Abstract
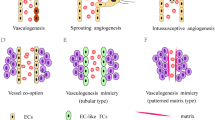
Vasculogenic mimicry (VM), including tubular VM and patterned matrix VM, has been generally recognized as a new pattern of tumor neovascularization. Pilot studies of tubular VM showed that it was present in hepatocellular carcinoma (HCC) and associated with poor clinical prognosis. However, whether patterned matrix VM is clinically significant in HCC is unknown. To elucidate the effects of patterned matrix VM on prognosis of HCC and the mechanisms involved in VM formation, we examined 151 cases of surgically resected human HCC by immunohistochemistry and transmission electron microscopy and conducted hypoxic experiments on human HCC cell line MHCC97-H. We observed 31 of 151 (20.5%) cases exhibited evidence of patterned matrix VM. The expression of patterned matrix VM was associated with larger tumors (P = 0.042), vascular invasion (P = 0.016), high-grade HCC (P = 0.022), and late-stage HCC (P = 0.013). Kaplan–Meier survival analysis revealed that cases of the VM group had lower overall survival (OS) rate (P < 0.001) and disease-free survival (DFS) rate (P = 0.002) than that of the non-VM group. Univariate and multivariate analysis indicated that the presence of patterned matrix VM was independent adverse prognostic factor for both OS (P = 0.004) and DFS (P = 0.011). Expression of hypoxia-inducible factor 1 alpha (HIF-1alpha), matrix metalloproteinase (MMP)-2, and MMP-9 were higher in the VM group than in the non-VM group (P = 0.001, P = 0.030, P = 0.007, respectively). After VM formation was induced by hypoxia, up-regulated expression of HIF-1α, MMP-2, and MMP-9 was also detected in cells cultured under hypoxia condition. Our results indicate that patterned matrix VM exists in HCC, and it might serve as an unfavorable prognostic factor for HCC patients. It is possible that hypoxia via induction of expression of HIF-1alpha, MMP-2, and MMP-9 may enhance VM formation in HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer progression and metastasis depend on recruitment of new capillaries from preexisting vessels, a process known as angiogenesis [1]. Therefore, tumors with high vascularity are frequently associated with aggressive behavior and poor prognosis. In addition to the well-studied angiogenesis, recent reports have revealed several new patterns by which tumor tissues nourish themselves, including the pattern of mosaic vessels from both endothelium and tumor cells [2, 3] and the pattern of vessels lined exclusively with tumor cells mimicking the presence and function of endothelial cells. This last process is termed vasculogenic mimicry (VM) [4]. So far, two distinctive types of VM have been described. In VM of the tubular type, non-endothelial cell-lined tubes resembling blood vessels are identified. In VM of the patterned matrix type, sheaths of extracellular matrix rich in laminin, collagens IV and VI, and fibronectin form loops surrounding packets of tumor cells [5, 6]. The VM has been observed in many tumors, including melanoma [4, 7, 8], breast cancer [9, 10], prostate cancer [11], ovarian carcinoma [12], multiple myeloma [13], Ewing sarcoma [14], osteosarcoma [15], glioblastoma [16], gastric adenocarcinoma [17], colorectal cancer [18], clear cell renal cell carcinoma [19], and acute leukemic bone marrow stromal cells [20]. The presence of VM has been associated with more aggressive tumor biology and increased tumor-related mortality in some tumors [5, 7, 14, 18, 19, 21].
There are limited data regarding VM in hepatocellular carcinoma (HCC). “A partial vasculogenic mimicry”, observed in a transgenic mouse model of HCC as early as 2003 by Dupuy et al. [22], appeared to be mosaic vessels, because the tumoral sinusoids described by them were lined by endothelial cells and HCC cells inserted between these endothelial cells. Perhaps for the first time, the team led by Sun [21, 23] identified tubular VM in human HCC by immunohistochemical and periodic acid-Schif histochemical double-staining. In 2007, evidence for patterned matrix VM was reported in human HCC; however, the pilot study on the VM only included 20 HCC patients who underwent orthotopic liver transplantation [6]. Therefore, patterned matrix VM should be subject to validation in larger patient samples for its true existence and clinical significance.
