Abstract
Litterfall is a key process in forests which is sensitive to climatic conditions like precipitation and temperature, and management practices. Therefore, knowledge about litterfall patterns and its associated variables is important for the conservation of Mediterranean ecosystems under conditions of climate change. We aimed to quantify the temporal pattern of litterfall and to investigate the influence of abiotic variables and pruning on litter production. Litterfall was collected at monthly intervals for 2 years in trees subjected to different pruning intensities in two locations. The effect of pruning, abiotic variables and tree size on litter production was analyzed using a mathematical model. Leaf fall was strongly seasonal with a peak occurring in the wettest month of the year in this area. The variability in leaf fall was mainly related to rainfall and soil water in 2 years and locations. Pruning reduced the amount of litter production during the first year following this practice, and might have negative effect on soil fertility and crop productivity in dehesas ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litterfall is a key process in the dynamics of forest ecosystems, being a linkage between the tree and soil nutrient pools and therefore the starting point for nutrient cycling (Gray and Schlesinger 1981). As primary production is influenced by the availability of nutrients, the rate of forest litterfall and its gradual decay will affect the productivity and the energy flow in forest ecosystems (Aerts and Chapin 2000). Litterfall improves the nutritional status of trees by increasing the nutrient availability, especially for N and P, but it also plays other important roles in forest ecosystems, buffering changes in soil water content and temperature, reducing erosion, thus augmenting the diversity of plant, fungi and soil organisms (Sayer 2006).
Holm oak (Quercus ilex L. subsp. ballota [Desf.] Samp) is one of the most important species in forest communities of the Mediterranean Basin. This species dominates one of the most representative and valuable forest communities in Southern Europe: the dehesas. Dehesas are agroforestry systems created by thinning of the original forests, in areas with low fertility and acidic soils. They are mainly used for livestock breeding (pigs, cattle and sheep), firewood, coal and other agro/silvi/pastoral purposes (Joffre et al. 1988; Olea and San Miguel-Ayanz 2006). Dehesas are an example of sustainability, where productive processes are linked to environmental benefits, reducing nutrient and soil losses from agriculture, increasing carbon sequestration or enhancing biodiversity (Regueiro-Rodríguez et al. 2009). Therefore, they are of great economic, ecological, social and aesthetic value in the Southern Europe. Scenarios for climate change in Mediterranean areas predict increasing temperatures and decreasing rainfall (Christensen et al. 2007). Such changes could affect both tree growth and phenology. Thus, any change in litter production or seasonality could affect soil fertility and net primary production. Hence, knowledge of the relationship between meteorological conditions and litter production is fundamental to sustainable management of these agroforestry systems.
Pruning is a common management practice in Mediterranean areas that was traditionally carried out to obtain fuel wood and because it was thought to improve acorn production in agroforestry systems (Alejano et al. 2011), but its impact on the latter has been questioned in the last decade (Alejano et al. 2008; Cañellas et al. 2006). Holm oak pruning involves removing branches every 6–10 years; the extent to which it is carried out is sometimes very extensive, and in some cases excessive (Cañellas and Montero 2002). Pruning reduces the above ground biomass, and consequently litter production patterns. As pruning is realized for fuel wood production, only small components of branches stay on the soil, therefore nutrient return to the soil and nutrient availability for the trees may be reduced in long term. In Mediterranean regions, where the economy of resources including water and nutrients is critical (Arianoutsou 1989), a reduction of biomass and nutrients provided by litterfall because of continuous pruning could have long-term effects on growth, acorn production and the stability of dehesas.
