Abstract
The influence of forest fragmentation (habitat isolation) on biological and ecological diversity of aquatic insects was investigated in streams of fragmented forests in Hulu Gombak (6 streams) and Gunung Angsi (5 streams) and un-fragmented forest of Berembun (6 streams) in peninsular Malaysia. Several environmental parameters including canopy cover, DO, temperature and pH differed significantly among the three catchments (P < 0.05). We found that taxonomic richness in Berembun forest was significantly different from Gunung Angsi (P < 0.05), but not with Hulu Gombak forests (P > 0.05). Nestedness pattern that measures the effect of habitat isolation on taxonomic assemblages showed that aquatic insect’s community in un-fragmented forest (Berembun) was less nested (T = 54.4), indicating high diversity compared to highly nested (less diverse) in the two fragmented forests (Hulu Gombak, T = 30.45 and Gunung Angsi, T = 35.45). Taxa similarity in Berembun streams was negatively correlated with the geographical distance among streams (Mantel test, r = − 0.462, P < 0.05). Such correlation was absent in both Gunung Angsi and Hulu Gombak streams. Forest fragmentation in Hulu Gombak and Gunung Angsi measured as the distance of the forests from the nearest forested area had negative effect on aquatic insects diversity (r 2 = − 0.149, P < 0.05), but not on their abundances (r 2 = 0.003, P > 0.05). We concluded that local habitat conditions were the most important in shaping the aquatic insects community among streams of both unfragmented and fragmented forests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deterioration of aquatic biodiversity is the main concern in recent conservational and ecological studies in various parts of the world including Europe (e.g., Heino 2009; Song et al. 2009), Hong Kong (e.g., Dudgeon 2006), Japan (e.g., Mori et al. 2010), Australia (e.g., Boyero et al. 2006) and Africa (e.g., Kasangaki et al. 2008). Although loss of global biodiversity issue has been highlighted in recent studies, less attention was paid to investigate the loss of biodiversity in tropical aquatic ecosystems (Strayer and Dudgeon 2010). Aquatic habitats in the tropics differ in several aspects from those in temperate areas (e.g., biodiversity, ecological functioning and evolutionary history) (Gopal 2005; Dudgeon 2008; Boyero et al. 2009).
Habitat fragmentation is an important conservation issue in preservation of natural biodiversity (Fahrig 2003; Monaghan et al. 2005). It could occur naturally or as consequences of human activities including road construction, timber logging, construction of dams and agricultural land-use (Fahrig 2003). Eradication of natural habitat and isolation of habitat fragments were reported to reduce species biodiversity and to alter the community structure in both terrestrial and aquatic ecosystems (Andren 1994; Monaghan et al. 2005).
Fragmentation diminishes the total area of the contiguous habitat and splits it into small and disjointed patches or fragments leading to changes in the spatial configuration and modifications in the original habitat characters (Fahrig 2003). Consequently, the inhabitant species remained within the fragment will be exposed to the conditions of a modified surrounding ecosystem (known as edge effect) (Eikaas et al. 2005).
Although habitat isolation would result in less connectivity among the isolates leading to reduction in taxonomic richness (Forbes and Chase 2002; Chase 2003; Kneitel and Miller 2003; Ims et al. 2004), some studies suggested that habitat isolation has no effect on the diversity (see Hoyle and Gilbert 2004). Despite this contrary, habitat isolation in the forested areas through road construction and agricultural land-use is known to have an adverse effect on local faunal diversity (Negishi et al. 2006; Azhar et al. 2011). For example, forest fragmentation due to unmanaged oil palm expansion was found as a major threat to the natural biodiversity in Southeast Asia (Fitzherbert et al. 2008; Koh and Wilcove 2008; Corley 2009; Azhar et al. 2011).