The microenvironment within tumors is significantly different from that of normal tissues. A major difference is that many regions within tumors are hypoxic; blood supply is insufficient because of structurally defect vessels and tumor cells that grow faster than the developing tumor vascularity [24]. The transcriptional response of tumor cells to hypoxia relies on multiprotein complexes regulated by several transcription factors, the most well studied of which is hypoxic-inducible factor-1α (HIF-1α), which then enhances the expression of the hypoxia-responsive gene and, thus, allows improved cell survival in conditions of limited oxygen availability [25]. Although the mechanistic association between hypoxia and VM formation has been studied in several tumors [14, 26], its effect on VM formation in HCC has not been reported.
The objective of the current study was to investigate whether patterned matrix VM occurred and has a prognostic impact in HCC. Further tissue samples detection and in vitro hypoxic experiments were performed to reveal the regulation of hypoxia on the expression of HIF-1α, matrix metalloproteinase (MMP)-2, and MMP-9, leading to the formation of patterned matrix VM in HCC.
Materials and methods
Patient tissue materials
Tumor tissues from 151 patients from Anhui Provincial Hospital were studied, who underwent curative resection by the same surgical team for pathologically confirmed HCC between January 2003 and December 2008. Tissue specimens from 8 normal livers served as controls. Detailed pathological and clinical data were collected by reviewing medical charts and pathological records for all of the samples, including age and gender, HbeAg, cirrhosis, Child-Pugh grade, AFP, tumor nodules, capsule and size, vascular invasion, histological grade, pTNM stage, metastasis and recurrence, and survival duration. None of the patients had received treatment before operation. Clinical outcome was followed from the date of surgery to the date of death or until March 2010. Cases lost during follow-up were regarded as censored data for the survival analysis. The use of these tissues in this study was approved by the ethics committee at Anhui Provincial Hospital.
Immunohistochemistry
Formalin-fixed paraffin-embedded samples were sectioned at 4 μm and stained with hematoxylin and eosin for identification. Sections adjacent to the hematoxylin and eosin-stained sections were used for immunohistochemical staining. All slides were dewaxed in xylene, rinsed in graded ethanol, and finally rehydrated in double-distilled water. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide methanol at ambient temperature for 20 min. Antigen retrieval was carried out with 20-min microwave treatment in 10 mmol/l citrate buffer (pH 6.0) for CD34, hepatocyte, HIF-1α, and MMP-2 and MMP-9, while it was digested with 0.3 mg/ml proteinase K (Sigma–Aldrich, St. Louis, MO, USA) 6 min for laminin. After washing in 0.1 M phosphate-buffered saline (PBS), pH 7.4, and exposure to 3% normal goat serum for 20 min to reduce non-specific binding, the slides were incubated for 2 h at 37°C with a 1:100 dilution of anti-laminin monoclonal antibody (Millipore, Bedford, MA, USA), or a 1:100 dilution of anti-CD34 antibody (Santa Cruz Biotechnology, CA, USA), or a working solution of anti-hepatocyte antibody (Zhongshan Goldenbridge Biotechnology CO Ltd, Beijing, China, ready to use), or a 1:300 dilution of anti-HIF-1α antibody (Santa Cruz Biotechnology, CA, USA), or a 1:300 dilution of anti-MMP-2 antibody (Santa Cruz), or a 1:300 dilution of anti-MMP-9 antibody (Santa Cruz). Then, the sections were treated with biotin-labeled anti-IgG secondary antibody (Santa Cruz) at a dilution of 1:100 for 1 h at room temperature. Finally, peroxidase activity was visualized with 0.05% diaminobenzidine plus 0.015% hydrogen peroxide for 10 min, and sections were counterstained with hematoxylin. Appropriate positive and negative controls for each antibody were used.