Research on litter production in Mediterranean areas is scarce, and the studies conducted on Mediterranean Quercus species has been focused on litter production and nutrient return to the soil, and its seasonality (Andivia et al. 2010; Bussotti et al. 2003; Rapp et al. 1999). Only Caritat et al. (2006) has reported the relationship between litter production and meteorological conditions, in a Q. suber forest. However, as far as we know, there is a lack of information about the characterization of litterfall in dehesas. Although some studies have addressed nutrient cycling in dehesas, they have been focused on the influence of trees (Moreno and Obrador 2007; Moreno et al. 2007) or shrubs (Rolo et al. 2012) on soil fertility but the litter production and their seasonality have not been considered. In addition, no studies have explored the effect of pruning on litter production in Mediterranean areas. Due to the lack of information about litter processes in dehesas, our study aims to be the basis for futures studies about nutrient cycling or about modeling the influence of climate conditions in litter production in dehesas. For this purpose, this study tries to achieve the following major objectives: (i) to quantify temporal changes in the amount of litterfall, (ii) to study the effect of pruning intensities on the pattern of litterfall at two sites in relation to control, and (iii) to understand the role of abiotic variables on the intraannual pattern of litterfall. To this end the effects of three pruning intensities and no-pruning were tested at two locations, taking into account meteorological and other variables.
Materials and methods
Experimental plots
The study was conducted in two locations (southwest Spain) structured as dehesas, and largely covered by Q. ilex trees at similar density and size. The CA location is in Calañas, Huelva (UTM: zone 29: X, 681349; Y, 4156557), the plot covers an area of 2.9 ha, and has a density of 35 trees ha−1 with a mean height of 6.2 ± 1.7 m and a mean diameter of 0.32 ± 0.11 m at 80 cm height. The tree diameter was not measured at breast height because tree crosses were always below 1.30 m due to formative pruning traditionally conducted in this species. The SB location is in San Bartolomé de la Torre, Huelva (UTM: zone 29: X, 669638; Y, 4145966), the plot covers an area of 2.7 ha, and has a density of 36 trees ha−1 with a mean height of 6.5 ± 1.1 m and a mean diameter of 0.35 ± 0.07 m at 80 cm height. Study sites have not been fertilized in the last decade, although livestock dejections occurred in both plots.
Both locations have a characteristic Mediterranean climate with a similar interannual rainfall distribution. Daily climatic data at each location was monitored by two automatic weather stations of the Consejería de Agricultura y Pesca (Regional Government of Andalusia, Spain). The climatic conditions at each location during the study period are shown in Table 1. Two soil profiles were opened at each plot to characterize soil properties (Table 2). The SB location is on a substrate consisting primarily of shale, and the CA location is on a substrate consisting primarily of shale and greywacke. In general, the soil of the CA location are less developed, because of its more uneven relief, more acid and showed higher concentration of soil organic matter than the soil of the SB plot.
Pruning treatments
Trees in each location were exposed to four pruning treatments; three trees per treatment at the CA location and four trees per treatment at the SB location. The trees were selected by stratified sampling based on the stem diameter measured at 80 cm height. The pruning treatments tested were: light (L), moderate (M), heavy (H) and control (C). Pruning treatments were discriminated in terms of intensity, based on the branch dry weight removed from the trees (DW) to tree diameter (D) ratio; thus, DW/D was <0.8 for light pruning, >1.7 for heavy pruning, and intermediate between these two levels for moderate pruning (Alejano et al. 2008). No pruning occurred for the control group (DW/D = 0).
To more accurately compare the effects of pruning during the period of study, pruning treatments were carried out at different time in each location, January 2001 at the CA location and February 2003 at the SB location. Previous pruning in each location occurred seven years before the pruning conducted in this study (1994 for CA, 1996 for SB).
