguirre and Watson 2002; Galluzzi et al. 2010; Huai and Hamilton 2009).
According to Molebatsi et al. (2010), rural and peri–urban home gardens are different as they play different roles in family livelihoods, which also vary between both conditions. Whereas peri–urban home gardens tend to accentuate services of ornamental species, rural home gardens maintain self–sufficiency functions and therefore contain species that provide for a wide range of uses: food, medicine, shade, fences, wood, and energy. However, home gardens in rural settings did not contain a greater overall species richness, but rather contained more individuals of useful species, and more native species, than those in peri–urban settings (Molebatsi et al. 2010). This indicates that increased livelihood dependence on urban centers does not always reduce agrobiodiversity in home gardens, but rather changes it.
The relation between rural conditions and agrobiodiversity takes complex forms. In indigenous peasant communities in the Peruvian Amazon, far from urban centers, home gardens exhibited low diversity (Wezel and Ohl 2005). The day–to–day transiting to swiddens reduced the need for diverse home gardens. Along this line of argument, increasing urban conditions could then lead to a higher agrobiodiversity, to compensate for the loss of opportunity to bring in products from the farms (Moreno–Black et al. 1996). Further, whereas in more urban environments most species may have been introduced, and hence native species make up only a small fraction of total species richness (Peng and Xuehua 2007; Thompson et al. 2003), it has also been documented that native species richness is sustained with the introduction of species (Akinnifesi et al. 2010).
Agrobiodiversity in home gardens across the rural—peri–urban gradient requires further research (Galluzzi et al. 2010). Based on fieldwork in rural, semi–rural, and peri–urban villages in the Maya area in the north of the state of Campeche, located in the central western part of the Yucatán Peninsula, México, we describe local livelihoods and try to answer the question: is there is a gradual decline of home gardens’ agrobiodiversity from rural to peri–urban livelihoods? We also explore questions on the character of agrobiodiversity in the three rurality conditions: do floristic composition, biogeographic origin of plants (native, neotropical, and introduced species), and plant uses vary among rural, semi–rural, and peri–urban home gardens? We hypothesize that agrobiodiversity of trees and shrubs decreases from rural to peri–urban conditions, in particular its native component, parallel to a decrease in the diversity of uses from rural to peri–urban livelihoods. We also expect that the richness of herbaceous species increases in the same direction, mainly due to introduced ornamental species, with a parallel decrease in the number of uses and richness of the native component. We discuss possible implications of our findings for agrobiodiversity conservation in the context of urbanization and livelihood change.
Methods
Study Area
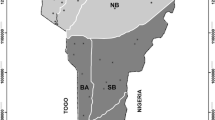
We classified communities as rural when between 50% and 90% of families work in agriculture, semi–rural when this percentage is between 10% and 50%, and as peri–urban when less than 10% is dedicated to agriculture (Molebatsi et al. 2010). We selected six localities from the northern section of the territory of the state of Campeche, on the Yucatán Peninsula, in southeast México: three rural localities (Sodzil, Chunkanán, and Chunhuas), two semi–rural (Pomuch and Hecelchakán), and one peri–urban (Chiná) (Fig. 1). All six localities have Maya origins, daily communication in Maya language being general in the rural and semi–rural communities and less common in the peri–urban community. The peri–urban community lies on the edge of the city of Campeche, while the semi–rural communities are at distances of 50 and 52 km along the Campeche–Mérida highway. The rural communities communicate with the semi–rural communities through secondary roads.
Home Garden Selection
The home gardens from the six communities were numbered using images available from Google Earth. We then selected 12 home gardens randomly for each condition of relative rurality, distributing the number of home gardens evenly over the localities in each condition. We visited the selected home gardens and, after confirming that they were actively managed, asked for the owner’s cooperation. We only included home gardens that belonged to nuclear families, that is, couples and their unmarried children, as the inclusion of extended families could result in biases favoring species richness in rural conditions, where this family type is more common.
Data Collection
We applied a questionnaire to quantify the physical, financial, social, human, and natural capital of the home garden owners’ families according to Poot–Pool et al. (2012), and used the obtained data to characterize livelihoods for each condition. We recorded all plants in the 36 home gardens between August 2012 and January 2013, and measured diameter at breast height (DBH), crown diameter, and height of trees and shrubs. We counted the individuals of cultivated herbaceous species. We collected specimens of species that we did not know, for identification by a senior botanist at the Universidad Autónoma de Yucatán (Yucatán Autonomous University). The biogeographical origin of each species was determined according to Barrera (1980), Ibarra–Manríquez (1996), Durán et al. (1998), the Flora of Yucatán websites of the Centro de Investigaciones Científicas de Yucatán (CICY 2010) (Center of Scientific Research of Yucatán, http://www.cicy.mx/sitios/flora%20digital/), and the Royal Botanic Gardens. We distinguished between native species, which are commonly found in the natural vegetation and have a distribution that is limited to the Yucatán Peninsula; neotropical species, which originate from and are widely distributed over the American tropics; and introduced species that do not originate from the Americas (Barrera 1980; Caballero 1992; Herrera–Castro 1994). The use of tree and herbaceous species was determined by questioning their owners, distinguishing the following categories: fruit, timber, fiber, firewood, shade providers, medicine, ornamental, condiment, fodder, edible, ceremonial, and “other uses.”
