Abstract
Although aerobiological data are often used in phenological research as an indicator of flowering, airborne pollen concentrations are influenced by a number of factors that could affect pollen curves. This paper reports on a study of various aspects of reproductive biology in Q. ilex subsp. ballota, together with environmental factors influencing pollen release and transport, with a view of achieving reliable interpretation of Quercus pollen curves in Ourense (NW Spain). Aerobiological data were recorded from 2002 to 2004 at two sites in the province of Ourense. From 1st February to the end of the flowering period, phenological observations were carried out on 19 trees from the Q. ilex subsp. ballota population found in the Ourense area. Pollen production was calculated for the same trees. The chilling and heating requirements for triggering development were also calculated. The mean flowering period lasted 11-15 days. Reduced pollen output per catkin and, especially, a reduced number of catkins per tree in 2003 and 2004, prompted a marked decline in overall pollen production. Major differences observed in Q. ilex subsp. ballota pollen curves were attributed to the considerable influence both of weather conditions during pollination and pollen production. In years with high pollen production and weather conditions favouring pollen release, Q. ilex subsp. ballota contributed almost 10% to the total Quercus pollen curve. Around 20% of the pollen trapped was captured before or after flowering periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Knowledge of pollen production can be very useful in determining the maximum amount of pollen that a population can spread. This can be used to help to establish an airborne forecasting system (Hidalgo, Galán, & Domínguez, 1999) and to assess the relative importance of every taxa included in a given pollen type. Quercus (L.) pollen production is very high, although it varies by species; findings for the number of pollen grains per flower range from 1.9 × 104 to 1.3 × 106 (Moore & Webb, 1983; Tormo, Muñoz, Silva, & Gallardo, 1996). Quercus is considered a moderate cause of pollinosis in many areas of Europe, especially those with high pollen counts. In Northwest Spain, between 4% and 12% skin sensitisation to Quercus pollen has been reported among pollen-allergy sufferers (Belmonte, Roure, & March, 1998; Ferreiro, Núñez, Rico, Soto, & López, 1998). Symptoms are often recorded over prolonged periods, due both to the overlapping of flowering in the various species present in the environment and cross-reactivity with the pollen of Alnus, Betula, Castanea, Olea and Poaceae (Ickovic & Thibaudon, 1991). The highest Quercus pollen counts in Galicia (Northwest Spain) are recorded in the areas displaying the greatest diversity of Quercus species, i.e., at the confluence of the Eurosiberian and Mediterranean areas, where daily mean pollen counts exceed 200 pollen grains/m3 and annual values are over 2,000 pollen grains (Aira, Jato, & Iglésias, 2005). The study of Quercus pollen data is not only useful because of their allergenic potential but also essential for evaluating the ecological conservation of Spain’s woodland. Possible changes in the distribution of oak due to hotter spring temperatures over recent years (Emberlin et al., 1997; Corden & Millington, 1999) are of major economic importance to the timber and livestock industries.
In aerobiological studies, the Quercus pollen curve usually represents total Quercus pollen: pollen released by all the species to be found in the area. The contribution of each species is governed not only by its local abundance but also by its pollen production, the distance from the pollen source to the pollen trap and the possibility of pollen transport over long and medium distances.
Pollen curve patterns do not always match those of the flowering phenophase, and a lack of correlation may be observed between flowering dates and pollen concentrations (Latorre, 1997). Moreover, a given pollen type may include different species. Knowledge of the flowering sequence, abundance and pollen production of each species is therefore essential in order to assess its contribution to the overall pollen curve in space and time. Moreover, aerobiological research must take into account not only phenological data but also other environmental variables, including topographical and weather-related factors, the ability of the pollen grain to be transported over long distances, pollen production and distance from the source to the sampler.
Temperature is the factor exerting the clearest influence on the onset of flowering, especially in tree species that flower at the start of spring. Q. ilex subsp. ballota (Desf.) Samp., is cited as the most relevant example of adaptation to different climatic and environmental conditions (Garcia-Mozo et al., 2002b). Identification of the chilling and heating required to overcome the period of dormancy enables the determination of the onset of pollination (Faust, 1989; Frenguelli & Bricchi, 1998; Galán, Cariñanos, García-Mozo, Alcázar, & Domínguez, 2001; Richardson, Seeley, & Walker, 1984). Since the presence of pollen in the air is the result of flowering, aerobiological data may be used to calculate these chilling and heating requirements (Frenguelli & Bricchi, 1998; Garcia-Mozo, Galán, Gomez-Casero, & Domínguez-Vilches, 2000, Garcia-Mozo et al., 2002b; Rodríguez-Rajo, Méndez, & Jato, 2000; Galán et al., 2001; Jato, Rodríguez-Rajo, Dacosta, & Aira, 2004). Over the last few years, prompted by the growing importance of global warming, a certain amount of phenological research has been carried out using aerobiological data to determine the timing of flowering (Chuine, Cour, & Rousseau, 1998; Emberlin et al., 1997; Emberlin et al., 2002; Galán et al., 2005; Garcia-Mozo, Galán, & Domínguez-Vilches, 2002a). Using long aerobiological data series, it is possible to chart delays or advances in the onset of pollination in the major wind-pollinated species. However, in order to achieve a full understanding of the pollen curve and thus ensure the correct application of pollen data, it is essential to take into account the phenology and reproductive biology of the plants, particularly those in the vicinity of the spore trap.
