Abstract
Background
Vasculogenic mimicry (VM) is the formation of vascular channels by tumor cells or tumor cell-derived, trans-differentiated cells in highly aggressive, solid tumors. However, the disease features and prognostic value of VM for overall survival of cancer patients remain controversial.
Method
To systematically investigate the roles of VM in cancer progression and its prognostic values, we performed a meta-analysis based on 36 studies (33 eligible articles) including 3609 patients. The pooled hazard ratios (HRs) with 95 % confidence intervals (95 % CIs) were used to assess the relationship between VM and overall survival in cancer patients.
Results
Vasculogenic mimicry was significantly associated with cancer differentiation, lymph node metastasis, distant metastasis, and TNM stage. The prognostic value of VM was significant in overall survival (HR 2.16; 95 % CI 1.98–2.38; P < 0.001). Analyses stratified by confounders, such as cancer type, ethnicity, VM detection methods, sample size, and Newcastle–Ottawa quality score, found similar significant results.
Conclusions
The presence of VM predicts poorer survival outcomes in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
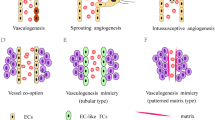
The tumor vasculature system supplies blood for tumor growth and hematogenous dissemination, which have long been regarded as hallmarks of tumorigenesis [1, 2]. The well-known theory of vascularization in tumors is a complex process that involves angiogenesis (sprouting new vessels from existing vessels), vasculogenesis (recruiting circulating endothelial progenitor cells), co-option (hijacking of the existing vasculature) [3–6], and tumor cell-derived vasculature development known as vasculogenic mimicry (VM) [6–9].
Tumor VM refers to the plasticity of highly aggressive cancer cells that imitate endothelial cells and form vessel-like structure with the characteristic of positive PAS and negative endothelial cell markers, including CD31 or CD34 [8]. VM has been identified in many malignancies, such as melanoma [7, 10–13], gastric cancer [14–17], sarcoma [10, 18], ovarian tumor [19–21], hepatocellular carcinoma [22, 23], breast cancer [24, 25], head and neck cancer [26, 27], colorectal cancer [28, 29], glioma [30–33], lung cancer [34, 35], testicular germ cell malignant tumors [36], gallbladder cancer [37], prostate cancer [38], esophageal cancer [39], renal carcinoma [40], and osteosarcoma [41].
Vasculogenic mimicry may be important in the biological behavior of various tumors because it can establish an adequate vascular supply to enhance tumor growth and metastasis [8]. Growing evidence supports the notion of VM as a prognostic factor for poor clinical outcomes in various types of cancer, such as melanoma [7, 10, 42], sarcoma [10], ovarian tumor [20, 21], hepatocellular carcinoma [22, 23], and breast cancer [24, 25]. Nonetheless, some of these studies have not confirmed the association between VM and cancer survival [10, 12, 18, 19, 38, 43]. As a result, the prognostic value of VM in tumors is still inconclusive.
Therefore, we conducted a meta-analysis to determine the prognostic value of VM on overall survival in cancer patients.
Methods
Search strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [44–46]. Relevant articles were identified from PubMed, EMBASE, and the Web of Science databases with the following keywords: “cancer,” “tumor,” “neoplasm,” “vasculogenic mimicry,” “vascular mimicry,” “VM,” “tumor cell-lined vessels,” “tumor derived endothelial cells,” “prognosis,” “survival,” and “outcome.” The last search was performed up to March 10, 2015, without restriction on language. All relevant publications in reference lists were also searched manually to find the additional eligible articles.
Selection criteria
To be included, studies had to have met several criteria: (1) the diagnosis of cancer must have been confirmed histologically or pathologically; (2) the association between VM and overall survival (OS) or disease features, such as tumor differentiation, TNM stages, lymph node metastasis, and distant metastasis, had to be evaluated; and (3) data had to be sufficient to estimate hazard ratios (HRs) for overall survival and risk ratios (RRs) for disease features. In case of duplicated articles [10, 47], only the most complete ones were included [10]. Narrative reviews, abstracts, letters, comments, editorials, and case reports were excluded.