HIF-1α, MMP-2, and MMP-9 levels were quantified according to the method described by Mattern et al. [27], and both the percentage and intensity of positive cells were measured. Ten visual fields of each tissue section were selected randomly under the microscope at 400× magnification, and 100 cells in each visual field (total of 1,000 cells) were counted. The average percentage of positively stained cells in 10 visual fields of each section was converted into a score as follows: 0 for <10% positive cells, 1 for <25% positive cells, 2 for <50% positive cells, and 3 for >50% positive cells. The score of the staining intensity of each section was completed as follows: 0 for no staining, 1 for light yellow, 2 for moderate yellow, and 3 for brown. The staining index (SI) equals the sum of staining intensity and positive cell scores, which were used to determine the final result for each section. Three independent investigators blind to the clinicopathologic data assessed the immunohistochemistry. Discrepancies were resolved by consensus.
Transmission electron microscopy
Tissue samples were fixed in 2.5% buffered glutaraldehyde and then postfixed in 1% osmium tetroxide. After dehydration in ethanol and propylene, cells were embedded in Epon epoxy resin. The presence of red blood cells or blood platelet in the channels without endothelium was used as a criterion for authentic VM. The presence of red blood cells in the channels with endothelium was used as a criterion for an authentic endothelium-dependent vessel. Ultra-thin sections stained with uranyl acetate and lead citrate were examined with a JEM-1230 (JEOL, Tokyo, Japan) transmission electron microscope.
Three-dimensional cultures
The human hepatocellular carcinoma cell line MHCC97-H was purchased from Fudan University, maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mmol/l l-glutamine, and cultured under a humidified atmosphere of 5% CO2. The following experiments were performed with 70–80% confluent cultures. A 24-well tissue culture plate was evenly coated with 200 μl/well growth factor-reduced matrigel (BD Biosciences, Bedford, MA, USA), which was allowed to solidify at 37°C for 60 min, before cells were plated. The cell suspension was added (6 × 105 cells/well) onto the surface of the matrigel and incubated at 37°C for varying times. When tissue culture plate was evenly coated with 25 μl/well matrigel, the 2 × 105/well cells were seeded onto the gels. The cells were then cultured in humidified incubators at 37°C for 48 h in normoxia (95% air and 5% CO2) or in hypoxia (1% oxygen, 5% CO2, and 94% nitrogen). Cells were photographed using an Olympus IX51 inverted microscope (Olympus, Tokyo, Japan); the images were then imported as TIFF files into National Institutes of Health (NIH) Image software. A second observer measured the total length of the tube-like structures (defined as those exceeding 200 μm in length [28]) per each image. Cells were also collected from matrigel by trypsin, to which Trizol or RIPA buffer was isolated to isolate cells of total RNA or protein.
Reverse-transcription polymerase chain reaction (RT–PCR)
Total cellular RNA was extracted using Trizol reagent (Invitrogen, Life Technologies, USA) and reverse transcribed into single-stranded cDNA as a template for subsequent PCR. The primer sequences were as follows: HIF-1α (393 base pairs [bp]) 5′- GAAACTTCTGGATGCTGGTG -3′ (forward) and 5′- TATACGTGAATGTGGCCTGT -3′ (reverse); MMP-2 (597 bp) 5′- GATGATGCCTTTGCTCG -3′ (forward) and 5′- AGTGGACATGGCGGTCT -3′ (reverse); MMP-9 (175 bp) 5′- GAGGTTCGACGTGAAGGCGCAGATG -3′(forward) and 5′- AGGTCACGTAGCCCACTTGGTC -3′(reverse); β-actin (546 bp) 5′- GGG ACC TGA CTG ACT ACC TC -3′(forward) and 5′- ACT CGT CAT ACT CCT GCT TG -3′ (reverse). Conditions for amplification were as follows: pre-heating at 94°C for 5 min followed by 35 cycles of denaturing at 94°C for 40 s, annealing at 55°C for 30 s, extending at 72°C for 45 s using a thermal cycler (Biometra, Germany), and a final extension at 72°C for 10 min. After the reaction was complete, 5 μl of PCR products was detected separately by 1% agarose gel electrophoresis. The gray value for each band was measured using a gel image analysis system (Bio-Rad, Hercules, CA, USA).