Effect of habitat isolation on community structure of stream invertebrates can be examined through studying the alteration in diversity measures (alpha, beta and gamma). Nestedness patterns of stream invertebrate communities is also suggested as another indicator for diversity in response to effects of habitat isolation. Originally, nestedness was proposed by Hulten (1937) and developed by Patterson and Atmar (1986) to describe species composition patterns among segregated habitats within a region including islands and landscape fragments. Unfortunately, many of available studies on freshwater organisms showed contradictory nestedness patterns in the aquatic habitats. Several studies found that freshwater communities are either nested (Heino 2005; Heino and Muotka 2005; Monaghan et al. 2005; Heino et al. 2009) or non-nested (Malmqvist et al. 1999; Urban 2004). Generally, nested pattern is encountered when species in less-diverse communities are ideal subsets of those in more-diverse communities. Thus, common species are found in all communities, while rare species are likely to occur only in diverse communities (Patterson and Atmar 1986; Heino 2011). It was assumed that local conditions affecting species richness such as habitat isolation would also shape the nestedness patterns (Heino and Muotka 2005; Heino 2011). Hence, nestedness measure can be applied as a proxy for taxonomic richness and indicator for possible adverse effect of habitat isolation on diversity.
The problems associated with habitat fragmentation and its effects on adjacent aquatic environment are well documented in temperate region (Monaghan et al. 2005; Ostman et al. 2006). However, such research efforts in tropical forest streams of Southeast Asia and Malaysia, in particular, are scarce (Sodhi and Brook 2006). Although there are several studies highlighting the adverse effects of anthropogenic disturbance on aquatic macroinvertebrates in Southeast Asia (see Dudgeon 2006; Al-Shami et al. 2011; Hamid and Rawi 2011), ecological aspects concerning the effects of forest fragmentation and habitat isolation on local aquatic diversity are rarely addressed (Sodhi and Brook 2006).
The present study aimed to investigate the local effects of forest fragmentation on biological and ecological diversity of aquatic insects in two fragmented forests: Hulu Gombak (isolated by Gombak highway road network) and Gunung Angsi (isolated by oil palm plantations). Biological and ecological data were compared with natural forest streams of Berembun. We assessed the stream isolation by measuring the map distance among the sampled streams within the catchment. The degree of the isolation which may affect the local aquatic habitat (i.e., connectivity) was estimated by measuring the geographical distance of a stream from the nearest forested area within the catchment. We assumed that both streams isolation and connectivity would alter the structure of aquatic insect’s communities. The nestedness patterns of aquatic insect assemblages were also explored and used as a proxy for diversity. We hypothesized that insect’s assemblages in the isolated streams would show nested patterns driven by low-diverse communities with dominance of common species and scarcity of rare species.
Materials and methods
Study sites
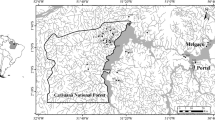
The headwater streams in southwestern part of Malaysia Peninsula were sampled in three catchments: unfragmented catchment of Berembun and fragmented catchments of Hulu Gombak and Gunung Angsi (Fig. 1). In this study, 6 streams were sampled in both Berembun (BR) and Hulu Gombak (HG) catchments and 5 streams in Gunung Angsi catchment (GA, Fig. 1) during rainy season in May and August 2008. A 10 sample replicates were collected from each stream. The mean altitudes of the streams in BR, HG and GA were 354.2, 515.33 and 367.33 meter above sea level (m.a.s.l.), respectively. The physical and chemical parameters of the three catchments are shown in Table 1. Despite the continuous efforts of the Malaysian government to keep these two forestry areas minimally impacted, extensive Gombak highway network in Hulu Gombak and large oil palm plantations in Gunung Angsi vicinities created fragmented habitats in these two areas.
Sampling of aquatic insects
Aquatic insects (mostly immatures) were sampled using a D-frame net of 0.3 m diameter and 0.15 m (radius) high and 300 μm-mesh net using the kick sampling technique (see Merritt and Cummins 1996). Benthic samples required to yield a representative estimate of macroinvertebrate populations were estimated using the index of precision (Elliott 1971) based on a preliminary collection.