Statistical Analysis
We used the PAST program (Hammer et al. 2001) for most statistical analysis. We analyzed rural, semi–rural, and peri–urban livelihoods with univariate statistics, and tested some differences using Kruskal–Wallis and Mann–Whitney tests and H and U statistics. We calculated pooled Shannon diversity indexes for each situation, both separately for trees/shrubs and herbs, and for all plants together. Depending on the results of the Shapiro–Wilk normality tests, we used ANOVA with the F statistic, and Tukey’s HSD test with the Q statistic, or non–parametric Kruskal–Wallis and Mann–Whitney tests, with the H and U statistics, to determine differences in the mean number of tree/shrub and herbaceous species and of subsets of species organized by use or origin, and differences in structural data between rurality conditions. We used individual–based rarefaction to determine if species richness of equally sized samples of trees/shrubs, herbs, and both in each rurality condition was different. We applied regression analysis on the natural logarithm transformed rarefaction data, and considered that species richness was different if the species accumulation curves had different slopes according to the t–test (Infante and Zárate 1997). We performed analysis of similarity (ANOSIM) on the matrix of percentage abundance data with the Euclidean measure of distance and Bonferroni sequential significance to determine if composition varied between the three conditions of rurality. If composition was significantly different, we performed SIMPER (Similarity Percentage) analysis to determine which species contributed the most to variation, using the Euclidean distance measure.
Results
Livelihoods of Rural, Semi–Rural, and Peri–Urban Families
Livelihoods largely varied among the three rurality conditions (Table 1). Monetary income was different among rural, semi–rural, and peri–urban conditions (H = 12.9, P = 0.001). Peri–urban families had higher incomes than rural families (U = 13, P < 0.001), while the income of semi–rural families was above that of rural families (U = 37.5, P = 0.04). Rural families’ incomes were relatively homogenous (11 families had an income near $20,000), in contrast with incomes in the peri–urban setting. Natural capital, as measured by home gardens’ extension, varied between groups (H = 15.7, P < 0.001). Rural home gardens were larger than peri–urban home gardens (Bonferroni corrected pairwise Mann–Whitney tests P < 0.001), whereas other pairwise differences were not significant (P = 0.07). Available land area for agriculture varied among groups (H = 7.8, P = 0.007), and was larger in rural and semi–rural conditions than in the peri–urban condition (U = 26.5 and 40.5, Bonferroni corrected P = 0.003 and 0.03). Families in rural conditions accessed more subsidy programs than families in semi–rural and peri–urban conditions (H = 8.0, P = 0.01; U = 36 and 25, P = 0.03 and 0.004). Human capital, as measured by the number of adults, was similar in all conditions. Most families had Maya roots, these being stronger in the rural and semi–rural conditions than in the peri–urban condition. All rural families, with the exception of one, were peasants, against seven in the semi–rural setting. In the peri–urban setting, families had economic activities such as mechanic, bus driver, construction worker, shop owner, watchman, and servant; only two were peasants.
Species and Structural Diversity in Rural, Semi–Rural, and Peri–Urban Home Gardens
We identified a total of 94 botanical families and 316 species of cultivated plants. Of the latter, 103 were trees, shrubs, palms, bananas, and 213 herbs, epiphytes, and vines (Appendix 1—Electronic Supplementary Material). Total species richness, considering all growth forms, was greatest in the peri–urban home gardens. This was due to the large number of herbaceous species, which tripled the number of tree species. The families with most species were Fabaceae, Euphorbiaceae, Araceae, Rutaceae, Solanaceae, Lamiaceae, and Asteraceae (Table 2). The number of Fabaceae species was remarkably higher and the number of Araceae species lower in rural and semi–rural conditions than in the peri–urban condition.