Western blot analysis
The preparation of whole-cell lysates was performed by resuspending cells in a buffer containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, and 0.1% sodium dodecyl sulphate (SDS). Fifty micrograms of protein extract was electrophoresed on a sodium dodecyl sulphate–polyacrylamide (SDS–PAGE) gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). After incubation with 5% non-fat milk to block non-specific binding, the membranes were exposed to specific anti-HIF-1α, anti-MMP-2, anti-MMP-9, or control anti-β-actin (all 1:1,000, Santa Cruz) monoclonal antibodies overnight at 4°C. Membranes were then washed and exposed to peroxidase-conjugated anti-IgG secondary antibody (1:5,000), and the antigen–antibody complex was visualized using an enhanced chemiluminescence detection system according to the manufacturer’s instructions (GE Healthcare, Chalfont St Giles, UK).
Statistical analysis
Statistical analyses were performed using SPSS 13.0 for Microsoft Windows (SPSS Inc., Chicago, IL). Continuous variables were expressed as the means ± SD and were compared between groups by using the Student’s t-test. Categorical variables were compared by using the chi-square test. The Mann–Whitney test was for non-normal distributive data. The analyses of overall survival and disease-free survival were calculated by the Kaplan–Meier method, and the differences in survival between groups were compared using the log-rank test. Univariate analysis, to identify predictors of survival, was performed by the Kaplan–Meier method and compared by the log-rank test. Multivariate analysis was carried out to identify the independent prognostic factors for survival using Cox regression hazard model. P < 0.05 was considered statistically significant.
Results
Patterned matrix VM expression in HCC detected by immunohistochemical staining
Patterned matrix VM in HCC was identified by the detection of laminin-positive loops surrounding clusters of 3–15 tumor cells (Fig. 1a) [5, 6]. The same HCC lesion in Fig. 1a showing VM expression by laminin immunostain did not express the endothelial cell marker CD34, indicating that the laminin-positive loops were not hepatic sinusoids. Hepatic sinusoids were CD34 positive (Fig. 1b). The cells external to the lumen of the patterned channels were positive for hepatocyte, which indicated the channels were formed by HCC cells (Fig. 1c). In comparing Fig. 1a, b, the laminin-positive loops seemed to connect to hepatic sinusoids. Patterned matrix VM was observed in 31 of 151 (20.5%) HCC samples. None of the control tissues from 8 normal livers (Fig. 1f) contained patterned matrix VM.
Immunohistochemical staining for patterned matrix VM in HCC and normal liver tissues. a Patterned matrix VM expression was identified in an HCC lesion by laminin immunostain (red arrow). b The same HCC lesion in (a) showing patterned matrix VM expression by laminin immunostain did not express the endothelial cell marker CD31. Hepatic sinusoids were CD34 positive (yellow arrow). c The same HCC lesion in (a) was positive for hepatocyte. d Patterned matrix VM in the same HCC lesion in (a) was viewed at low magnification. e A HCC lesion without patterned matrix VM expression by laminin immunostain. f Patterned matrix VM was absent in hepatocytes in a section of normal livers stained by laminin. Normal portal bile duct and vascular basement membranes were positive for laminin (blue arrow). Bars: (a, b, and c) 25 μm; (d, e, and f) 50 μm
Transmission electron microscopic observations
VM channel structure in 31 VM-positive patients was further validated by transmission electron microscopy, which showed that tumor cells, and not endothelial cells, formed the walls of VM channels. Red blood cell appeared in the middle of the VM channels (Fig. 2a). Compared with VM channels, endothelium-dependent vessels were lined externally by endothelial cells, and not tumor cells, and red blood cells can also be observed in vessels (Fig. 2b).