Data extraction
Two investigators (JP Yang and YD Liao) separately extracted the data on authors, year of publication, races (Asian or non-Asian), tumor types, VM assay methods, disease information (including cancer differentiation, TNM stages, lymph node metastasis, and distant metastasis), sample sizes, and number of VM-positive or VM-negative patients. HRs with 95 % CIs were extracted from multivariable analyses. When HRs and 95 % CIs could not been directly extracted from the original studies, they were calculated using the method of Tierney et al. [45].
Two investigators assessed the quality of included studies using the Newcastle–Ottawa scale [48]. This scale consists of eight items assessing three aspects of a study: patient selection, comparability of study groups, and ascertainment of outcome. Each item could be awarded 1 point except for the item on comparability, which allowed 2 points. Studies with scores of five to nine were considered high quality; those with scores of zero to four were considered low quality.
Tumor differentiation grade was categorized as well (grade 1) or moderately/poorly differentiated (grades 2 and 3). TNM stage was dichotomized as I and II or III and IV. Lymph node metastasis and distant metastasis were both scored as positive or negative, respectively. Discrepancies were resolved by consensus.
Statistical methods
Hazard ratios (HRs) and 95 % CIs were used to evaluate the impact of VM on overall survival [49, 50]. In addition, risk ratios were used to summarize the association between VM and disease features: TNM stage, differentiation, lymphatic metastasis, and distant metastasis [49, 50]. The heterogeneity of pooled HRs and RRs was estimated using the Chi-square-based Cochrane’s Q test (P < 0.10 was considered significant) and the I 2 index [51]. When heterogeneity was not so severe (I 2 values <50 %), a fixed-effect model was used. Otherwise, the random-effect model was applied to pooled data. A Galbraith plot was constructed to identify the main sources of heterogeneity [52].
Sensitivity analysis that assesses the stability of results was conducted by sequentially removing each individual study and recalculating the results [53]. Cumulative meta-analysis was conducted by publication times [54]. Publication bias was evaluated by the Begg’s funnel plot and the Egger’s test [55, 56].
All data were analyzed with the STATA software (version 12.0; STATA Corporation, College Station, TX). Unless otherwise stated, all P values were two-tailed and alpha was 0.05.
Results
Study characteristics
Of the 1056 articles identified in the literature search, 36 studies (described in 33 articles) representing 3609 patients were included in the meta-analysis (Fig. 1) [7, 10, 12, 14–31, 34–43, 57]. The most common cancers studied were melanoma and gastric cancers (Table 1). Vascular mimicry was detected in 8–56 % of patients. All but four studies were of Asian populations. Methods for detecting VM were PAS (n = 7), CD31/PAS (n = 14), CD34/PAS (n = 14), and pan-cytokeratin/CD34 (n = 1) staining. Of the 36 studies, 18 (50 %) were high-quality studies (Table 1) [12, 15–17, 20, 25, 27, 30, 31, 34, 37–41, 57]. Hazards ratios with 95 % CIs were extracted directly from 13 studies [14, 16, 17, 20, 23, 25, 34, 36, 37, 40, 41, 57] and calculated for the remaining 23.
Disease features associated with vascular mimicry
Several studies evaluated associations between VM and cancer differentiation status (14 studies [15, 17, 19, 21, 23, 25, 27, 29, 30, 34, 37, 39, 40, 42]; 2032 patients), lymph metastasis (10 studies [15, 17, 24, 25, 27, 34, 35, 37–39]; 1735 patients), distant metastasis (13 studies [14, 15, 18, 19, 21, 22, 24, 27, 36–38, 40, 42]; 1616 patients), and TNM stage (13 studies [15, 17, 19, 21, 23, 25, 27, 29, 34, 35, 37, 39, 40]; 1915 patients).