All aquatic insects were sorted and preserved in 80 % ethanol. Thereafter, they were identified using available keys of Morse et al. (1994) and Yule and Yong (2004). In most cases, identifications of the specimens to species were extremely difficult in the lack of aquatic insect species records from Malaysian streams. In such situations, we conformed to Heino and Soininen (2007) suggestion that using the genus-level data in biodiversity studies was satisfactorily useful.
Functional feeding groups (FFGs)
Merritt and Cummins (1996) categorize five functional feeding group (FFG): shredders, collector–gatherers, collector–filterers, scrapers and predators. Generally, collector–gatherers feed on fine particulate organic matter (FPOM; <1 mm diameter) deposited in the stream. This group equipped with specialized anatomical structure such as setae, fans, mouth brushes or silk secretions that act as sieves to capture suspended FPOM in the water column (Merritt and Cummins 1996). Scrapers adapt themselves to graze or scrape materials such as periphyton and attached algae on the stream substrates, while shredders are organisms that comminute primarily large pieces of decomposing vascular plant tissue (>1 mm diameter) along with the associated microflora and fauna. They also can feed directly on vascular macrophytes and decomposing wood (Merritt and Cummins 1996). Meanwhile, predators feed primarily on animal tissue either by engulfing or piercing their prey, or sucking their body fluids.
Collected aquatic insects in this study were classified into their relevant FFGs following Merritt and Cummins (1996) and Yule and Yong (2004). Although Merritt and Cummins (1996) categorize the FFGs of North American aquatic insects, it is the main reference available to us. The book edited by Yule and Yong (2004) provides taxonomical description of most of the freshwater macroinvertebrates in Malaysia and Singapore. Many contributing authors identify some groups of the FFGs for this region. An expert opinion for other minor groups of aquatic insects such as Plecoptera (Ignac Sevic, personal communication) was also sought after.
Physicochemical parameters of the forested streams
At each stream, measurements of physicochemical parameters such as water depth, river width, water pH, water temperature, velocity and dissolved oxygen (DO) content in the water were carried out in situ at three randomly selected locations. Dissolved oxygen and temperature were measured with aYSI-57 meter (YSI Inc., Yellow Springs, Ohio), and measurement of water pH was recorded using a Termo-Orion Model 210 pH meter. The depth and width of the river were measured using a metal measuring tape. To analyze selected chemical parameters (BOD, COD and ammonium-N), three replicates of water samples were randomly collected using 500-ml plastic bottles. Thereafter, all samples were transported to the laboratory under cool conditions and stored at 4 °C for further analysis. The ammonium-N contents of the water were measured at appropriate wavelength using the YSI 9100 photometer test kit. The BOD and COD were measured using a Hach reactor following the manufacturer’s procedures.
Measuring the effects of forest fragmentation on aquatic habitat isolation
In this context, we considered two main criteria: (1) degree of stream isolation and (2) effect of forest fragmentation on stream isolation. Firstly, the degree of stream isolation was assessed by measuring the distance among the sampled streams within the catchment. Secondly, the effect of forest fragmentation on stream isolation was determined by measuring geographical distance of each stream from the nearest forested area in each catchment. Additionally, three other parameters of each stream; substrate embeddedness; canopy cover; and total habitat score, were assessed to compare the effect of habitat isolation with local habitat alteration. The total habitat score was calculated based on the composite scores of habitat assessments (epifaunal substrates, embeddedness, velocity, sediment deposition, flow channel status, catchment alteration, frequency of riffle, bank stability, vegetative protection and width of riparian vegetative zone) following the method of Barbour et al. (1999).
Data analysis
The statistical software of SPSS (version 13) was used to conduct the ANOVA test (at P < 0.05) to compare the physical and chemical parameters among the three investigated catchments. The ANOVA test followed by Tukey’s multiple mean comparison (at P < 0.05) was employed to examine whether the taxa richness differed among the catchments investigated.