Transmission electron microscopy of VM channel and endothelium-dependent vessel. a Electron micrograph of a VM channel with a red blood cell identified within its lumen and surrounded externally by HCC cells. b Electron micrograph of an endothelium-dependent vessel lined with endothelial cells and containing red blood cells. Original magnifications: a × 8000; b × 3000. Abbreviations: HCC, hepatocellular carcinoma; RBC, red blood cell; EC, endothelial cell
Clinicopathologic correlation of patterned matrix VM expression in HCC
To elucidate its biologic significance, we correlated the VM expression with clinicopathologic features of HCC. As shown in Table 1, the incidence of VM in HCC was associated with tumor size (P = 0.042), vascular invasion (P = 0.016), Edmondson grade (P = 0.022), and pTNM stage (P = 0.013), but not with age, gender, HbeAg, cirrhosis, Child-Pugh grade, serum AFP, tumor number, and tumor encapsulation. Larger tumors, vascular invasion, high-grade HCC, and late-stage HCC were more frequently noted in HCC patients with patterned matrix VM than in patients without patterned matrix VM (Table 1).
Prognostic significance of patterned matrix VM expression
The cumulative 1-, 3-, and 5-year OS rates were 67.7, 34.6, and 11.5%, respectively, in the VM group and 86.7, 64.7, and 40.3%, respectively, in the non-VM group. As far as DFS was concerned, the cumulative 1-, 3-, and 5-year survival rates were 41.9, 19.4, and 6.5%, respectively, in the VM group and 63.3, 40.6, and 19.6%, respectively, in the non-VM group. In the VM group, the OS and DFS rates were significantly lower than in the non-VM group (P < 0.001 and P = 0.002) (Fig. 3).
Kaplan–Meier analysis of OS and DFS for patterned matrix VM expression. Patterned matrix VM expression was associated with both shortened survival and increased recurrence. The P values were determined by the log-rank test. Abbreviations: OS, overall survival; DFS, disease-free survival; VM, vasculogenic mimicry
Univariate analyses of prognostic factors indicated that age, tumor size, tumor number, vascular invasion, and VM had significant prognostic influence on OS, while tumor size, tumor number, vascular invasion, and VM had significant prognostic influence on DFS (Table 2). These factors were the potential candidates for multivariate analysis for survival. After multivariate analyses, only tumor number, vascular invasion, and VM were independent prognostic factors for OS, while vascular invasion and VM were independent prognostic factors for DFS (Table 3).
Expression of HIF-1α, MMP-2, and MMP-9 between the VM and non-VM groups
We performed immunohistochemical staining for HIF-1α, MMP-2, and MMP-9 in the VM and non-VM groups to further identify their association with VM formation. The positive expression of HIF-1α was either in the nucleus or in the cytoplasm of HCC cells and that of MMP-2 and MMP-9 was in the cytoplasm (Fig. 4a–f). The SI of HIF-1α, MMP-2, and MMP-9 was all higher in the VM group than that of non-VM group (P = 0.001, 0.030, and 0.007, respectively) (Table 4).
Immunohistochemical analysis of HIF-1α, MMP-2, and MMP-9 expression in the VM and non-VM groups of HCC. a and b Expression of HIF-1α in the VM and non-VM groups. c and d Expression of MMP-2 in the VM and non-VM groups. e and f Expression of MMP-9 in the VM and non-VM groups. The percentage of positive cells and staining intensity was stronger in the VM group (a, c, and e) than in the non-VM group (b, d, and f)
Effect of hypoxia on tube formation in MHCC97-H cells
To demonstrate the effect of hypoxia on tube formation, MHCC97-H cells were grown in matrigel gels and incubated under normoxic or hypoxic conditions for 48 h. Cells grown in thick (200 μl/well) matrigel gels failed to form tumor cell-lined, matrix-rich networks (patterned matrix VM) [5, 29] under normoxic condition (Fig. 5a), while obvious patterned matrix VM was produced under hypoxic condition (Fig. 5b). In addition, we also observed that longer tube-like structures (tubular VM) [5] were formed under hypoxia than under normoxia, when cells were cultured in thin (25 μl/well) matrigel gels (Fig. 5c–e).