A previous survey of Quercus phenology was carried out in 2001 at Ourense (Northwest Spain) in order to analyze the phenological behaviour of the various local Quercus species (Jato, Rodríguez-Rajo, Méndez, & Aira, 2002) and to test the match between flowering and airborne pollen counts. Quercus robur L. and Q. pyrenaica Willd. were the largest contributors to the Quercus pollen curve. The heat requirement to trigger flowering in Q. ilex subsp. ballota was similar to that obtained for both Q. robur and Q. faginea Lam. The study found that transported pollen could have a marked effect on Quercus pollen curves. The present study was designed to determine pollen production and reproductive biology of Q. ilex susbp. ballota,, and to evaluate the contribution of this species to the overall Quercus pollen curve.
2 Material and methods
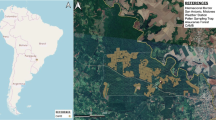
The study was carried out in the city of Ourense, situated in a depression at 139 m above sea level in Northwest Spain (42°20′ N and 7°52′ W), and in Santomé, 220 m above sea level and 3 km from the city of Ourense (Fig. 1). The climate of Ourense is oceanic, with a strong Mediterranean influence. Records for the last 30 years show a mean annual temperature of 14.2°C, maximum average temperature of 20.2°C and minimum average temperature of 8.2°C. Annual rainfall is 794 mm, with a very irregular distribution through the year; average summer rainfall is only 21.6 mm (Martínez & Pérez, 1999).
Location of the studied area and the different samplers in the field monitoring: 1—Ourense: 42°20′41.08′′ N, 7°51′19.62′′ W, 139 m. a.s.l.; 2—Bamio: 42°20′43.98′′ N, 7°50′42.99′′ W, 120 m. a.s.l.; 3—Santomé: 42°20′47.77′′ N, 7°49′48.79′′ W, 220 m. a.s.l.; 4—Santomé Sur: 42°20′44.51′′ N, 7°49′48.73′′ W, 210 a.s.l.; 5—Ceboliño: 42°20′45.87′′ N, 7°49′24.09′′ W, 145 a.s.l
In the Eurosiberian oak forests of the northwestern Iberian peninsula, several deciduous species predominate. The evergreen species Q. ilex L. is widely distributed in the Spanish Mediterranean region, and is thought to display considerable ecological and geobotanic variations. It is found in most Spanish provinces, though it is scarcer in northern and western Spain, in the arid southeast, in high mountains and in extremely continental high moorlands (Costa, Morla, & Sainz, 1998). It includes two subspecies: Q. ilex subsp. ilex L. and Q. ilex subsp. ballota (Desf.) Samp. (=Q. ilex subsp. rotundifolia) (holm oaks). Part of the Ourense province belongs to the Eurosiberian region, while lower-altitude areas and especially the Miño, Sil and Támega river valleys are considered part of the Mediterranean region. Confluence of species representative of both regions is therefore normal in the area. Oak forests predominate in the area surrounding the city, particularly Quercus pyrenaica and Quercus robur. Q. suber L. is found in the lowest areas around the city, while there is only one Q. ilex subsp. ballota population, in the Santomé area. There are also a few ornamental American oaks: Q. rubra L. and Q. palustris Muenchn.
The Q. ilex subsp. ballota population studied here is underdeveloped, due to certain ecological peculiarities prompted by steep slopes and frequent forest fires. Small stands of this species are intermingled with other shrub species.
2.1 Aerobiological survey
Pollen monitoring was conducted in the city of Ourense and in Santomé. In Ourense, pollen data was recorded in 2002, 2003 and 2004 using a seven-day LANZONI VPPS 2000 continuous volumetric pollen trap, located on the roof of the sciences faculty (approximately 20 m above ground level). The methodology recommended by the Spanish Aerobiological Network (REA)—was used (Domínguez, Galán, Villamandos, & Infante, 1992). The onset of the airborne pollen season was defined according to three different criteria: the date on which 2.5% (Andersen, 1991) or 5% (Nilsson & Person, 1981) of annual total pollen was recorded, and the date from which the first pollen grains were recorded on a continual basis with no more than two pollen-free days (Jato et al., 2002). Finally, the day on which the maximum daily mean pollen concentrations were recorded (peak day) was taken as the mean date of anthesis for the population around the sampling station, as suggested by Chuine, Cour, and Rousseau (1999).
Monitoring of airborne Quercus pollen concentrations usually includes all Quercus species, since morphological similarities hinder any ready distinction between them. However, observed differences in the size and, more particularly, the ornamentation of the exine, together with known differences in flowering dates between local Quercus species, enabled Q. ilex subsp. ballota pollen to be distinguished from that of other Quercus species. For the purposes of this study, therefore, the Quercus pollen type includes pollen from all local Quercus species, mainly Q. robur, Q. pyrenaica, Q. ilex subsp. ballota, Q. suber and also Q. faginea and Q. rubra, (both from a very small number of trees), while the Q. ilex subsp. ballota pollen type includes only pollen from this species.