We observed a total richness of 79 tree and shrub species in rural, 73 in semi–rural, and 53 species in peri–urban conditions. In terms of herbaceous species richness, we observed a total of 161 species in peri–urban, 104 in semi–rural, and 82 in rural conditions (Fig. 2). The pooled Shannon diversity index for all trees and shrubs was significantly higher for semi–rural home gardens (3.51) than for rural (3.35) and peri–urban (3.34) home gardens (Shannon diversity t–test, P < 0.01). Equitability—similarity of abundances of species—was significantly higher in semi–rural and peri–urban home gardens (0.88 and 0.94) than in rural home gardens (0.77) (Kruskal–Wallis H = 21.3, P < 0.001; Mann–Whitney P < 0.05 in both cases). Rural home gardens also had a lower pooled Shannon diversity index of herbaceous species than semi–rural (Shannon diversity t–test P < 0.001) and peri–urban home gardens (P < 0.001), whereas the peri–urban herb community had a higher index than the semi–rural (P = 0.02). When we considered herbs and trees and shrubs together, the pooled Shannon diversity Index in peri–urban home gardens was 4.68, significantly higher than in rural (4.12, P < 0.001) and semi–rural home gardens (4.40, P < 0.001). Also the difference between rural and semi–rural home gardens was significant (P < 0.001). We conclude that diversity does not decline continuously from rural to peri–urban conditions: when considering only trees and shrubs, rural and peri–urban home gardens were quite similar, and the highest diversity occurred in semi–rural conditions; when considering only herbs, diversity increases from rural to peri–urban conditions, as was the case when considering all cultivated plants.
The observed total species richness of trees and shrubs was related to the number of sampled trees/shrubs: 1,103, 723, and 296 in rural, semi–rural, and peri–urban home gardens, respectively. This biases comparison of species richness among conditions, as we may expect to observe more species when we observe more plants. We therefore tested if there were differences in species richness of equally sized samples, applying individual based rarefaction. The sample of 296 trees and shrubs from the peri–urban home garden had 53.0 species, and random samples of 296 trees and shrubs from rural and semi–rural home gardens had 53.7 and 58.6 species respectively. Regression analysis on natural logarithm transformed rarefaction data showed no significant differences of slopes of the species accumulation curves of rural, semi–rural, and peri–urban home gardens and semi–rural home gardens (t < |1| in all comparisons, t0.025 = 2.05). Equally sized samples of the aggregate communities of trees and shrubs in the rural, semi–rural, and peri–urban communities thus had equal species richness, and we therefore conclude that on the level of the communities of trees and shrubs, richness does not decrease from rural to peri–urban conditions.
The total number of herbaceous cultivated plants was 589 in rural home gardens, 338 in semi–rural, and 844 in peri–urban home gardens. Rarefaction showed that 338 herb individuals had a richness of 68.3 species in rural home gardens, 104.0 species in semi–rural, and 112.5 species in peri–urban home gardens. Regression analysis on log transformed rarefaction data of herbs showed significantly smaller slopes of the species accumulation curves in rural than in peri–urban and semi–rural home gardens (t > |6| , t0.025 = 1.98), and no difference between peri–urban and semi–rural conditions. We conclude that richness of herbs decreases from peri–urban and semi–rural to rural conditions, but is not different between semi–rural and peri–urban conditions.
When taking all cultivated plants—trees/shrubs and herbs—together, we obtained similar rarefaction results: 1,051 plants (trees and herbs) in rural home gardens had a richness of 140.6 species, 178.4 in semi–rural home gardens, and 207.0 species in peri–urban home gardens. Regression analysis on log transformed rarefaction data of trees and herbs showed a significant lower slope of the species accumulation curves in rural, than in peri–urban and semi–rural home gardens (t > |3|, t 0.025= 1.984), whereas there was no difference of slopes between peri–urban and semi–rural conditions. Species richness of all plants in equally sized samples and diversity was thus higher in peri–urban home gardens and semi–rural home gardens, than in rural home gardens. Richness on a community basis thus increases from rural to semi–rural and peri–urban conditions, but is not different between semi–rural and peri–urban conditions.
Mean species richness of home gardens showed a different pattern. Mean richness of trees and shrubs varied between rurality conditions (F = 5.853, P = 0.007), and was significantly higher in rural than in peri–urban home gardens (Q = 4.81, P = 0.005), whereas there were no significant differences between rural and semi–rural (P = 0.36) and semi–rural and peri–urban home gardens (P = 0.12). Also the mean species richness of herbs varied between rurality conditions (ANOVA on log transformed data, F = 4.718, P = 0.02). There were on average 32.6 herb species in peri–urban home gardens, significantly more than the 13.8 in rural (Tukey’s Q = 3.845, P = 0.027) and 16.5 in semi–rural home gardens (Tukey’s Q = 3.663, P = 0.04), whereas there was no difference between rural and semi–rural home gardens. The mean species richness of all cultivated plants (trees, shrubs, and herbs together), was not different among rurality conditions: 37.3 in rural, 35.8 in semi–rural, and 45.6 in peri–urban home gardens (ANOVA, Welch F = 0.81, P = 0.46). We conclude that the average species richness of trees and shrubs decreases from rural to peri–urban conditions, whereas semi–rural home gardens are intermediate. Herb species richness decreases from peri–urban to rural conditions, with again semi–rural home gardens in an intermediate position. Finally, the average number of all species did not vary among rurality conditions. Thus, there is neither an overall decline nor an overall increase of agrobiodiversity as measured by species richness from rural to peri–urban conditions.