To describe the biodiversity patterns of the aquatic insects in unfragmented and fragmented habitats, alpha and gamma diversity measures were calculated following Whittaker (1960) and Heino et al. (2009). Alpha diversity is expressed as mean taxa richness. However, gamma diversity is simply the total number of taxa detected in all streams for each catchment. Species Diversity and Richness software (version 4.1.2) (Seaby and Henderson 2006) was used to calculate alpha and gamma diversities.
The degree of nestedness of insect’s communities in each basin was quantified using the temperature calculator of Atmar and Patterson (1993). The presence/absence data were reordered to maximize matrix nestedness and used for temperature calculator. The temperature value that reflects the matrix deviation from a typical nested community ranges from 0 to 100 °C. The ideally nested matrices with rare taxa in rich locations have T = 0 °C, while totally random matrices have T = 100 °C (Larsen and Ormerod 2010). The statistical significance of the observed temperature value was estimated using a Monte Carlo probability tests as the observed temperature was compared with simulated temperatures of 500 randomly simulated matrices. As recommended by Heino (2009), the null model of “fixed–fixed” for testing the null hypothesis was considered, which is less sensitive to variation in taxa richness and occurrences (Heino et al. 2009; Larsen and Ormerod 2010). Furthermore, we also calculated the percentage of idiosyncratic species in each matrix because they have the potential to increase the matrix temperature (Heino et al. 2010).
Species turnover between the sites within the catchment was measured using the Sorensen’s similarity index C: C = 2j/(a + b), where j = the number of taxa found in both sites, a = the number of taxa in site a, and b = the number of taxa in site b (Magurran 1988). The scores of C index range from 0 to 1. The maximum value of 1 indicates an identical species composition, and the minimum value of 0 indicates that the sites have no taxa in common. The Sorensen’s similarity index (C) was calculated using Community Analysis Package (version 4.1.3, PISCES Conservation Ltd., UK).
For each catchment, the relationship between the geographical distance (m) among streams and the Sorensen’s similarity index (C) of the species was examined. Both matrices for each catchment (distance and species similarity) were compared using the Mantel test with Pearson correlation (Mantel 1967; McCune and Grace 2002). Significance in the resulted Pearson correlation coefficient (r) was tested at P < 0.05 with 1,000 permutations using Monte Carlo test.
All streams of the three catchments were used in the linear regression model (at P < 0.05) to investigate the relationship between the geographical distance of each stream from the nearest forested area and diversity and abundance of aquatic insects. The linear regression model was also applied to examine the effect of distance from forest on abundance of each ecological group (collector–gatherer, collector–filterer, predators, shredders and scrapers).
Results
Biological and ecological diversity of aquatic insects
As shown in Table 2, the highest number of taxa was recorded in un-fragmented Berembun forest and fragmented forest Gunung Angsi had the lowest number of taxa collected. The scores of gamma diversity were 85, 71 and 54 in Berembun, Hulu Gombak and Gunung Angsi, respectively. The alpha diversity showed similar trend with values of 40.83, 36.83 and 34.6 in Berembun, Hulu Gombak and Gunung Angsi, respectively. Interestingly, abundance of aquatic insects differed significantly among the three catchments (ANOVA, P < 0.05). The highest abundance of aquatic insects was reported in Hulu Gombak. However, the total number of collected individuals in Berembun and Gunung Angsi were 1,635 and 1,432, respectively (Table 2 and see also Table S1). Total taxonomic richness (gamma diversity) was significantly different among the three catchments (ANOVA, P < 0.05, df = 16). The results of Tukey’s test showed that taxonomic richness was significantly different between Berembun and Gunung Angsi (P < 0.05), but not between Berembun and Hulu Gombak (P > 0.05).