In the area of Santomé (3 km from the city of Ourense), home to the only Q. ilex subsp. ballota population in the Ourense area, an aerobiological survey was carried out over three years using a seven-day LANZONI VPPS 2000 continuous volumetric pollen trap (running from 12:00 h to 20:00 h every day), together with a LANZONI VPPS 1000 and Burkard portable samplers. The location of the pollen trap and the sampling dates were modified every year, as a function of the results obtained in the previous year and the flowering period of Q. ilex subsp. ballota (Fig. 1). Spearman’s correlation test was applied in order to prove the association level between pollen concentrations and meteorological parameters registered in this area.
Pollen dispersion maps were fulfilled by using the Surfer mapping program. The maps were constructed by interpolating the pollen data onto a grid, using the powerful Kriging gridding method that produces grids with up to 100 million nodes.
2.2 Phenological survey
To characterize the floral phenophase, trees were sampled at least once a week from 1 February to the end of flowering, from 2002 to 2004. Nineteen randomly chosen Quercus ilex subsp. ballota trees were sampled. For each tree, the dates of bud development (the bud has increased of size up to 2–4 mm and the catkin is still not distinguished), catkin development (the catkin begins to develop although it measures less than 1 cm and its pedicelum is distinguished), catkin lengthening (the catkins measure 2 cm and the flowers are distinguished although they are still closed), start of flowering (as the first day a male catkin distributed its pollen, Wielgolaski, 1999), and end of flowering (drying-up of all catkins) were noted. The same trees were observed every year.
The average length of the flowering phenophase was limited by the calculated onset and ending of the average flowering date. Moreover the earlier and later dates for the onset and end of flowering mark the total length of the flowering phenophase.
2.3 Pollen production
The methods developed by Cruden (1977) and Hidalgo et al. (1999) were used to calculate pollen production by anther, by flower, by catkin and by tree. The 19 trees randomly chosen for phenological observation were used for this purpose.
Pollen production per anther, catkin and tree, number of flowers per catkin and number of catkin per tree were calculated. Since the study area was confined, it was possible to count the total number of Q. ilex subsp. ballota trees, and thus to quantify total pollen released from this source. A direct count was made of all accessible trees; inaccessible trees were photographed after flowering, and the different colours of the new branches allowed them to be counted. Since trees were underdeveloped due to the specific ecological characteristics of the area, pollen production per square meter was not calculated; it was considered that production might be different in trees of the same species growing in optimal areas.
2.4 Chilling and heat requirements
The model developed by Aron (1983) was used to determine the chill accumulation expressed as chilling hours:
where B = 24D [(7.2 − t min)/(t max − t min)], D is the number of days in the period, and t max and t min are the mean maximum and minimum temperatures, respectively, for the period.
The period selected ranged from the day on which the mean temperature fell below the threshold temperature, to the day (in late December or early January) on which the minimum temperature was recorded and a change in the temperature trend was detected. Only those days on which weather conditions allowed no physiological activity were taken into account (Jato et al., 2002); this occurs when the daily maximum temperature is equal to or less than the threshold temperature (Montero & Gonzalez, 1983). The threshold temperature was set at 7°C, since previous studies have shown this to be the most effective temperature for chilling (Jato et al., 2002).
Two methods for determining the heat requirement were tested taking into account the period from the day after the chill period to the start of the airborne pollen season (using aerobiological data) or the start of each phenophase (using phenological data).
-
(1)
The heat requirement (HR) was calculated as a function of the sum of the daily maximum temperatures (Galán et al., 2001): HR = ∑t max
-
(2)
The heat requirement was also calculated by taking into account the days after chilling when weather conditions enabled plant physiological activity, either totally (t min > t h) or partially (t max > t h > t min) (referred to by Jato et al. (2002) as the vegetative activity or VA). HR was considered as a function of the sum of the difference between the daily maximum temperatures and the threshold temperatures: HR = ∑(t max − t h).
When t max was less than or equal to the threshold temperature (t max < t h), the values were set to 0.
Different threshold temperatures from 6°C to 8°C were tested, in 0.5°C increments, for heat requirement.
2.5 Meteorological data
Weather data for Ourense over the study period were provided by the National Meteorological Institute; weather data during sampling in Santomé were recorded using two Weatherlink portable weather stations.
3 Results
3.1 Phenological results
Using the mean dates of onset and ending, the flowering period lasted between 11 and 15 days, depending on the year. The longest flowering period was in 2002 and the shortest in 2004 (Table 1 and Fig. 2).
The minimum and maximum dates for the onset and end of flowering mark the total duration of the period when the oaks were releasing pollen from the anthers into the atmosphere. For all studied trees as a whole, the pollen-release period in Ourense lasted from late March/early April to late April/early May, depending on the year (Fig. 2).
The standard deviation coefficient was less than 5% in each year, thus showing very homogeneous behaviour with regard to the flowering dates observed.