Available area limits species richness and abundances in peri–urban and semi–rural home gardens. The Spearman non–parametric correlations between home garden size and the numbers of trees and shrubs species and individuals were significant in peri–urban and semi–rural home gardens (species rho = 0.81 and 0.77, P = 0.001 and 0.003; individuals rho = 0.85 and 0.83, P < 0.001 in both cases). This was not the case in rural home gardens (P = 0.48 and 0.10 for species and individuals). In the largest rural home gardens, where the available area is not a limiting factor, wide spacing and conserving large trees is common, whereas in the smallest rural home gardens farmers compensate small area with a higher tree density and avoid large trees. Instead of a significant correlation between the number of trees and rural home garden area, we therefore find a significant negative correlation between the tree density and the available area (Pearson r = – 0.58, P < 0.05). Such correlation is not found in peri–urban and semi–rural home gardens, where density is as high as possible as long as it does not affect the production of individual trees.
The average density of trees and shrubs was significantly higher in semi–rural home gardens than in rural and peri–urban home gardens (F = 5.2, P < 0.02; one sided Tamhane test, P < 0.05). This was mainly due to trees and shrubs with a DBH between 10 and 20 cm (F = 7.1, P < 0.01; Tamhane test P < 0.05), whereas average densities of both thinner and thicker trees were not different (Fig. 3). Basal area per hectare was also significantly different between rurality conditions (F = 5.3, P = 0.01), being higher in semi–rural (18.2 m2) than in rural and peri–urban home gardens (11.3 and 9.7 m2) (P < 0.05).
Species Composition in Rural, Semi–Rural, and Peri–Urban Home Gardens
ANOSIM showed small but significant variation in tree/shrub species composition among the groups of home gardens (R = 0.13, P = 0.001), both between rural and peri–urban home gardens (P < 0.001), and between semi–rural and peri–urban home gardens (P < 0.02). There were no significant differences in composition between the rural and semi–rural home gardens (P = 0.40). SIMPER showed that Piscidia piscipula (L.) Sarg., Spondias purpurea L., and Citrus aurantium L. were more abundant in the rural home gardens, and Musa paradisiaca L. in the peri–urban home gardens. Together these species explained 50% of the variation in species compositions between the rural and peri–urban settings. M. paradisiaca, S. purpurea, and C. aurantium were more abundant in semi–rural home gardens, and C. sinensis and Anona muricata in peri–urban home gardens. Together these species explained 50% of the variations between the semi–rural and peri–urban settings.
ANOSIM also showed small differences in herbaceous species composition (R = 0.06, P < 0.001), where only the differences between rural and peri–urban home gardens were significant (P = 0.000). SIMPER analysis indicated that Zea mays L., Digitaria insularis (L.) Fedde, Bromelia caratas Hill, Allium schoenoprasum Hill, Ipoemoea batatas (L.) Lam, Aloe vera (L.) Burm. f., and Agava sisilana Perine explained 50% of the variation, all having higher abundances in rural home gardens.
Species’ Biogeographical Origins
Thirty–nine percent of tree and shrub species were native to the Yucatán Peninsula, 29% were neotropical, and 32% were introduced. The overall percentages of introduced, native, and neotropical tree and shrub species were not different between rural, semi–rural, and peri–urban conditions (chi–squared < 5), but the abundances were (chi–squared > 90). The mean percentage of native tree and shrub species was higher in rural home gardens (37.2%) than in peri–urban home gardens (20.9%) (H = 7.4, P < 0.05; U = 26.5, P < 0.01), whereas the mean percentage of introduced tree and shrub species was higher in peri–urban (54.7%) than in rural home gardens (37.6%) (F = 3.7, P < 0.05; Q = 3.8, P < 0.05). Fifty percent of trees and shrubs (individuals) in rural home gardens belonged to native species, compared with 42% in semi–rural and 15% in peri–urban home gardens (Fig. 4).
Only 3.8% of all herbaceous species were native, 49.3% were neotropical, and 46.9% were introduced. The percentage of herbaceous species that are introduced was 39% in rural home gardens, and 52% in semi–rural and peri–urban home gardens, but this difference was not statistically significant. The percentage of introduced herbaceous plants (individuals) was 45% in rural home gardens, 53% in semi–rural, and 63% in peri–urban home gardens (Fig. 4). The mean percentage of introduced herbaceous species was higher in peri–urban than in rural and semi–rural home gardens (H = 5.95, P < 0.05; U = 29.5, P = 0.01).