Litter collection
Litterfall from those trees selected for the pruning treatments was collected using the trapping method (Andivia et al. 2010; Caritat et al. 2006; Rapp et al. 1999). For each tree, four 0.16 m2 circular plastic traps were placed on each cardinal point at a distance corresponding to three quarters of the crown radius, as measured from the stem (Alejano et al. 2008). Samples were collected monthly at each plot from January 2004 to January 2006. They were oven dried at 65 °C over 2 days, separated into various fractions (leaves, twigs, catkins and female flowers), and weighed. Acorns were collected and used to estimate acorn production by trapping method (see Alejano et al. 2008, 2011; Carevic et al. 2010). Litterfall data were expressed as g m−2 of the orthogonal projection of the crown on the ground, by dividing the dry weight of litter collected from each tree by the surface area of the four litter traps (0.64 m2). Annual litter production was calculated as follow: \( LP_{ij} = (LF_{ij} \cdot CS_{i} )/S_{i} \); where LP ij is the litter production of location i at the year j, LF ij is the annual litterfall (g m−2 of crown) in location i at the year j, CS i is the total crown surface of the location i calculated as sum of elliptical crown surface of all trees of the plot (4798.0 m2 for CA location and 6137.5 m2 for SB location), and Si is the area of each plot (ha).
Data analysis
We considered that the following factors affected leaf and twig fall: location (CA, SB), tree (within location), pruning treatment (C, L, M, H), year (2004 and 2005) and month (1–12). Thus the initial model was: \( y_{ijklm} = \mu + \alpha_{i} + b_{j(i)} + \tau_{k} + \eta_{l} + \gamma_{m} + (\alpha |\tau |\eta |\gamma )_{iklm} + e_{ijklm} \)where y ijklm is the mean dry mass of fallen leaves or twigs (g m−2) from tree j in location i under pruning treatment k in month l of year m; μ is the general mean; α i is the location fixed effect (i = 1, 2); b j(i) is the tree (within location) random effect, with j = 1, 2, …16 and i = 1, 2 with the initial hypothesis b j(i) ~ N(0, \( \sigma_{b}^{2} \)); \( \tau_{k} \) is the pruning treatment fixed effect, with k = 1, 2, 3, 4; η l is the month fixed effect, with l = 1, 2, …12; \( \gamma_{m} \) is the year fixed effect, with m = 1, 2; (α|τ|η|γ) iklm represents all the possible interactions among fixed effects; and \( e_{ijklm} \) is the error term under the assumption of normality.
To select the best variance–covariance structure we assessed (1) the presence of temporal correlation and heteroscedasticity among observations in different months for a particular tree and year; (2) the significance of the tree random effect; and (3) the significance of spatial correlation among observations of trees within locations. To assess (1) we tested different variance–covariance structures among observations for different months for a particular tree and year (Wolfinger 1996). Variance components for each structure were estimated by restricted maximum likelihood (REML) and the best structure for each model was selected based on the Akaike information criteria (AIC; Akaike 1974) with a lower value indicated a better model.
After selecting the best variance–covariance structure we estimated fixed effects using generalized least squares (GLS), and determined the significance of each factor using an F test. Only significant effects (α = 0.05) were retained in the final model. Comparisons among levels were performed using the Scheffé test. For significant effects we determined whether the introduction of covariates could explain a significant part of the observed variability. The levels and the covariates considered at each level were: Tree level: we considered variables related to tree size (i) circumference at 80 cm height (CH80), and (ii) canopy area (SCROWN); microtopography variables and competition indexes were initially considered, but finally removed from the model due to the lack of significance. Location × year × month level: We considered the values for climatic variables per month, per year and per location for the study period.
The significance of the covariates was tested using a likelihood ratio test that considered the reduction of the –2LL statistic after introducing the covariate. In this case the variance components were estimated by maximum likelihood (ML). Statistical analysis was performed with SAS (version 9.2, SAS Institute Inc., North Carolina, USA).