3.2 Chilling and heat requirements
Taking 7°C as the threshold temperature, the chilling requirement was accumulated in Ourense from 2nd November and 17th to 25th December, respectively, in 2001–2002 and 2003–2004 and from 5th December to 15th January in 2002–2003. Accumulated chilling ranged from 639 to 715 CH.
Heat requirements were calculated for every phenophase; values were lower in 2003 for all phenophases. Average totals of 1,855, 1,409 and 1,932, calculated as ∑t max, were accumulated from the end of chilling to the mean flowering start date in 2002, 2003 and 2004, respectively (Fig. 3). Totals of between 907 (in 2004) and 1,110 (in 2002), also calculated as ∑t max, were accumulated from the start of bud development to flowering onset.
Heat requirements were also calculated taking into account the onset of the Quercus ilex subsp. ballota airborne pollen season, delimited using different criteria and also taking into account the date on which maximum pollen counts were detected, i.e., the peak pollen date (Table 2). Regardless of the method used, values were lower in 2003. The results obtained using the method developed by Galán et al. (2001), which is calculated as a function of the sum of daily maximum temperatures applied to the peak date, most closely matched those obtained using phenological data for flowering onset (Table 2).
3.3 Pollen production
Pollen production per anther was similar in 2002 and 2003, but declined by roughly 25% in 2004 (average 4,646 pollen grains in 2004 versus 6,064 pollen grains in 2003. Pollen production per catkin was similar in 2003 and 2004, but roughly 40% higher in 2002 (777.698, 530.829 and 543.207 pollen grains, respectively, in 2002, 2003 and 2004). The most striking difference, however, was in the number of catkins per tree, which in 2002 was ten times than in 2003 and approximately twice as high as in 2004. Thus, the average number of pollen grains per tree was 2.6 × 1010 in 2002, compared with 1.6 × 109 in 2003 and 6.02 × 109 in 2004 (Fig. 4). The variation in size of the trees studied accounts for the high standard deviation and coefficient of standard deviation for number of catkins per tree and pollen production per tree.
Given the total number of trees in the area (558) and the average pollen production per tree each year, total Q. ilex subsp. ballota pollen production in the area was 14.40 × 1012 pollen grains in 2002, 0.88 × 1012 pollen grains in 2003 and 3.36 × 1012 pollen grains in 2004.
3.4 Aerobiological results
3.4.1 Ourense monitoring station
The pollen trap located in the city of Ourense provided information on pollen captured at a linear distance of 3 km from the source area of Q. ilex subsp. ballota pollen. Total annual Quercus and Q. ilex subsp. ballota pollen counts were highest in 2002 (5,027 and 460 pollen grains, respectively). In 2003 and 2004, very similar values were recorded for total annual Quercus pollen (2,713 and 2,724), while 85 and 50 Q. ilex subsp. ballota pollen grains were identified. Pollen from Q. ilex subsp. ballota accounted for 9.15%, 3.13% and 1.83% of total Quercus pollen in 2002, 2003 and 2004, respectively.
Airborne Quercus pollen was detected in Ourense from mid-March to mid-June, while Q. ilex subsp. ballota pollen was detected from mid-March to mid-May; in both cases, pollen concentrations were highest in April (Fig. 5).
Quercus pollen accounted for 16.6%, 16% and 14.5% of total annual pollen in the three study years, while Q. ilex subsp. ballota accounted for 1.5% in 2002 and <1% in 2003 and 2004. Quercus pollen concentrations of over 100 grains/m3 were recorded over 23 days in 2002, 9 days in 2003 and 7 days in 2004. Maximum daily mean Quercus pollen concentrations—recorded in early April—were 361, 218 and 147 pollen grains/m3 respectively. In the case of Q. ilex subsp. ballota they were 40, 10 and 23 pollen grains/m3, recorded at the middle or end of the same month every year.
The Quercus pollen curve displayed a number of peak counts, especially marked in 2002, separated by periods with low pollen concentrations.
The airborne Quercus pollen season started later in 2002, especially when calculated using the criteria proposed by Jato et al. (2002) (Table 3), and lasted between 65 days and 104 days depending on the year and on the criteria used. Airborne Q. ilex subsp. ballota pollen season, calculated following Jato et al. (2002), lasted 44 days in 2002. Rainfall in 2003 split the pollen season into two periods separated by a five-day interval with no airborne pollen. In 2004, although a few airborne pollen grains were observed in mid-April, the start of the pollen season was delayed until late April, and it lasted only eight days.
3.4.2 Santomé area
In 2002, one Lanzoni VPPS 2000 trap was placed in the Q. ilex subsp. ballota area of Santomé from 15 to 25 April (12:00 h to 20:00 h), and another one was placed halfway between Santomé and Ourense city (Bamio) (Fig. 1). The results obtained (expressed as average concentrations for the 8-h period) indicated a marked gradient in Q. ilex subsp. ballota pollen concentrations (Fig. 6). The maximum concentration was 3664 pollen grains/m3 in Santomé (18 April, i.e., at the start of flowering) and in the same date was recorded in Bamio (74 pollen grains/m3). However the maximum concentration in Ourense (50 pollen grains/m3) was recorded later, on 25 April, i.e., at the end of flowering. Q. ilex subsp. ballota pollen concentrations were higher in Bamio and Ourense city than in Santomé (the source area) at the end of the period. No significant correlation was found between the counts registered at the three sites.