Species’ Uses
The distribution of species over uses varied between rural, semi–rural, and peri–urban home gardens. The mean percentage of fruit tree species in home gardens was higher in the peri–urban than in the rural and semi–rural home gardens (ANOVA, F = 5.8, P < 0.01; Q = 4.7, P < 0.01). The mean percentage of timber species was higher in the rural and semi–rural than in the peri–urban home gardens (H = 12.6 and P = 0.001, U = 9.5 and P < 0.001, and U = 33.5 and P < 0.01), as was the case of fodder species (H = 6.04, P < 0.05, U = 36 and 39, P < 0.03 and P < 0.05). Also the mean percentage of “other use” species was higher in rural than in semi–rural and peri–urban home gardens (H = 14.14, P < 0.001, U = 20 and 26 and P < 0.01 in both cases).
The distribution of tree and shrub individuals over uses also varied between the rurality conditions (Fig. 5). The mean proportion of fruit individuals of the total number of trees and shrubs was higher in the peri–urban (81%) than in the rural home gardens (61%) (F = 5.3 and P = 0.01; Q = 4.3 and P = 0.01). The percentage of fodder tree individuals was higher in the rural and semi–rural than in the peri–urban home gardens (H = 6, P = 0.04; U = 33, P = 0.01; H = 6 and P = 0.04, and U = 36, P = 0.02). The mean proportion of timber trees was significantly higher in the rural than in the peri–urban home gardens (H = 12.7, P = 0.00; U = 9.5, P = 0.00), as was the case of trees and shrubs for “other use” (H = 6.5 y P = 0.03, and U = 32 and P = 0.01) and firewood (F = 7.8, P = 0.004; Q = 4.2, P = 0.01). The mean percentage of individual trees and shrubs used for medicinal purposes was similar in the rural, semi–rural and peri–urban home gardens.
In the three rurality conditions, most herbaceous species and individuals were ornamentals, varying the proportions of this and other uses between rurality conditions. The mean proportion of ornamental species was higher in the peri–urban (81%) than in the rural (58%) and semi–rural (64%) home gardens (H = 11.8, P = 0.002; U = 17, P = 0.001). The mean proportion of edible species (spices, tomatoes, etc.) was higher in the rural home gardens than in the peri–urban home gardens (F = 6.9, P = 0.006; Q = 4.2, P = 0.01). The mean percentage of herbaceous ornamental individuals was greater in the peri–urban and semi–rural home gardens than in the rural home gardens (F = 15.2, P = 0.00; Q = 7.7, P = 0.000; and Q = 4.7, P = 0.005) (Fig. 5).
Discussion
The relation between the degree of rurality and attributes of diversity of home gardens is a complex one, and to address it we have to clearly distinguish the scales of the community of plants in the aggregate sample of home gardens for each condition, and the scale of individual home gardens. At both scales, relations differ according to the growth forms (trees and shrubs, and herbs, respectively) under consideration. We therefore cannot draw one simple conclusion regarding an increase or decrease of agrobiodiversity along the rural—peri–urban gradient. We must look for answers that take into account the most important expressions of agrobiodiversity.
The overall species richness of the aggregated sample of trees and shrubs of each condition is smaller in peri–urban than in rural and semi–rural home gardens, the latter having similar richness, and less herbaceous species in rural than in semi–rural and peri–urban home gardens. This is in agreement with earlier findings (Arifin et al. 1998; Michon and Mary 1994; Rico–Gray et al. 1990), and confirms the general notion of a decreasing tree and shrub species richness and an increasing richness of herbs along the rural to peri–urban gradient. This notion refers to the richness of all plants in a sample of home gardens, within their socially defined confines. It is a socially rather than an ecologically defined parameter. Molebatsi et al. (2010) indicate that farmers’ families in “deep” rural conditions, who maintain traditional ways of living, cultivate more local species covering more uses. Richness of species depends here on the local knowledge of the uses of plants, as well as on poverty (Poot–Pool et al. 2012). Poor families in semi–rural conditions maintain tree and shrub species of different uses to reduce the need to buy raw materials and manufactured products. As observed (see Table 1), families in rural conditions have extremely low incomes, and this incentivizes overall tree and shrub species richness.
Richness of equally sized samples of plants is an ecological attribute of the socially defined communities of plants. With regard to trees and shrubs, we find no differences in richness of equally sized samples of the aggregate communities from each rurality condition, whereas the communities of cultivated herbs, and the aggregate community of all cultivated plants (herbs, trees, and shrubs) in peri–urban and semi–rural home gardens are richer than their equivalent in rural home gardens. To our knowledge, this has not been reported earlier for tropical home gardens.
The pooled Shannon diversity index, a frequently used indicator of diversity that combines richness and abundance, of the tree and shrub component of all sampled home gardens (socially defined) was higher for semi–rural than for peri–urban and rural home gardens. This is because the mosaic of semi–rural home gardens contains many species of similar abundances. The mosaic of rural home gardens contains more species, but as some of these have high abundances, a lower diversity index results; whereas the mosaic of peri–urban home gardens contains a small number of species with low abundances, resulting in a diversity index that is similar to that found in rural home gardens. Diversity of trees and shrubs thus peaks in semi–rural home gardens and does not gradually decline from rural to peri–urban home gardens.