Morphological analyses of MHCC97-H following culture under normoxic or hypoxic conditions for 48 h. Phase-contrast microscopy demonstrated the ability of MHCC97-H cells to form patterned matrix VM (b) in thick matrigel gels or longer tubular VM (d) in thin matrigel gels than normoxia group with no exposure to hypoxia, when cultured under hypoxic condition for 48 h. MHCC97-H cells following culture under normoxic condition for 48 h were unable to form patterned matrix VM (a) in thick matrigel gels or shorter tubular VM (c) in thin matrigel gels. The white arrowheads indicate vasculogenic-like networks (objective magnification, × 40). e Comparison of tubular VM formation in response to normoxic and hypoxic culture. * P < 0.05, compared with the normoxia group
Effect of hypoxia on HIF-1α, MMP-2, and MMP-9 production in MHCC97-H cells
We hypothesized that, under hypoxic condition, the stronger ability to form VM could be involved in the induction of the gene and protein expression of HIF-1α, MMP-2, and MMP-9 in MHCC97-H cells. To test this hypothesis, experiments were conducted under normoxic and hypoxic conditions, and RT–PCR and Western blot were used to assess hypoxia-induced modulations of gene and protein expression levels. Results from these experiments showed that when cells were cultured for 48 h, HIF-1α, MMP-2, and MMP-9 gene expression were strikingly higher under hypoxia than under normoxia (Fig. 6a, b). In accordance with RT–PCR data, hypoxia also up-regulated HIF-1α, MMP-2, and MMP-9 protein expression (Fig. 6c, d).
Effect of hypoxia on HIF-1α, MMP-2, and MMP-9 expression. a The level of HIF-1α, MMP-2, and MMP-9 mRNAs was detected by semi-quantitative RT–PCR in MHCC97-H following culture under normoxic or hypoxic conditions for 48 h. b The level of HIF-1α, MMP-2, and MMP-9 mRNA was normalized to β-actin. c The level of HIF-1α, MMP-2, and MMP-9 proteins was examined by Western blot analysis in MHCC97-H following culture under normoxic or hypoxic conditions for 48 h. d The level of HIF-1α, MMP-2, and MMP-9 proteins was normalized with respect to β-actin. Experiments were repeated at least three times with similar results. * P < 0.05, ** P < 0.01, compared with the normoxia group
Discussion
It has been reported that patterned matrix VM was found in 55% of HCC specimens from 20 liver explants of consecutive patients who underwent orthotopic liver transplantation for HCC [6]. However, in our retrospective study of 151 HCC patients, we found that patterned matrix VM was present in 31 of 151 (20.5%) cases who underwent radical hepatectomy. Possible explanations for the discrepancy may be that there exists difference in sample size and inclusion criteria of cases. Nevertheless, our result was similar to another study in which tubular VM was found in 18 out of 97 (19%) HCC samples from patients who underwent hepatectomy for HCC [23].
As a non-vascular and thus non-endothelial cell-lined channel, tumor cells in the patterned matrix VM may be exposed to blood flow, which may facilitate metastasis. The tumor cells also migrate along extracellular matrix scaffolds, and the physical connection between these patterns and vessels may facilitate hematogenous dissemination of cancer [5, 30]. In addition, extravascular matrix patterns of patterned matrix VM are generated by highly invasive tumor cells, and poorly invasive tumor cells do not generate such patterns [4]; therefore, matrix patterns are markers of the presence of a highly invasive tumor cell phenotype that can be targeted [5]. All of these imply that patterned matrix VM existence in tumor may indicate that the tumor has potentially more malignant biological behavior and poorer prognosis.