Results
Litter production
The mean total litterfall (LF) for both locations and years was 228.0 g m−2/year (acorns not included), corresponding to a litter production (LP) of 450.2 kg ha−1. In 2004 LF was greater at CA location, but in 2005 the opposite occurred, although LP was always greater in SB location due to the higher total crown surface of SB plot (Table 3). Leaves and twigs were the main fractions of the litterfall, comprising 69 and 22 % of the total, respectively. Catkins and female flowers constituted 9 and 0.3 % of the total litterfall, respectively, and showed similar values between years and locations with the exception of catkins in 2005 at the SB site, where a large peak was observed. Mean litter production was 656.1 kg ha−1 (including acorns), with more similar values among locations in 2004 (694.0 kg ha−1 in SB and 564.3 kg ha−1 in CA) but with much higher production in SB in 2005 (1035.7 kg ha−1 in SB and 330.6 kg ha−1 in CA) due to the differences in acorn production among locations.
Litterfall patterns
The significance (p < 0.0001) of the fixed effect month and the interaction month × year (Table 4) shows the seasonality of the litterfall. The selected variance–covariance structures for both models were nonhomogeneous (unstructured for leaf fall and unstructured correlations for twig fall), demonstrating the heterogeneity of variances among months. In addition, the selection of structures with four bands for leaf fall and two bands for twig fall provided evidence of a correlation in litter production between consecutive months (three for leaf fall and one for twig fall) but not for temporally more distant months.
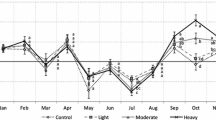
Litterfall was maximum during spring (March–June), with a small peak between October and November coinciding with leaf fall seasonality (Fig. 1). Although twigs constituted 22 % of the total litterfall, the seasonality of this fraction was not as pronounced as that of leaf fall; relatively constant values were found through the year, although the maximum occurred in April and minimum in summer. Of the total leaf fall 60 % occurred during spring, but the distribution among spring months was different for each year (Fig. 1). In 2004 the period of maximum leaf fall had two peaks (March and June), while in 2005 the period of maximum leaf fall occurred in only one month (April).
Some differences were also found between the two locations (Fig. 1; Table 4). In 2004 two peaks occurred at the CA location (March and June), while at the SB location there was only a small peak in March, an unexpected peak in January, and little leaf fall in autumn. In 2005 the spring peak at both locations occurred in April, but was larger at the SB site. A second period of leaf fall that during October and November only occurred at the SB location.
The seasonality of catkins and female flowers in the litterfall was different between the 2 years (Fig. 2). In 2004 catkins fell earlier (Mar–Apr) than in 2005 (Apr–May), and the amount of catkins in the litterfall was less in 2004 than in 2005 because of the large quantity of catkins that fell at the SB location in 2005 (Table 3). The female flowers fell earlier in 2005 (Apr–May) than in 2004 (May–Jun), although the values for both years were similar.
Influence of meteorological parameters on litterfall patterns
For twig fall none of the meteorological variables was statistically significant; while for leaf fall, precipitation (P) (F = 18.06, p < 0.0001, estimated coefficient = 0.063) and ET0 (F = 13.13, p = 0.0003, estimated coefficient = 0.296) were the most statistically significant covariates, each showing a positive estimated coefficient. Mean wind speed (Ws) was also statistically significant (F = 5.34, p = 0.0212, estimated coefficient = 5.096). Differences between locations were evident in the periods of maximum leaf fall (spring and autumn) for both years (Fig. 1). In these periods statistically significant covariates showed differences between locations. The most statistically significant covariate, P, was always greater in months prior to the peaks of leaf fall in the location where the peaks were also larger. In February 2004 P was 23 % greater at the CA location, while in February and March 2005 it was greater at the SB location (76.2 mm vs 56.2 mm). In addition, in October 2004 and 2005 P was greater in locations where leaf fall was also greater. Ws and ET0 were almost always greater at the CA location; the exception was ET0 between March and May 2004, when it was similar for both locations.