Over six days during the flowering period, two Burkard portable samplers were used at 14:00 h, 17:00 h and 20:00 h for 10 min. The first was used in Santomé area in close proximity to the Q. ilex subsp. ballota trees, while the second was located just across the canyon from Santomé, at a linear distance of 200 m (South Santomé). A marked gradient was observed in pollen counts, and only in four samplings were the pollen concentrations higher in South Santomé than in Santomé (Fig. 7). No significant correlations were found between pollen concentrations and weather-related parameters recorded at the sampling site.
Similar surveys were performed in 2003 and 2004, although the number of samplers and their location were modified in order to widen the study area in other directions and over greater distances (Fig. 1). In both years, rainy days were frequent, and the washing-out effect—together with lower pollen release—prompted a marked decline in airborne pollen production compared with 2002; this hindered interpretation of the data. However, it is noteworthy that the maximum mean counts between 14:00 and 20:00 h in Ourense city in 2003 (9 pollen grains/m3) and Santomé (279 pollen grains/m3) were recorded on the same date at the beginning of the flowering period. However in Ceboliño (located upstream at a linear distance of 200 m from Santomé and at a lower altitude) the mean count was detected eight days later (41 pollen grains/m3 recorded on 23 April), when most Q. ilex subsp. ballota trees had finished flowering. Weather data recorded in Santomé showed three rainy days just after the maximum pollen concentration was recorded; after that, there was a mild wind that constantly changed direction, although was predominantly south or southwesterly (in contrast, over the preceding days the wind had been constantly northerly or northeasterly). This may have favoured turbulence and a decreased limit boundary layer, helping pollen grains to rise from the surfaces where they had previously been deposited by the rain. In 2004, pollen concentrations recorded in Santomé, by means a sampler located at a distance of 50 m from the Q. ilex subsp. ballota stand, were compared with those recorded in South Santomé and Ourense city. The gradient between Santomé and South Santomé was lower than for the other study years. In 2004, the maximum pollen concentration in Ourense city was detected at the end of the flowering period; higher counts were also recorded in Ceboliño at the end of the flowering period in 2004.
Using the results obtained at the various sampling sites, several maps were made showing the pollen concentration gradient (Fig. 8). Every year, the maximum pollen concentrations were recorded either at the start or at the full flowering phenophase in the sampler nearest to the pollen source. However, in the samplers located further upstream, maximum counts were delayed until the end of flowering.
4 Discussion
Knowledge of the flowering phenology and potential pollen-release patterns of the various Quercus species may be of value in assessing the contribution of each species to total airborne Quercus pollen, as well as in predicting pollen counts over the year. One aim of the present study was to measure Quercus ilex subsp. ballota pollen production, in order to evaluate the dispersion and transport potential of this pollen and its contribution to the Quercus pollen curve. Since there is only one small Quercus ilex subsp. ballota population near Ourense (where the pollen trap has been in use since 1993), it was possible to count the total number of trees and thus quantify total pollen production. Identification of Q. ilex subsp. ballota pollen in the Ourense monitoring station enabled a determination of the percentage of pollen coming from the source area.
Of the six Quercus species to be found in Ourense, Q. robur is the first to flower (Jato et al., 2002). Q. ilex subsp. ballota starts to flower a few days later. Q. robur and Q. pyrenaica are the most widespread species in the area and might be expected to be the major sources of airborne Quercus pollen (Jato et al., 2002). This study showed that, despite the small number of trees involved, the contribution of Q. ilex subsp. ballota to the total Quercus pollen count may be significant. Airborne Q. ilex subsp. ballota pollen appears around 20-30 days after atmospheric Quercus pollen season onset. Its contribution to the Quercus pollen curve may be as high as 10% in years with high pollen production and weather conditions that favor the release and dispersal of pollen into the air.
A marked decline in Quercus ilex subsp. ballota pollen per catkin was recorded in 2003. The small size of local Quercus ilex subsp. ballota trees led to extremely low pollen production per tree. Although pollen production per anther appears to be genetically fixed (Subba-Reddi, 1986), total pollen production per plant is also influenced by other factors (Stanley & Linskens, 1974) and varies between years (Rogers, 1993). Calculated pollen production per anther was higher than that reported by Gómez-Casero et al. (2004) for the same species and similar to that cited by Tormo et al. (1996) for Q. rotundifolia. Despite the small size of the trees, pollen production per tree recorded in 2002 was also higher than that reported by Gomez-Casero, Hidalgo, Garcia-Mozo, Domínguez, and Galán (2004). However, considerable year-on-year differences were observed in pollen production per tree, and particularly in number of catkins per tree; these may be due to weather-related factors and to a system of resource allocation similar to that reported for other species (Moe, 1998; Ranta, Oksanen, Hokkanen, Bondestam, & Heino, 2005). The study population grows close to its altitudinal limit, under low-resource conditions that might affect pollen production. Moreover the pathogenic fungi observed on catkins in 2004 undoubtedly influenced total pollen production that year. Fluctuations in pollen production may account for the biannual or triannual cycles widely reported for Quercus pollen (Corden & Millington, 1999; Garcia-Mozo et al., 1999). Here, there was a sharp fall in catkins per tree in 2003 and 2004, which might help to account for the marked decline in Q. ilex subsp. ballota airborne pollen counts for both years; however, this is unlikely to be the sole factor influencing airborne pollen concentrations. Anther dehiscence and pollen dispersal was favored by the warm and dry weather during flowering in 2002, increasing the amount of pollen in the air. If these conditions are not met, anthesis can be delayed or reduced, as happened in 2003 and 2004.