Several clinical studies have reported the relationship between the expression of VM, including tubular VM and patterned matrix VM, and cancer progression and poor clinical outcome [14, 19, 21]. However, controversial study has also found patterned matrix VM to not always be related to clinicopathological features and poor prognosis in pT3 and pT4 cutaneous melanoma [31]. In the present study, we observed that HCC tumors with patterned matrix VM were larger and were more frequently, vascular invasion, grade III–IV and stage III–IV than HCC tumors without patterned matrix VM. Further research demonstrated that OS and DFS were better in patients with non-VM than in patients with patterned matrix VM. Kaplan–Meier and multivariate analysis showed that the presence of patterned matrix VM was independent predictors of poor prognosis for both OS and DFS. These results also indicated that patterned matrix VM had an adverse influence on the HCC patient’s prognosis. Thus, patterned matrix VM can be used to assess the prognosis of HCC patients.
As is well-recognized, hypoxia is a common mechanism in HCC as the solid tumor owing to aberrant vascularization [32]. Work from many laboratories has elucidated that hypoxia was able to stimulate proliferation, induce angiogenesis, accelerate invasion and was responsible for treatment resistance in HCC [33, 34]. The adaptation of HCC cells to tissue hypoxia is of central importance for tumor progression, where inducing the ubiquitous transcription factor HIF-1α expression appears to be a critical step [35]. Study has shown that melanoma cells in a hypoxic microenvironment increased HIF-1α expression and induced the formation of VM channels to acquire an adequate blood supply [14]. Besides, it was found that the tumor cells surrounding VM in Ewing sarcoma tissue samples did express HIF-1α, which was indicative of inefficient oxygen delivery by these structures. When Ewing sarcoma cells were cultured under low oxygen tension, an increased expression of genes involved in VM was also observed [14]. Thus, we anticipate that hypoxia via induction of HIF-1α is able to enhance VM.
Remodeling of extracellular matrix (ECM) is one of the three factors governing VM channel formation. ECM remodeling provides the space needed for VM and is associated with the MMP secretion by tumor cells [12, 36]. Among MMPs, the expression and activity of MMP-2 and MMP-9, which can degrade various extracellular matrix proteins and facilitate tumor angiogenesis, invasion and metastasis, have positively correlation to VM formation [10, 12, 23, 26, 37]. It seemed quite possible that hypoxia-induced expression of MMP-2 and MMP-9, although the results from some studies about the effects of hypoxia on MMP-2 and MMP-9 were controversial [26, 38, 39]. In view of the above, we speculate that MMP-2 and MMP-9 may be involved in the possible mechanism of VM formation induced by hypoxia.
When assessing the HCC samples for hypoxia by immunohistochemical staining, we found that HIF-1α, MMP-2, and MMP-9 expression levels in the VM group were higher than in non-VM group. This finding is in agreement with a very recent study in which there are more abundant expression of HIF-1α, MMP-2, and MMP-9 in VM group of gastric adenocarcinoma than in non-VM group [17]. Furthermore, we employed a HCC cell line MHCC97-H to investigate the effect of hypoxia on the formation of patterned matrix VM. Results from our study showed that patterned matrix VM was produced under hypoxic condition, while cells cultured under normoxic condition for 48 h failed to form patterned matrix VM. Interestingly, tubular VM was also induced by hypoxia when cells were cultured in thin matrigel gels. With the knowledge of the inducibility of VM formation by hypoxia, we conjectured whether hypoxic condition might induce the expression of HIF-1α, MMP-2, and MMP-9 in MHCC97-H cells. Our in vitro experiments revealed that hypoxia was able to increase the gene and protein expression of HIF-1α, MMP-2, and MMP-9 in MHCC97-H cells cultured in thick matrigel gels. Together, these results demonstrated that hypoxia might induce VM formation by HCC cells through up-regulation of the expression of HIF-1α, MMP-2, and MMP-9. However, it remains to be determined whether hypoxia may activate MMP-2 and MMP-9 proteinases to induce HCC cells to form VM. In addition, our initial work, although promising, requires confirmation in large-scale, multicenter, and preferably, population-based studies to delineate the true biologic role of patterned matrix VM in HCC.
This study shows that patterned matrix VM exists in HCC. Its presence is associated with larger tumor size, vascular invasion, and higher tumor grade and stage. Patterned matrix VM might serve as an unfavorable prognostic factor for HCC patients undergoing radical hepatectomy. It is possible that hypoxia via induction of expression of HIF-1α, MMP-2, and MMP-9 may enhance VM formation. These findings may be important in understanding the angiogenesis patterns in HCC, which may ultimately lead to the development of effective therapeutic strategies for HCC.