Effect of tree level variables
For both models tree random effect (p = 0.042 for leaf fall; p = 0.007 for twig fall) and spatial correlation (p < 0.0001) were significant. For leaf fall the variables related to tree size CH80 (F = 4.8891; p = 0.0275, estimated coefficient = 0.024) and SCROWN (F = 4.7412; p = 0.0299, estimated coefficient = 0.021) explained 27.6 % and 27.8 % of the variability at the tree level respectively. The estimated coefficients for both covariates were positive, showing that leaf fall was greater for larger trees. CH80 and SCROWN were linearly correlated (r = 0.840, p < 0.0001, n = 31). For twig fall no variable related to tree size or the topographic position of trees was significant.
Effect of pruning
At the CA location the leaf fall generally showed the same pattern for each pruning treatment in each year. However, at this location significant differences were found among treatments at some times, particularly during April–June 2004, when unpruned trees showed less leaf fall than those in the other treatments (Fig. 3).
Least squares means estimates of leaf fall for each pruning treatment (g m−2 tree−1 ± standard error) at each location in each year. The pruning treatments tested were: light (L), moderate (M), heavy (H) and unpruned (C). The figure shows the standard error for only one of the treatments because the standard error in any month was the same for all treatments
At the SB location the patterns of leaf fall among pruning treatments differed during the first 6 months of 2004. For unpruned trees the period of maximum leaf fall was during May and June, whereas for pruned trees the maximum leaf fall occurred earlier. From May to October 2005 trees from the L treatment showed greater leaf fall than the other treatments, while in November the trees from the M treatment had the most leaf fall (Fig. 3).
Discussion
Our results show that litter production in the evergreen species Quercus ilex is strongly seasonal, with a major peak in spring (March–June) and another much smaller peak in autumn (October–November). Similar results have previously been reported for Q. ilex (Bellot et al. 1992; Bussotti et al. 2003; Rapp et al. 1999) and Q. suber (Andivia et al. 2010; Caritat et al. 2006). Litterfall seasonality is mainly determined by leaf fall, which constitutes approximately 60 % of total litter production. Twig fall was not as strongly seasonal, but the periods of maximum and minimum yield were similar. Catkins and female flowers showed marked interannual variability, especially catkins, according to Bellot et al. 1992. Female flowers fell earlier in 2005 than in 2004 in our study area may be due to severe drought recorded during 2005 (highest for the last 60 years) (European Environmental Agency 2007). The level of precipitation was particularly low in spring, which may have interfered with the proper development of female flowers, leading to their premature fall (Misson et al. 2011). However, the fall of catkins occurred earlier in 2004 than in 2005 probably because the development of catkins was less affected by drought because of their earlier development (Misson et al. 2011). The catkin amount considerably varied between years and locations. Jato et al. (2007) suggested that large year-to-year differences in catkin production may be a consequence of weather-related factors or resource allocation. To understand differences in flower production among years a complementary long-term phenology study that considers the evolution of phenophases in trees, and other resource-consuming process such as acorn production or growth, will be necessary.
In our study the peaks of leaf fall followed the wettest months (generally February and October), which account for almost 60 % of annual rainfall. The main cause of leaf fall in spring is the renewal of foliar cover, which is a typical process in evergreen Mediterranean species (Escudero and Del Arco 1987). Trees in Mediterranean climates have to take advantage of the spring, the most physiologically favorable period (Bussotti et al. 2003), to renew their crown prior to the onset of the dry summer, which is the most stressful period. The development of new leaves requires high temperatures and good water status (a pre-dawn water potential >–3.0 MPa) to maintain cell turgor and construct new xylem tissue (Lo Gullo and Salleo 1993; Tognetti et al. 1998); these conditions usually occur in this area in spring and autumn.