Finally, when looking at average numbers of cultivated species in home gardens in the three conditions, we find higher richness of trees and shrubs in rural than in peri–urban home gardens, and no difference of both with semi–rural home gardens; whereas average species richness of herbs was higher in peri–urban than in rural and semi–rural home gardens. There was no net difference of average species richness of all plants between the rurality conditions.
The contrast between findings regarding richness is related to the influence of home garden size on the abundance of trees and shrubs. Large rural home gardens contain more trees and shrubs than small peri–urban home gardens, and therefore we find on average more species in the former. Rural home gardens do not pose a limitation of space on cultivated tree diversity, as indicated by a lack of correlation between extension and the richness of tree and shrub species. Widely available space encourages families to cultivate some dominants, as reflected in a low equitability. This reduces species richness in subsamples and equals it to richness in peri–urban conditions.
The floristic composition of the tree and shrub component, and of the herbaceous component, varied between rural, semi–rural, and peri–urban home gardens, in agreement with earlier findings (Arifin et al. 1998; Bernholt et al. 2009; Molebatsi et al. 2010). Regarding tree and shrub species, semi–rural and rural home gardens were different from peri–urban home gardens, mainly due to differences in the abundance of common species. However, it should also be noted that many non–abundant species are only found in large rural home gardens (Van der Wal and Bongers 2012). Differences in herbaceous plant composition between rural and peri–urban home gardens were due to a large number of non–abundant and non–frequent ornamental species in peri–urban home gardens. Peri–urban dwellers are keener on introduced ornamental species (Cilliers et al. 2012; Kumar and Nair 2004).
The number of species of Fabaceae was quite high in rural home gardens, as compared to semi–rural and peri–urban home gardens. This family is particularly useful, as several of its species are renowned for the quality of their wood, firewood, fodder, and environmental services. The high number of species of Fabaceae was also found in Sahcabá (Xuluc–Tolosa 1995), Xuilub (Herrera–Castro 1994), and in other villages in the Yucatán Peninsula (García de Miguel 2000). As for herbaceous species, the Araceae family had most species, and these mainly occurred in peri–urban home gardens. This family was also reported to provide most ornamental species in Tehuacán, Oaxaca, México (Blanckaert et al. 2004).
The differences in floristic composition and structure between rurality conditions were related to plant use and home garden functions in livelihoods. Rural and semi–rural families organize their home garden in such a way that it supplies a variety of products for daily use. The mean number of species of timber, fodder, and firewood and other uses was therefore higher in rural and semi–rural home gardens than in peri–urban home gardens. The peri–urban families have relatively high incomes from a diversity of non–agricultural economic activities, and the economic contribution of home gardens is less relevant. Its contribution is rather hedonic and aesthetic (Arifin et al. 1998; Cilliers et al. 2012; Kehlenbeck et al. 2007). The relationship between economic level, livelihoods, and home garden composition has been highlighted earlier (Azurdia et al. 2000; Michon and Mary 1994). Focusing on the functions of the rural home garden for daily needs, there was a greater proportion of native tree and shrub species, and less introduced herbaceous species than in the peri–urban home gardens. Semi–rural home gardens presented intermediate values. This is in agreement with studies in India and South Africa (Das and Das 2005; Molebatsi et al. 2010; Nemudzudzanyi et al. 2010).
In previous work in one of the semi–rural communities, relatively wealthy peasant families concentrated on fruit and ornamentals, as did the peri–urban families in the present study, whereas relatively poor peasants cultivated a conglomerate of species for different uses in their home gardens, as in the rural and semi–rural home gardens in the present study. Economic conditions also influenced tree density in the semi–rural home gardens: poor families maintained higher tree densities than more affluent families (Poot–Pool et al. 2012). In agreement with this, we also found higher density and biomass in semi–rural than in peri–urban home gardens.
Our study confirms that the process of increasing biodiversity in cities through an increased global flow of exotic plants, as documented for the global north (Niinemets and Pañuelas 2008), is also taking place with regard to herbaceous, mainly ornamental species in the global south (Akinnifesi et al. 2010; Bernholdt et al. 2009). In our study area, it occurs in semi–rural and in peri–urban communities, and less in rural communities. It seems therefore necessary to analyze the risks of biological invasions by species introduced in home gardens (Niinemets and Peñuelas 2008; Vila–Ruiz et al. 2014). The low number of native species, particularly among herbs in all three conditions, and trees and shrubs in peri–urban conditions, indicates the need for an effort that focuses on the conservation of native species as a part of regional (rural, semi–rural, and peri–urban) agrobiodiversity. We conclude that agrobiodiversity decline along the rural—peri–urban gradient is not general, but that evidently changes are ongoing, and these serve to differentiate the home gardens between the three conditions.