References
Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60.
Chang YS, et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97(26):14608–13.
Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41(5):864–80.
Maniotis AJ, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–52.
Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS. 2004;112(7–8):508–25.
Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007;131(12):1776–81.
Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–21.
Lissitzky JC, et al. Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Res. 2009;69(3):802–9.
Basu GD, et al. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res. 2006;8(6):R69.
Robertson FM, et al. Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostanoid receptors EP3 and EP4. Cancer. 2010;116(11 Suppl):2806–14.
Sharma N, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50(3):189–201.
Sood AK, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190(4):899–909.
Scavelli C, et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27(5):663–74.
van der Schaft DW, et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65(24):11520–8.
Zhang LZ, et al. The role of VE-cadherin in osteosarcoma cells. Pathol Oncol Res. 2010;16(1):111–7.
El Hallani S, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133(Pt 4):973–82.
Li M, et al. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res. 2010;16(2):259–66.
Baeten CI, Hillen F, Pauwels P, de Bruine AP, Baeten CG. Prognostic role of vasculogenic mimicry in colorectal cancer. Dis Colon Rectum. 2009;52(12):2028–35.
Vartanian AA, et al. Prognostic significance of periodic acid-Schiff-positive patterns in clear cell renal cell carcinoma. Can J Urol. 2009;16(4):4726–32.
Mirshahi P, et al. Vasculogenic mimicry of acute leukemic bone marrow stromal cells. Leukemia. 2009;23(6):1039–48.
Sun B, et al. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16(4):693–8.
Dupuy E, et al. Tumoral angiogenesis and tissue factor expression during hepatocellular carcinoma progression in a transgenic mouse model. J Hepatol. 2003;38(6):793–802.
Sun T, et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51(2):545–56.
Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70(4):478–90.
Liu SY, et al. Clinicopathologic significance of tumor cell-lined vessel and microenvironment in oral squamous cell carcinoma. Oral Oncol. 2008;44(3):277–85.
Sun B, et al. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249(2):188–97.
Mattern J, Koomagi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid lung carcinoma. Br J Cancer. 1996;73(7):931–4.
Yamamoto K, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95(5):2081–8.
Sood AK, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158(4):1279–88.
Folkman J. Can mosaic tumor vessels facilitate molecular diagnosis of cancer? Proc Natl Acad Sci USA. 2001;98(2):398–400.
Massi D, et al. Vasculogenic mimicry has no prognostic significance in pT3 and pT4 cutaneous melanoma. Hum Pathol. 2004;35(4):496–502.
Vengellur A, Phillips JM, Hogenesch JB, LaPres JJ. Gene expression profiling of hypoxia signaling in human hepatocellular carcinoma cells. Physiol Genomics. 2005;22(3):308–18.
Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: The therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22(8):1178–82.
Dai CX, et al. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer. 2009;9:418.
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–75.
Zhang S, Zhang D, Sun B. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254(2):157–64.
Seftor RE, et al. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61(17):6322–7.
Annabi B, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21(3):337–47.
Ottino P, et al. Hypoxia activates matrix metalloproteinase expression and the VEGF system in monkey choroid-retinal endothelial cells: Involvement of cytosolic phospholipase A2 activity. Mol Vis. 2004;10:341–50.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30972892), the Science and Technology Key Project of Anhui Province (07010302193), Anhui Provincial Natural Science Foundation (090413107), Anhui Provincial College Natural Science Key Project (KJ2009A178), the Science and Technological Fund of Anhui Province for Outstanding Youth (08040106818), and the Anhui Provincial ‘‘115’’ Industrial Innovation Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wen-bin Liu and Wei-dong Jia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Wb., Xu, Gl., Jia, Wd. et al. Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol 28 (Suppl 1), 228–238 (2011). https://doi.org/10.1007/s12032-010-9706-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9706-x