Although leaf fall showed a strong seasonal peak in spring, the distribution of leaf fall within the spring months differed among years. Leaf emergence in Q. ilex tends to be concentrated in a single flush at the beginning of the growth period (Mediavilla and Escudero 2004). It is also well known that new leaf production is related to litterfall (Bellot et al. 1992; Bussotti et al. 2003), and that production of foliar biomass has to be balanced with the amount of rainfall and the soil water reserves (Hoff and Rambal 2003). Under stressful environmental conditions the production of new leaves in Holm oak may be reduced and associated with increased the leaf retention and leaf longevity, and consequently decreased litter production (Misson et al. 2011). Drought is the main stress factor in Mediterranean areas, and ecophysiological traits are influenced by water availability. Because of the low level of rainfall in February 2005 (Fig. 1), leaf fall was delayed until the single peak observed in April, as a consequence of delaying production of new leaves, while leaf fall maximum in 2004 occurred in March and June, probably because of the more favorable climatic conditions (Rodá et al. 1999). In addition, litter amount in 2004 was greater than in 2005 at CA location, but at SB location litter amount was similar between the 2 years. These unexpected differences might be related with the different pruning timing, at the CA location litterfall was greater in 2004 than at the SB location because the pruning conducted at the latter site reduced the aboveground biomass, and hence the litter production. Our results suggest that leaf fall patterns between the 2 years of the study were mainly influenced by precipitation, although these results should be corroborated in long-term studies and considering more locations climatically contrasted.
Litter production varied significantly among individual trees, although it was mainly influenced by phenology. Because of the significant tree effect we introduced covariates at this level to attempt to explain some of the variability, as we found that leaf fall was influenced by variables related to the size of the trees. These variables (CH80 and SCROWN) were clearly correlated, as thicker trees have larger canopies. In this regard, larger trees have more leaf fall because of the greater thickness of the crown, and consequently litter traps in larger trees collect more leaves per crown surface projection. We also found a significant spatial correlation among trees at the same site, indicating that adjacent trees have more similar values of litter production than do trees that are further apart. Litter production can be influenced at the local level by topographic factors, nutrient and water distribution and availability (Blanco et al. 2008); therefore, it is predictable that nearby trees have more similar values of litter production.
Pruning involves removing aboveground biomass and therefore the litterfall and nutrients reaching the forest floor could be reduced. The effects of pruning in Mediterranean oak woodlands have long been controversial, and there is an inadequate scientific basis on which to recommend this practice (Alejano et al. 2008; Cañellas et al. 2006). Our data show different trends in leaf fall under different pruning timing. In the first part of 2004 the patterns of leaf fall among pruning treatments were different at SB location. From July 2004 until the end of the study at SB location, and throughout the study at CA location, the leaf fall patterns were similar for the various pruning treatments. These differences occurred because pruning treatments at SB location were carried out in February 2003, while at CA location they were done in January 2001. Therefore, pruning seems to only have a marked influence on leaf fall during the year immediately following this practice; the subsequent patterns of leaf fall among treatments then appear to converge. Regarding the differences found in SB plot, unpruned trees showed a normal pattern of leaf fall, with a maximum occurring between May and June, but pruned trees showed an earlier and smaller peak of leaf fall in the March–April period. This was probably because pruning increased early sprout formation as a consequence of branch removal. However, the quantity of leaf fall was less because of the smaller number of leaves in the canopy of pruned tress, with heavily pruned (H) trees showing a smaller amount of leaf fall during the following spring. The reduction of the litterfall reaching the soil in dehesas ecosystems might have a negative effect on soil fertility and crops (Gallardo 2003; Moreno et al. 2007), as Holm oak trees act as a “nutrient pump” bringing up nutrients from the soil and recycling via litterfall (Moreno and Obrador 2007). In addition the pruning effect on sprouting involves a reallocation of resources in the tree to rebuild the aboveground biomass, and might also be related to the decrease in acorn production reported in other studies to occur during the year following pruning (Cañellas et al. 2006). However, it is evident that other factors are involved, because Alejano et al. (2011) did not find a decreasing acorn crops after pruning for the same trees here involved.
Conclusions
Litter production in Q. ilex shows a strong seasonal pattern with peaks occurring in spring and autumn, but maximum occurring in spring. These periods coincided with the wettest months in the 2 years of the study, during which the trees took advantage of the favorable conditions to renew their crowns.