We found statistically significant associations between VM and cancer differentiation (well vs. moderate/poor: RR: 1.08; 95 % CI 1.04–1.12; p < 0.001), lymph node metastasis (N1 vs. N0: RR: 1.81; 95 % CI 1.43–2.29; p < 0.001), distant metastasis (M1 vs. M0: RR: 1.94; 95 % CI 1.56–2.41; P < 0.001), TNM stage (III/VI vs. I/II: RR: 1.81; 95 % CI: 1.48–2.23; P < 0.001) (Table 2, Figure S1).
Vascular mimicry and overall survival
The meta-analysis revealed that the risk of mortality was significantly higher for cancer patients with VM than without VM (HR, 2.16; 95 %CI 1.95–2.38; P < 0.001) (Table 3, Fig. 2). There was moderate heterogeneity among studies (P het = 0.055 and I 2 = 29.4 %) (Table 3), so we conducted subgroup analyses according to confounders, such as cancer types, ethnicity, VM detection methods, patient number, and quality score. After stratifying by cancer type, VM was still associated with statistically significantly poorer overall survival from melanoma, gastric cancer, sarcomas, ovarian tumor, hepatocellular carcinoma, breast cancer, head and neck cancer, colorectal cancer, glioma, lung cancer, and other types of cancer (Table 3). After stratifying by ethnicity, the pooled HRs of Asians and non-Asians were 2.13 and 3.45, respectively (Table 3). In the subgroup analysis based on VM detection methods, the pooled HR was 2.25 for PAS staining, 2.11 for CD31/PAS staining, 2.15 for CD34/PAS staining, and 2.84 for pan-cytokeratin/CD34 staining (Table 3). In studies with larger samples (n ≥ 100) and smaller samples (n < 100), the pooled HRs were 2.01 and 2.43, respectively (Table 3). Stratification by quality scores revealed significant associations for both high- and low-quality studies (Table 3).
Test of heterogeneity
We created a Galbraith plot to identify potential sources of heterogeneity. The main contributors to heterogeneity were the studies by Liu and Wang [25, 36] (Fig. 3). When these two studies were omitted, I 2 % decreased from 29.4 to 0 %, and the statistical significance of the combined HRs in the overall and subgroup analyses was not substantially altered (data not shown.)
Galbraith plot analysis of the amount of heterogeneity from all the included studies. The Y-axis shows the ratio of the log HR to its standard error (SE), and the X-axis shows the reciprocal of the SE. At a 2 standard deviation distance parallel to the regression line, the 2 lines create an interval. Studies lacking in heterogeneity would lie within the 95 % confidence interval. Studies by Liu and Wang were identified as potential sources of heterogeneity in a meta-analysis of the effects of vasculogenic mimicry on cancer survival
Sensitivity analysis, cumulative analyses, and publication bias
The pooled HRs remained the same in a sensitivity analysis in which each study was sequentially excluded (Figure S2). Moreover, cumulative meta-analyses suggested that the association has been significant since the first study was published in 1999 (Figure S3). These results indicate stabile and reliable findings. No indication of publication bias was found using Egger’s test (P = 0.82), and the funnel plot seemed symmetrical (Fig. 4), indicating no marked publication bias.
Discussion
Our meta-analysis summarized the results of 36 clinical studies representing 3609 patients. Overall, positive VM status strongly predicted lower overall survival in patients with malignant cancers. Moreover, similar, significant results were found in the subgroup analyses by cancer types, ethnicity, VM detection methods, sample size, and study quality. Additionally, VM was also significantly associated with cancer differentiation, lymph node metastasis, distant metastasis, and TNM stage, which might adversely affect cancer survival.