Airborne pollen does not exactly reflect the flowering period (Latorre, 1999). During the mean phenological flowering period, less than 60% of the atmospheric annual total pollen was captured (44% in 2002, 37% in 2003 and 65% in 2004; between 68% and 77% taking into account the total length of flowering of Q. ilex subsp. ballota). This means that over 20% of airborne Quercus ilex subsp. ballota pollen was captured outside of the flowering periods, much of it afterwards. This fact could be a consequence of the earlier flowering of some unstudied individual trees (19 trees were studied from 558 in the population). Weather conditions during flowering strongly influence pollen release and dispersal. Conditions varied considerably in the three study years, especially with regard to rainfall. The weather was sunny and dry (only 14 mm recorded in three days) in the full flowering period (16 April to 1 May) in 2002; however, in 2003 (8–20 April) and 2004 (16–27 April) flowering periods were characterized by abundant rainy days (64 mm were recorded in nine days in 2003 and 20 mm in five days in 2004). Such unfavourable meteorological conditions, especially the abundant rainfall in 2003 and 2004, disturbed pollination and prompted rapid air cleansing and a consequent decline in pollen counts. However, when weather conditions are favorable, airborne pollen remains in the air after flowering, and the atmospheric pollen season may be longer than the flowering period. Here, the atmospheric Q. ilex subsp. ballota pollen season lasted for only eight days in 2004 and 44 days in 2002; however, the total length of the flowering phenophase was 33 days in 2002 and 20 days in 2004.
Quercus pollen morphology appears not to facilitate transport (Mandrioli, Negrini, Cesari, & Morgan, 1984); however some authors report a considerable, though varying, capacity for transport (Ducousso, Michaud, & Lumaret, 1993; Recio, Trigo, Toro, & Cabezudo, 1999; Trigo, Recio, Toro, & Cabezudo, 1997). Weather conditions may play a major role in facilitating transport; here, while only to a limited degree, resuspension would appear to be a possibility, and might account for the increase in Q. ilex subsp. ballota pollen concentrations once flowering finished. Maximum pollen concentrations were recorded at the start of full flowering. However, a recovery of pollen concentrations was noted every year at the end of flowering, and even after the flowering period, especially in the pollen traps located furthest from the source. Turbulence generated by atmospheric changes probably favoured a reduction of the boundary layer, lifting pollen grains previously deposited on the ground into the air and depositing them close to the source in conditions of low wind speeds.
Local topography may also play an important role in the transport and deposition of pollen grains (Hjelmroos, 1991). Khanduri et al. (2002) have suggested that the long-distance transport of Pinus roxburghii pollen is easier downhill than uphill. Similarly, transport upstream appears to have been more favored in the present study, prompting an increase in Q. ilex subsp. ballota pollen counts at the end of flowering periods.
Pollen transport is a complex phenomenon involving various physical factors, such as pollen grain size, density, and morphology, as well as source-related factors (location, structural aspects, pollen production) (Mandrioli, Negrini, & Zanotti, 1982; Mandrioli, Magnani, & De Nuntiis, 1999) and weather conditions (wind speed and direction, temperature and relative humidity). The presence of turbulence prompted by heating of the air modifies the vertical structure of the atmosphere helping to raise pollen and/or prompting its deposition (Giostra, Mandrioli, Tampieri, & Trombetti, 1991). Here, a marked gradient in pollen concentrations was recorded over the short distance from the source to the pollen trap located in Ourense city. The percentage of Q. ilex subsp. ballota pollen captured in this sampler in relation to total pollen production was 0.32 × 10−7% in 2002, 0.97 × 10−8% in 2003 and 0.15 × 10−8% in 2004. A correlation was noted between years with low pollen production and low pollen counts. However, the stable weather during flowering in 2002, with warm, sunny days, helped to keep pollen airborne and thus the percentage of pollen captured was higher. These findings serve to underline the influence of weather-related factors—particularly rainfall, which was more abundant and continued during full flowering and the end of flowering in 2002 and 2003—on airborne pollen concentrations.
5 Conclusions
Airborne pollen counts do not always match flowering periods, and considerable amounts of pollen may be captured before or after flowering periods. When pollen curves include pollen from different species, studies should be carried out to determine the flowering sequence and the contribution of each species to overall pollen counts. For an adequate interpretation of pollen counts, weather conditions, topographic and phenological factors, together with possible transport of pollen, should be taken into account. Phenological studies should be made of major wind-pollinated tree species in order to improve the interpretation of airborne pollen data.