The differences found in litter production between years seem to be related with precipitation and water availability. Nevertheless these results should be corroborated in future long-term studies.
Our results show that pruning only affects litter production during the year after pruning, with pruned trees showing smaller and earlier leaf fall.
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30(1):1–67
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723
Alejano R, Tapias R, Fernández M, Torres E, Alaejos E, Domingo J (2008) Influence of pruning and the climatic conditions on acorn production in holm oak (Quercus ilex L.) dehesas in SW Spain. Ann For Sci 65(2):209–217
Alejano R, Vázquez-Piqué J, Carevic F, Fernández M (2011) Do ecological and silvicultural factors influence acorn mass in Holm Oak (southwestern Spain)? Agroforest Syst 83(1):25–29
Andivia E, Fernández M, Vázquez-Piqué J, González-Pérez A, Tapias R (2010) Nutrients return from leaves and litterfall in a mediterranean cork oak (Quercus suber L.) forest in southwestern Spain. Eur J For Res 129(1):5–12
Arianoutsou M (1989) Timing of litter production in a maquis ecosystem of north–eastern Greece. Oecolog Plantar 10(4):371–378
Bellot J, Sánchez JR, Lledó MJ, Martínez P, Escarré A (1992) Litterfall as a measure of primary production in mediterranean holm oak forest. Vegetatio 99–100:69–76
Blanco JA, Bosco-Imbert J, Castillo FJ (2008) Nutrient return via litterfall in two contrasting Pinus sylvestris forests in the Pyrenees under different thinning intensities. For Ecol Manag 256:1840–1852
Bussotti F, Borghini F, Celesti C, Leonzio C, Cozzi A, Bettini D, Ferretti M (2003) Leaf shedding, crown condition and element return in two mixed holm oak forests in Tuscany, central Italy. Forest Ecol Manag 176:273–285
Cañellas I, Montero G (2002) The influence of cork oak pruning on the yield and growth of cork. Ann For Sci 59:753–760
Cañellas I, Roig S, Montero G (2006) Pruning influence on acorn yield in cork oak open woodland. In: Mosquera MR, McAdam J, Rigueiro A (eds) Silvopastoralism and sustainable land management. CABI Publishing, Oxfordshire
Carevic FS, Fernández M, Alejano R, Vázquez-Piqué J, Tapias R, Corral E, Domingo J (2010) Plant water relations and edaphoclimatic conditions affecting acorn production in a holm oak (Quercus ilex L. ssp. ballota) open woodland. Agrofor Syst 78(3):299–308
Caritat A, García-Berthou E, Lapeña R, Vilar L (2006) Litter production in a Quercus suber forest of Montseny (NE Spain) and its relationship to meteorological conditions. Ann For Sci 63(7):791–800
Christensen JH, Hewitson B, Busuioc A et al (2007) Contribution of working group I to fourth assessment report of the intergovernmental panel of climate change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Miller MTHL (eds) Climate change 2007: the physical science. Cambridge University Press, Cambridge
Escudero A, Del Arco JM (1987) Ecological significance of the phenology of leaf abscision. Oikos 49:11–14
European Environmental Agency (2007) Climate change and water adaptation issues. European Environmental Agency Technical Report no 2/2007
Gallardo A (2003) Effect of tree canopy on the spatial distribution of soil nutrients in a Mediterranean dehesa. Pedobiologia 47:117–125
Gray JT, Schlesinger WH (1981) Nutrient cycling in mediterranean type ecosystems. In: Miller PC (ed) Resource use by chaparral and matorral. A comparison of vegetation function in two mediterranean type ecosystems, vol 39. Springer-Verlag, Ecological Studies, New York, pp 259–286
Hoff C, Rambal S (2003) An examination of the interaction between climate, soil and leaf area index in a Quercus ilex ecosystem. Ann For Sci 60(2):153–161