Tumor growth and hematogenous dissemination depend on adequate blood supply. Tumor vasculature is highly complex and possibly derived from a variety of sources, including angiogenesis [58], vasculogenesis [59–61], co-option [62], and vasculogenic mimicry [8, 63]. In VM, highly aggressive cancer cells form vessel-like structures by their high plasticity, which enables them to function as endothelial-like cells.
Several recent studies [7, 10, 12, 14–31, 34–43, 57] have reported that VM exists in various malignant tumors and is associated with tumor progression, metastasis, treatment failure, and increases death in many [6, 9, 18–23, 38]. Although one meta-analysis [64] has reported on the prognostic value of VM in cancer, it has some shortcomings. Data from one study were published in two articles [10], one of which should have been excluded from the analysis. Despite evidence of remarkable heterogeneity and publication bias, there was no attempt to explore the sources of heterogeneity, which might have compromised the interpretation. Time-to-event outcomes were assessed with relative risks, rather than with hazards ratios. Additionally, 12 new studies [12, 16, 17, 21, 25, 31, 35, 39–42, 57] evaluating the relationship between VM and survival outcomes have been published since September 2013. Thus, it was important to update the meta-analyses. The pooled results of our meta-analysis showed that VM was significantly associated with poor OS in cancer patients. What was more, the presence of VM was also significantly associated with cancer differentiation, lymph node metastasis, distant metastasis, and TNM stage.
We found an association of VM with a more aggressive tumor phenotype. Several underlying mechanisms are possible. First, VM could provide a functional perfusion pathway for rapidly growing tumors and possibly a metastatic escape route by transporting blood from leaky vessels or by connecting with the endothelial-lined vasculature [65]. Second, accumulating evidence [66] indicates that cancer stem cells are involved in VM formation, have been associated with tumor invasion and metastasis, and are therefore important in tumor progression. Third, the formation of VM involves signaling pathways and some factors related to tumor cell migration, invasion, and matrix remodeling, including Notch [67] and Nodal [68] signaling pathways, VE-cadherin [69, 70], EPH receptor A2(EphA2) [71, 72], matrix metalloproteinases [20, 22, 73], and hypoxia-inducible factor-1alpha (HIF-1(alpha)) [37, 39, 69, 74, 75]. Forth, hypoxia can modulate the expression of genes essential for cell viability, tumor growth, metastasis, and VM in cancer [76–78]. These hypoxia-induced VM and VM-associated genes highlight the critical importance of hypoxia in tumor progression. Finally, VM is lined with tumor-derived endothelial-like cells that differ from conventional endothelial cells. Therefore, VM might present with a native resistance to anti-angiogenic compounds, such as bevacizumab, sorafenib, and sunitinib.
Our study has some limitations. The moderate heterogeneity among the studies was a potential problem when interpreting the results, although when the studies by Liu et al. and Wang et al. [25, 36] were deleted from the analysis, our results remained the same. A more accurate pooled HRs analysis with sufficient data was required to adjust for covariates, such as age, sex, histological type, tumor differentiation, TNM stage, lymphatic metastasis, and distant metastasis, if these data were available. Finally, although publication bias was not detected using Begg’s funnel plot and Egger’s test, it always remains a possibility. However, several studies with contradictory results would be required to refute or reverse our findings. Our meta-analysis also has some strengths. Sensitivity analysis revealed no significant difference when any individual article was omitted. Moreover, cumulative meta-analyses demonstrated that inclinations toward the significant association have been evident since the first study in 1999. These analyses indicate that our results are stable and reliable.
We found that VM is associated with a worse prognosis in several different tumor types. Vasculogenic mimicry is also significantly associated with cancer differentiation, lymph metastasis, distant metastasis, and TNM stage. These results suggest that developing strategies against the VM would be a promising therapeutic approach to solid tumors.