References
Aira, M. J., Jato, V., & Iglesias, I. (2005). Calidad del aire. Polen y esporas en la Comunidad Gallega. Xunta de Galicia. Santiago de Compostela.
Andersen, B. A. (1991). A model to predict the beginning of the pollen season. Grana, 30, 269–275.
Aron, R. (1983). Availability of chilling temperatures in California. Agricultural Meteorology, 28, 351–363.
Belmonte, J., Roure, J. M., & March, X. (1998). Aerobiology of Vigo, North-Western Spain: Atmospheric pollen spectrum and annual dynamics of the most important taxa, and their clinical importance for allergy. Aerobiologia, 14, 155–163.
Chuine, I., Cour, P., & Rousseau, D. D. (1998). Fitting models predicting dates of flowering of temperate-zone trees using simulated annealing. Plan Cell and Environment, 21, 455–466.
Chuine, I., Cour, P., & Rousseau, D. D. (1999). Selecting models to predict the timing of flowering temperate trees: implications for tree phenology modelling. Plant Cell and Environment, 22, 1–13.
Corden, J., & Millington, W. (1999). A study of Quercus pollen in Derby area. Aerobiologia, 15, 29–37.
Costa, M., Morla, C., & Sainz, H. (Eds.). (1998). Los bosques ibéricos. Ed. Planeta, Barcelona.
Cruden, R. W. (1977). Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution, 31, 32–46.
Domínguez, E., Galán, C., Villamandos, F., & Infante, F. (1992). Handling and evaluation of the data from the aerobiological sampling. Monografías Rea, 1, 1–18.
Ducousso, A., Michaud, H., & Lumaret, R. (1993). Reproduction and gene flow in the genus Quercus L. Ann Scic For, 50(suppl), 91–106.
Emberlin, J., Detandt, M., Gehrig, R., Jaeger, S., Nolard, N., & Rantio-Lehtimäki, A. (2002). Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperature across Europe. International Journal of Biometeorology, 46, 159–170.
Emberlin, J., Mullins, J., Corden, J., Millington, W., Brooke, M., Savage, M., & Jones, S. (1997). The trend to earlier Birch pollen seasons in the U.K.: A biotic response to changes in weather conditions? Grana, 36(1), 29–33.
Faust, M. (1989). Physiology of temperate zone fruits trees. Chichester, New York: John Wiley & Sons.
Ferreiro, M., Núñez, M., Rico, A., Soto, T., & López, R. (1998). Pólenes alergénicos y polinosis en el área de A Coruña Rev. Esp de Alergol. e Inmunol. Clín, 13, 98–101.
Frenguelli, G., & Bricchi, E. (1998). The use of pheno-climatic model for forecasting the pollination of some arboreal taxa. Aerobiologia, 14, 39–44.
Galán, C., Cariñanos, P., García-Mozo, H., Alcázar, P., & Domínguez, E. (2001). Model for forecasting Olea europaea L. airborne pollen in South-West Andalucía, Spain. International Journal of Biometeorology, 45, 59–63.
Galán, C., García-Mozo, H., Vázquez, L., Ruiz, L., Díaz de la Guardia C., & Trigo, M. M. (2005). Heat requirement for the onset of the Olea europaea L. pollen season in several sites in Andalusia and the effect of the expected future climate change. International Journal of Biometeorology, 49, 184–188.
Garcia-Mozo, H., Galán, C., Cariñanos, P., Alcázar, P., Méndez, J., Vendrell, M., Alba, F., Sáenz, C., Fernández, D., Cabezudo, B., & Domínguez, E. (1999). Variations in the Quercus sp. pollen season at selected sites in Spain. Polen, 10, 59–69.
Garcia-Mozo, H., Galán, C., Gomez-Casero, M. T., & Domínguez-Vilches, E. (2000). A comparative study of different temperature accumulation methods for predicting the start of the Quercus pollen season in Córdoba (South West Spain). Grana, 39, 194–199.
Garcia-Mozo, H., Galán, C., & Domínguez-Vilches, E. (2002a). The impact of future climate change in the start of Quercus flowering in the Iberian Peninsula. Quaternary climatic changes and environmental crises in the Mediterranean Region, July, 2002. Universidad de Alcalá de Henares. Madrid, 1–7.
Garcia-Mozo, H., Galán, C., Aira, M. J., Belmonte, J., Díaz de la Guardia, C., Fernández, D., Gutierrez, A. M., Rodríguez, F. J., Trigo, M. M., & Domínguez-Vilches, E (2002b). Modelling start of oak pollen season in different climatic zones in Spain. Agricultural and Forest Meteorology, 110, 247–257.
Giostra, U., Mandrioli, P., Tampieri, F., & Trombetti, F. (1991). Model for pollen immission and transport in the evolving convective boundary layer. Grana, 30, 210–214.
Gomez-Casero, M. T., Hidalgo, P., Garcia-Mozo, H., Domínguez, E., & Galán, C. (2004). Pollen biology in four Mediterranean Quercus species. Grana, 43, 22–30.