Jato V, Rodríguez-Rajo FJ, Aira MJ (2007) Use of Quercus ilex subsp. ballota phenological and pollen-production data for interpreting Quercus pollen curves. Aerobiologia 23(2):91–105
Joffre F, Vahar J, De las Llamas G, Long G (1988) The dehesa: an agrosilvopastoral system of the Mediterranean region with special reference to the Sierra Morena of Spain. Agrofor Syst 6:71–96
Lo Gullo M, Salleo S (1993) Different vulnerabilities of Quercus ilex L. to freeze- and summer drought-induced xylem embolism: an ecological interpretation. Plant Cell Environ 16:511–519
Mediavilla S, Escudero A (2004) Stomatal responses to drought of mature trees and seedlings of two co-occurring Mediterranean oaks. For Ecol Manag 187:281–294
Misson L, Degueldre D, Collin C, Rodriguez R, Rocheteau A, Ourcival JM, Rambal S (2011) Phenological responses to extreme droughts in a Mediterranean forest. Global Change Biol 17:1036–1048
Moreno G, Obrador J (2007) Effects of trees and understorey management on soil fertility and nutritional status of holm oaks in Spanish dehesas. Nutr Cycl Agroecosyst 78:253–264
Moreno G, Obrador J, García A (2007) Impact of evergreen oaks on soil fertility and crop production in intercropped dehesas. Agri Ecosyst Environ 119:270–280
Olea L, San Miguel-Ayanz A (2006) The Spanish dehesa. A traditional Mediterranean silvopastoral system linking production and nature conservation. Grassland Sci Eur 11:3–13
Rapp M, Santa-Regina I, Rico M, Gallego HA (1999) Biomass, nutrient content, litterfall and nutrient return to the soil in Mediterranean oak forest. For Ecol Manage 119:39–49
Regueiro-Rodríguez A, Fernández-Nuñez E, González-Hernández P, Mc Adams JH, Mosquera-Losada MR (2009) Agroforestry systems in Europe: productive, ecological and social perspectives. In: Regueiro-Rodríguez A, Mc Adams JH, Mosquera-Losada MR (eds) Agroforestry in Europe: current status and future prospects. Advances in agroforestry, vol 6. Springer, Berlin, pp 43–65
Rodá F, Retana J, Gracia CA, Bellot J (1999) Ecology of Mediterranean evergreen oak forests. Springer, New York
Rolo V, López-Díaz ML, Moreno G (2012) Shrubs affect soil nutrients availability with contrasting consequences for pasture understory and tree overstory production and nutrient status in Mediterranean grazed open woodlands. Nutr Cycl Agroecosyst 93:89–102
Sayer EJ (2006) Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol Rev 81:1–31
Tognetti R, Longobucco A, Raschi A (1998) Vulnerability of xylem to embolism in relation to plant hydraulic resistance in Quercus pubescens and Quercus ilex co-occurring in a Mediterranean coppice stand in central Italy. New Phytol 139:437–447
Wolfinger RD (1996) Heterogeneous variance-covariance structures for repeated measures. J Agric Biol Environ Stat 1:205–230
Acknowledgments
This study was supported by the Department of Innovation, Science and Business of the Regional Government of Andalusia (Spain) and the European Union (FEDER funds; ref: C03-192), and by the Ministry of Science and Innovation of Spain and the National Agriculture Research Institute (INIA; ref: SUM2006-00026-00-00). Currently E. Andivia is beneficiating of a postdoctoral grant supported by OP Education for Competitiveness (European Social Fund and the Czech Ministry of Education, Youth and Sport; ref: CZ.1.07/2.3.00/30.0017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andivia, E., Vázquez-Piqué, J., Fernández, M. et al. Litter production in Holm oak trees subjected to different pruning intensities in Mediterranean dehesas. Agroforest Syst 87, 657–666 (2013). https://doi.org/10.1007/s10457-012-9586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-012-9586-5