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Stacker SA, Achen MG (2013) The VEGF signaling pathway in cancer: the road ahead. Chin J Cancer 32:297–302
Liu J, Huang J, Yao WY, Ben QW, Chen DF et al (2012) The origins of vacularization in tumors. Front Biosci (Landmark Ed) 17:2559–2565
Qin L, Bromberg-White JL, Qian CN (2012) Opportunities and challenges in tumor angiogenesis research: back and forth between bench and bed. Adv Cancer Res 113:191–239
Qian CN (2013) Hijacking the vasculature in ccRCC–co-option, remodelling and angiogenesis. Nat Rev Urol 10:300–304
Qian CN, Tan MH, Yang JP, Cao Y (2016) Revisiting tumor angiogenesis: vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chin J Cancer 35:10
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM et al (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155:739–752
Qiao L, Liang N, Zhang J, Xie J, Liu F et al (2015) Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med 19:315–326
Chen YS, Chen ZP (2014) Vasculogenic mimicry: a novel target for glioma therapy. Chin J Cancer 33:74–79
Sun B, Zhang S, Zhao X, Zhang W, Hao X (2004) Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol 25:1609–1614
Zhang S, Li M, Zhang D, Xu S, Wang X et al (2009) Hypoxia influences linearly patterned programmed cell necrosis and tumor blood supply patterns formation in melanoma. Lab Invest 89:575–586
Van Beurden A, Schmitz RF, Van Dijk CM, Baeten CIM (2012) Periodic acid Schiff loops and blood lakes associated with metastasis in cutaneous melanoma. Melanoma Res 22:424–429
Dunleavey JM, Xiao L, Thompson J, Kim MM, Shields JM et al (2014) Vascular channels formed by subpopulations of PECAM1+ melanoma cells. Nat Commun 5:5200
Sun B, Qie S, Zhang S, Sun T, Zhao X et al (2008) Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol 39:444–451
Li M, Gu Y, Zhang Z, Zhang S, Zhang D et al (2010) Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res 16:259–266
Song YY, Sun LD, Liu ML, Liu ZL, Chen F et al (2014) STAT3, p-STAT3 and HIF-1alpha are associated with vasculogenic mimicry and impact on survival in gastric adenocarcinoma. Oncol Lett 8:431–437
Zhou L, Yu L, Feng ZZ, Gong XM, Cheng ZN et al (2015) Aberrant expression of markers of cancer stem cells in gastric adenocarcinoma and their relationship to vasculogenic mimicry. Asian Pac J Cancer Prev 16:4177–4183
Wang LGY, Zhang S, Sun B (2009) Pilot study and clinical prognosis significance of vasculogenic mimicry in leiomyosarcoma. Tianjin Med J 37:161
Gao Y, Zhao XL, Gu Q, Wang JY, Zhang SW et al (2009) Correlation of vasculogenic mimicry with clinicopathologic features and prognosis of ovarian carcinoma. Zhonghua Bing Li Xue Za Zhi 38:585–589
Yu L, Wu SW, Zhou L, Song WQ (2012) Correlation between bacterial L-form infection, expression of HIF-1alpha/MMP-9 and vasculogenic mimicry in epithelial ovarian cancer. Sheng Li Xue Bao 64:657–665
Sun Q, Zou X, Zhang T, Shen J, Yin Y et al (2014) The role of miR-200a in vasculogenic mimicry and its clinical significance in ovarian cancer. Gynecol Oncol 132:730–738
Sun B, Zhang S, Zhang D, Du J, Guo H et al (2006) Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep 16:693–698
Liu WB, Xu GL, Jia WD, Li JS, Ma JL et al (2011) Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol 28(Suppl 1):S228–S238
Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S et al (2002) Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer 99:821–828
Liu T, Sun B, Zhao X, Li Y, Gu Q et al (2014) OCT4 expression and vasculogenic mimicry formation positively correlate with poor prognosis in human breast cancer. Int J Mol Sci 15:19634–19649
Liu SY, Chang LC, Pan LF, Hung YJ, Lee CH et al (2008) Clinicopathologic significance of tumor cell-lined vessel and microenvironment in oral squamous cell carcinoma. Oral Oncol 44:277–285
Wang W, Lin P, Han C, Cai W, Zhao X et al (2010) Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res 29:60