Hidalgo, P. J., Galán, C., & Domínguez, E. (1999). Pollen production of the genus Cupressus. Grana, 38, 1–5.
Hjelmroos, M. (1991). Evidence of long-distance transport of Betula pollen. Grana, 30, 215–228.
Ickovic, M., & Thibaudon, M. (1991). Allergenic significance of Fagaceae pollen. In: G. D’Amato, F. Spieksma, & S. Bonini (Eds.), Allergenic pollen and pollinosis in Europe. Oxford.
Jato, V., Rodríguez-Rajo, F. J., Méndez, J., & Aira, M. J. (2002). Phenological behaviour of Quercus in Ourense (NW Spain) and its relationship with the atmospheric pollen season. International Journal of Biometeorology, 46, 176–184.
Jato, V., Rodríguez-Rajo, F. J., Dacosta, N., & Aira, M. J. (2004). Heat and chill requirements of Fraxinus flowering in Galicia (NW Spain). Grana, 43, 217–223.
Khanduri, V. P., & Sharma, C. M. (2002). Pollen production, microsporangium dehiscence and pollen flow in Himalaya cedar (Cedrus deodora Roxb ex D. Don). Annals of Botany, 89(5), 587–593.
Latorre, F. (1997). Comparison between phenological and aerobiological patterns of some arboreal species of Mar del Plata (Argentina). Aerobiologia, 13, 49–59.
Latorre, F. (1999). Differences between airborne pollen and flowering phenology of urban trees with reference to production, dispersal and interannual climate variability. Aerobiologia, 15, 131–141.
Mandrioli, P., Magnani, P., & De Nuntiis, P. (1999). Mesoscale transport of Castanea pollen and its survey in Italy. Paper presented in the Int. Symposium on Castanea. Lugano, Switzerland, pp. 25–26.
Mandrioli, P., Negrini, M. G., Cesari, G., & Morgan, G. (1984). Evidence for long transport of biological and anthropogenic aerosol particles in the atmosphere. Grana, 23, 43–53.
Mandrioli, P., Negrini, M. G., & Zanotti, A. L. (1982). Airborne pollen from the Yugoslavian coast to the Po Valley (Italy). Grana, 21, 121–128.
Martínez, A., & Pérez, A. (1999). Atlas climático de Galicia. Santiago de Compostela, España: Xunta de Galicia.
Moe, D. (1998). Pollen production of Alnus incana at this south Norwegian altitudinal ecotone. Grana, 37, 35–39.
Montero, J. L., & González, J. L. (1983). Diagramas bioclimáticos. Madrid: Instituto Nacional para la conservación de la Naturaleza.
Moore, P. P., & Webb, J. A. (1983). An illustrated guide to Pollen Analysis 2. Great Britain: Blackwell Sci Publ.
Nilsson, S., & Persson, S. (1981). Tree pollen spectra in the Stockholm region (Sweden), 1973–1980. Grana, 20, 179–182.
Ranta, H., Oksanen, A., Hokkanen, T., Bondestam, K., & Heino, S. (2005). Masting by Betula-species; applying the resource budget model to north European data set. International Journal of Biometeorology, 49, 146–151.
Recio, M., Trigo, M. M., Toro, F. J., & Cabezudo, B. (1999). Estimate and mapping of the activity of airborne pollen sources. Aerobiologia, 8, 69–74.
Richardson, E. A., Seeley, S. D., & Walker, D. R. A. (1984). Model for estimating the completion of rest for “Redhaven” and “Elverta” peach trees. HortoScience, 9(3), 331–332.
Rodríguez-Rajo, F. J., Méndez, J., & Jato, V. (2000). Influencia de la temperatura en la floración de Quercus en el sur de Galicia (Ourense y Vigo, 1994–98). Acta Bot Malacitana, 25, 153–163.
Rogers, C. A. (1993). Application of aeropalynological principles in Palaeoecology. Review of Paleobotany and Palynology, 79, 133–140.
Stanley, R. G., & Linskens, H. F. (1974). Pollen biology, biochemistry, management. New York: Springer-Verlag, Berlin, Heidelberg.
Subba-Reddi, C., & Reddi, N. S. (1986). Pollen production in some anemophilous angiosperms. Grana, 25, 55–61.
Tormo, R., Muñoz, A., Silva, I., & Gallardo, F. (1996). Pollen production in anemophilous trees. Grana, 35, 38–46.
Trigo, M. M., Recio, M, Toro, F. J., & Cabezudo, B (1997). Intradiurnal fluctuations in airborne pollen in Málaga (S Spain): A quantitative method. Grana, 36, 39–43.
Wielgolaski, F. E. (1999). Starting dates and basic temperatures in phenological observations of plants. International Journal of Biometeorology, 42, 158–168.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jato, V., Rodríguez-Rajo, F. & Aira, M. Use of Quercus ilex subsp. ballota phenological and pollen-production data for interpreting Quercus pollen curves. Aerobiologia 23, 91–105 (2007). https://doi.org/10.1007/s10453-006-9046-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-006-9046-7