Literature Cited
Abdoellah, O., B. Parekesit, and H. Hadikusumah. 2002. Home gardens in the Upper Citarum Watershed, West Java: A challenge for in situ conservation of plant genetic resources. In: Home gardens and in situ conservation of plant genetic resources in farming systems: Proceedings of the second International Home Gardens workshop, 17–19 July 2001, Witzenhausen, Federal Republic of Germany, eds., J. W. Watson and P. B. Eyzaguirre, 140–160. Rome: International Plant Genetic Resources Institute.
Abebe, T., F. J. Sterck, K. F. Wiersum, and F. Bongers. 2013. Diversity, composition and density of trees and shrubs in agroforestry home gardens in Southern Ethiopia. Agroforestry Systems. doi:10.1007/s10457-013-9637-6.
Akinnifesi, F. K., G. W. Sileshi, O. C. Ajayi, A. I. Akinnifesi, E. G. Moura, J. F. P. Linhares, and I. Rodrigues. 2010. Biodiversity of the urban home gardens of São Luís City, Northeastern Brazil. Urban Ecosystems 13:129–146.
Arifin, H. S., K. Sakamoto, and K. Chiba. 1998. Effects of urbanisation on the vegetation structure of home gardens in West Java, Indonesia. Japanese Journal of Tropical Agriculture 42:94–102.
Azurdia, C., J. M. Leiva, and E. López. 2000. Contribución de los huertos familiares para la conservación in situ de recursos genéticos vegetales II. Caso de la región de Alta Verapaz, Guatemala. TIKALIA (Guatemala) 18:35–78.
Barrera, A. 1980. Sobre la unidad de habitación tradicional campesina y el manejo de recursos bióticos en el área Maya Yucatanense. Biotica 5:115–129.
Bernholt, H., K. Kehlenbeck, J. Gebauer, and A. Buerkert. 2009. Plant species richness and diversity in urban and peri–urban gardens of Niamey, Niger. Agroforestry Systems 77:159–179.
Blanckaert, I., R. L. Swennen, M. Paredes–Flores, R. Rosas–López, and R. Lira–Saade. 2004. Floristic composition, plant uses and management practices in home gardens of San Rafael Coxcotlaán, Valle of Tehuacán–Cuicatlán, México. Journal of Arid Environments 57:179–201.
Caballero, J. 1992. Maya homegardens: Past, present and future. Etnoecológica 1:35–54.
Chandrashekara, U. M. and E. C. Baiju. 2010. Changing pattern of species composition and species utilization in home gardens of Kerala, India. Tropical Ecology 51:221–233.
CICY, 2010. Flora de la Península de Yucatán. http://www.cicy.mx/sitios/flora%20digital/ (15 June 2013).
Cilliers, S., J. Cilliers, R. Lubbe, and S. Siebert. 2012. Ecosystem services of urban green spaces in African countries: Perspectives and challenges. Urban Ecosystems 1–22, doi:10.1007/s11252–012–0254–3.
Das, T. and A. K. Das. 2005. Inventorying plant biodiversity in home gardens: A case study in Barak Valley, Assam, North East India. Current Science (Bangalore) 89:155–163.
Durán, R., J. C. Trejo–Torres, and G. Ibarra–Manríquez. 1998. Endemic phytotaxa of the Peninsula of Yucatán. Harvard Papers in Botany 3(2):263–314.
Eyzaguirre, P. and J. Watson. 2002. Home gardens and agrobiodiversity: An overview across regions. In: Home gardens and in situ conservation of plant genetic resources in farming systems: Proceedings of the second International Home Gardens workshop, 17–19 July 2001, Witzenhausen, Federal Republic of Germany, eds., J. W. Watson and P. B. Eyzaguirre, 10–14. Rome: International Plant Genetic Resources Institute.
Galluzzi, G., P. Eyzaguirre, and V. Negri. 2010. Home gardens: Neglected hotspots of agro–biodiversity and cultural diversity. Biodiversity and Conservation. doi:10.1007/s10531-010-9919-5.
García de Miguel, J. 2000. Etnobotánica maya: Origen y evolución de los huertos familiares de la Península de Yucatán, México. Ph.D. thesis, Instituto de Sociología y Estudios Campesinos, Universidad de Cordoba, Cordoba, España.
Hammer, Ø., D. A. T. Harper, and P. D. Ryan. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1–9.
Herrera–Castro, N. 1994. Los huertos familiares mayas en el oriente de Yucatán. In: Etnoflora Yucatanense, ed., J. S. Flores, 169. Mérida, Yucatán, México: Universidad Autónoma de Yucatán.