Baeten CI, Hillen F, Pauwels P, de Bruine AP, Baeten CG (2009) Prognostic role of vasculogenic mimicry in colorectal cancer. Dis Colon Rectum 52:2028–2035
Liu Z, Sun B, Qi L, Li H, Gao J et al (2012) Zinc finger E-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci 103:813–820
Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K et al (2011) Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol 105:173–179
Wang SY, Ke YQ, Lu GH, Song ZH, Yu L et al (2013) Vasculogenic mimicry is a prognostic factor for postoperative survival in patients with glioblastoma. J Neurooncol 112:339–345
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S et al (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153:139–152
Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE et al (2010) Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468:829–833
Wu S, Yu L, Cheng Z, Song W, Zhou L et al (2012) Expression of maspin in non-small cell lung cancer and its relationship to vasculogenic mimicry. J Huazhong Univ Sci Technol Med Sci 32:346–352
Li Y, Sun B, Zhao X, Zhang D, Wang X et al (2015) Subpopulations of uPAR+ contribute to vasculogenic mimicry and metastasis in large cell lung cancer. Exp Mol Pathol 98:136–144
Wang X, Wang L, Gu Y, Zhang S, Zhao X et al (2009) Vasculogenic mimicry in testicular germ cell malignant tumor and its significance for prognosis. Chin J Clin Oncol 36:78–82
Sun W, Shen ZY, Zhang H, Fan YZ, Zhang WZ et al (2012) Overexpression of HIF-1alpha in primary gallbladder carcinoma and its relation to vasculogenic mimicry and unfavourable prognosis. Oncol Rep 27:1990–2002
Liu R, Yang K, Meng C, Zhang Z, Xu Y (2012) Vasculogenic mimicry is a marker of poor prognosis in prostate cancer. Cancer Biol Ther 13:527–533
Chai DM, Bao ZQ, Hu JG, Ma L, Feng ZZ et al (2013) Vasculogenic mimicry and aberrant expression of HIF-lalpha/E-cad are associated with worse prognosis of esophageal squamous cell carcinoma. J Huazhong Univ Sci Technol Med Sci 33:385–391
Zhang Y, Sun B, Zhao X, Liu Z, Wang X et al (2013) Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol 108:414–419
Ren K, Yao N, Wang G, Tian L, Ma J et al (2014) Vasculogenic mimicry: a new prognostic sign of human osteosarcoma. Hum Pathol 45:2120–2129
Zhang X, Liu C, Luo L, Cai X (2013) Vasculogenic mimicry in tongue squamous cell carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 33:593–597
Hillen F, Baeten CI, van de Winkel A, Creytens D, van der Schaft DW et al (2008) Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother 57:97–106
Jang TL, Bekelman JE, Liu Y, Bach PB, Basch EM et al (2010) Physician visits prior to treatment for clinically localized prostate cancer. Arch Int Med 170:440–450
Knobloch K, Yoon U, Vogt PM (2011) Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 39:91–92
Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S (2013) Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 8:e83138
Sun B, Zhang S, Ni C (2005) The clinical significance study of vasculogenetic mimicry in 337 cases of bi-directional differential malignant tumors. Chin J Clin Oncol 32:64–67
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Whitehead A, Whitehead J (1991) A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med 10:1665–1677
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Galbraith RF (1988) A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 7:889–894
Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37:1148–1157
Mullen B, Muellerleile P, Bryant B (2001) Cumulative meta-analysis: a consideration of indicators of sufficiency and stability. Pers Soc Psychol Bull 27:1450–1462
Soeken KL, Sripusanapan A (2003) Assessing publication bias in meta-analysis. Nurs Res 52:57–60
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Qi H, Sun B, Zhao X, Du J, Gu Q et al (2014) Wnt5a promotes vasculogenic mimicry and epithelial-mesenchymal transition via protein kinase Calpha in epithelial ovarian cancer. Oncol Rep 32:771–779
Cao Y (2009) Angiogenesis in malignancy. Semin Cancer Biol 19:277–278
Silvan U, Diez-Torre A, Bonilla Z, Moreno P, Diaz-Nunez M et al (2015) Vasculogenesis and angiogenesis in nonseminomatous testicular germ cell tumors. Urol Oncol 33(268):e217–e268
Brown JM (2014) Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br J Radiol 87:20130686
Qing YL, Sha TH, Ji FY, Qin HK, Jun CX et al (2009) Angiogenesis, vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J Gynecol Obstet 107:S242
Pezzella F, Harris AL (2014) When cancer co-opts the vasculature. N Engl J Med 370:2146–2147
Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM et al (2012) Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol 181:1115–1125
Cao Z, Bao M, Miele L, Sarkar FH, Wang Z et al (2013) Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta-analysis. Eur J Cancer 49:3914–3923
Wagenblast E, Soto M, Gutierrez-Angel S, Hartl CA, Gable AL et al (2015) A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 520:358–362
Yao XH, Ping YF, Bian XW (2011) Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell 2:266–272
Vartanian A, Gatsina G, Grigorieva I, Solomko E, Dombrovsky V et al (2013) The involvement of Notch signaling in melanoma vasculogenic mimicry. Clin Exp Med 13:201–209
McAllister JC, Zhan Q, Weishaupt C, Hsu MY, Murphy GF (2010) The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. J Cutan Pathol 37(Suppl 1):19–25
Tang N, Shi H, Zhang J (2013) HIF-1alpha induces expression of VE-cadherin and modulates vasculogenic mimicry in ESCC cells. J Gastroenterol Hepatol 28:863
Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor REB et al (2006) VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther 5:228–233
Wang W, Lin P, Sun B, Zhang S, Cai W et al (2014) Epithelial-mesenchymal transition regulated by EphA2 contributes to vasculogenic mimicry formation of head and neck squamous cell carcinoma. Biomed Res Int 2014:803914
Margaryan NV, Strizzi L, Abbott DE, Seftor EA, Rao MS et al (2009) EphA2 as a promoter of melanoma tumorigenicity. Cancer Biol Ther 8:275–284
Lu XS, Sun W, Ge CY, Zhang WZ, Fan YZ (2013) Contribution of the PI3 K/MMPs/Ln-5gamma2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. Int J Oncol 42:2103–2115
Shi R, Jin HL, Zhang HJ (2011) Effect of RNA interference targeting for HIF-1(alpha) on vasculogenic mimicry in esophageal squamous cell carcinoma. Gastroenterology 140:S827
Comito G, Calvani M, Giannoni E, Bianchini F, Calorini L et al (2011) HIF-1alpha stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radic Biol Med 51:893–904
Du J, Sun B, Zhao X, Gu Q, Dong X et al (2014) Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol 133:575–583
Osinsky S, Zavelevich M, Vaupel P (2009) Tumor hypoxia and malignant progression. Exp Oncol 31:80–86
Sun B, Zhang D, Zhang S, Zhang W, Guo H et al (2007) Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett 249:188–197
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81472386, 81272340, and 81572901), the National High Technology Research and Development Program of China (863 Program) (No. 2012AA02A501), and the Science and Technology Planning Project of Guangdong Province, China (Grant Nos. 2014B020212017 and 2014A020209024).
Author information
Authors and Affiliations
Corresponding author
Additional information
J. P. Yang, Y. D. Liao and D. M. Mai have contributed equally to this work and should be considered as co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, J.P., Liao, Y.D., Mai, D.M. et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: a meta-analysis. Angiogenesis 19, 191–200 (2016). https://doi.org/10.1007/s10456-016-9500-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-016-9500-2