Huai, H. and A. Hamilton. 2009. Characteristics and functions of traditional home gardens: A review. Frontiers of Biology in China 4:151–157.
Ibarra–Manríquez, G. 1996. Biogeografía de los árboles nativos de la Península de Yucatán: Un enfoque para evaluar su grado de conservación. Ph.D. thesis, Facultad de Ciencias, UNAM, México D.F., México.
Infante G. S. and L. G. Zárate. 1997. Métodos estadísticos, un enfoque interdisciplinario, ed., Trillas, 643. México: Cuarta reimpresión.
Kehlenbeck, K., H. Arifin, and B. Maass. 2007. Plant diversity in homegardens in a socio–economic and agro–ecological context. In: Stability of tropical rainforest margins. Linking ecological, economic and social constraints of land use and conservation, ed., Teja Tscharntke, 297–319. Berlin: Springer.
Kumar, B. M. and P. K. R. Nair. 2004. The enigma of tropical home gardens. Agroforestry System 61:135–152.
Michon, G. and F. Mary. 1994. Conversion of traditional village gardens and new economic strategies of rural households in the area of Bogor, Indonesia. Agroforestry Systems 25:31–58.
Molebatsi, L. Y., S. J. Siebert, S. S. Cilliers, C. S. Lubbe, and E. Davoren. 2010. The Tswana tshimo: A home garden system of useful plants with a particular layout and function. African Journal of Agricultural Research 5:2952–2963.
Moreno–Black, G., P. Somnasang, and S. Thamathawan. 1996. Cultivating continuity and creating change: Women’s home garden practices in northeastern Thailand. Agriculture and Human Values 13:3–11.
Nemudzudzanyi, A. O., S. J. Siebert, A. M. Zobolo, and L. Y. Molebatsi. 2010. The Zulu muzi: A home garden system of useful plants with a specific layout and function. African Journal of Indigenous Knowledge Systems 9:57–72.
Niinemets, Ü. and J. Peñuelas. 2008. Gardening and urban landscaping: Significant players in global change. Trends in Plant Science 13(2):60–65.
Peng, Y. and L. Xuehua. 2007. Research progress in effects of urbanization on plant biodiversity. Biodiversity Science 15:558–562.
Poot–Pool, W. S., H. Van der Wal, J. S. Flores–Guido, J. M. Pat–Fernandez, and L. Esparza–Olguín. 2012. Economic stratification differentiates home gardens in the Maya village of Pomuch, México. Economic Botany 66:264–275.
Rico–Gray, V., J. G. Garcia–Franco, A. Chemas, A. Puch, and P. Sima. 1990. Species composition, similarity, and structure of Mayan home gardens in Tixpeual and Tixcacaltuyub, Yucatán, México. Economic Botany 44:470–487.
Royal Botanic Gardens. http://www.kew.org/scienceconservation/plants-fungi (19 June 2013).
Thompson, K., K. C. Austin, R. M. Smith, P. H. Warren, P. G. Angold, and K. J. Gaston. 2003. Urban domestic gardens (I): putting small–scale plant diversity in context. Journal of Vegetation Science 14:71–78.
Van der Wal, H. and F. Bongers. 2012. Biosocial and bionumerical diversity of variously sized home gardens in Tabasco, México. Agroforestry Systems 87:93–107.
Vila–Ruiz, C. P., E. Meléndez–Ackerman, R. Santiago–Bartolomei, D. García–Montiel, L. Lastra, C. Figuerola, and J. Fumero–Caban. 2014. Plant species richness and abundance in residential yards across a tropical watershed: Implications for urban sustainability. Ecology and Society 19(3):22.
Watson, J. W. and P. B. Eyzaguirre, eds. 2002. Home gardens and in situ conservation of plant genetic resources in farming systems: Proceedings of the second International Home Gardens workshop, 17–19 July 2001, Witzenhausen, Federal Republic of Germany, p. 184. International Plant Genetic Resources Institute, Rome.
Wezel, A. and J. Ohl. 2005. Does remoteness from urban centres influence plant diversity in home gardens and swidden fields: A case study from the Matsiguenka in the Amazonian rain forest of Peru. Agroforestry Systems 65:241–251.
Xuluc–Tolosa, F. 1995. Caracterización del componente vegetal de los solares de la comunidad de Sahcabá, Yucatán, México. Tesis de licenciatura. Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Yucatán, UADY.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
(DOCX 51 kb)
Rights and permissions
About this article
Cite this article
Poot–Pool, W.S., van der Wal, H., Flores–Guido, S. et al. Home Garden Agrobiodiversity Differentiates Along a Rural—Peri–Urban Gradient in Campeche, México. Econ Bot 69, 203–217 (2015). https://doi.org/10.1007/s12231-015-9313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12231-015-9313-z