Figure 2 illustrated the proportion of the functional feeding groups (FFGs) of aquatic insects in the three forest catchments (Berembun, Gunung Angsi and Hulu Gombak). Community of aquatic insects in Berembun streams showed remarkably diverse pattern indicated by ratios of FFGs with pronounced dominance of predators. However, collector–filterers dominated the aquatic insects community in Gunung Angsi; meanwhile, equal proportions of FFGs were the main pattern of community in Hulu Gombak.
Nestedness patterns of aquatic insects assemblages
Variations in nestedness (expressed as temperature value) of aquatic insects communities among the three catchments are shown in Table 3. All calculated temperature values were significant based on Monte Carlo test with 500 runs at P < 0.01. The highest temperature value of 54.4 °C was recorded in the streams of unfragmented Berembun forest. In fragmented catchment of Gunung Angsi, the observed temperature value of the aquatic insect’s community was 35.45 °C and Hulu Gombak scored the lowest. The highest percentage of idiosyncratic species was detected in Berembun (52 %) followed by Gunung Angsi (51 %) and the lowest (30 %) in Hulu Gombak.
The species turnover (Sorensen’s similarity index, C) values are shown in Table S2. Apparently, most investigated streams in Berembun catchment (except site B2) shared high number of taxa with other streams within the same catchment. Noteworthy, other streams in Berembun catchment (such as B1, B3 and B4) have higher number of taxa in common compared with streams of Gunung Angsi (A1 and A2). The streams in Hulu Gombak catchment especially G1 and G2 exhibited similar taxonomic composition with other streams within the same catchment. Other streams in Hulu Gombak catchment such as G4 and G6 as well as most streams in Gunung Angsi catchment showed lower number of shared taxa.
Based on results of Mantel test, matrices of species similarity (Sorensen’s similarity index, C) and distance among the streams in Berembun were negatively correlated (Z = 5.398, r = −0.462, P < 0.05). However, no significant relationship (P > 0.05) was detected between each pair of matrices (distance and similarity) in Gunung Angsi and Hulu Gombak.
Interestingly, the distance from the nearest forested area showed negative impact on diversity of aquatic insects (Fig. 3, r 2 = 0.149, P < 0.05), but not the abundance (Fig. 3, r 2 = 0.003, P = 0.838). The FFGs proportions showed different relationships with variation in distance from forested area. Based on linear regression models, significant negative relationships (P < 0.05) were found between abundance of collector–filterers (r 2 = 0.357) and predators (r 2 = 0.0.329) with distance variability from the nearest forested area.
Discussion
The present study revealed that the physical and chemical parameters varied among all investigated catchments in unfragmented (Berembun) and fragmented forests (Hulu Gombak and Gunung Angsi). In Hulu Gombak forested streams, low DO concentration was mainly attributed to the influence of the highway network (built in 90s). Similar findings were obtained from another area (Bukit Tarek Experimental Watershed) in peninsular Malaysia by Negishi et al. (2006). The highway network was suggested to change the concentrations of DO in the streams, increased the suspended sediments and soil erosion (Negishi et al. 2006). Increasing the amount of suspended solids reduces light penetration in the streams, thereby restrains primary production and resulting in deterioration in DO levels (Ali and Ahmad 1988). It is an acceptable fact that modification of natural aquatic habitats leads to invincible changes in physical and chemical parameters of rivers. For example, deforestation is always associated with profound changes in water pH, DO levels and temperature regime (Douglas et al. 1992; Iwata et al. 2003; Wantzen 2006; Lorion and Kennedy 2009). Deforestation and elimination of canopy cover will expand the exposure surface of the streams increasing the water temperature which lead to deterioration in DO concentration (Gopal 2005).
Agricultural land-use has been proven to affect the biological and ecological diversity of aquatic insects in both tropical (Gucker et al. 2009; Gopal 2005; Dudgeon 2010; Al-Shami et al. 2011) and temperate streams (Delong and Brusven 1998; Song et al. 2009). The oil palm expansion resulted in apparent alteration in physical and chemical characteristics of the natural stream habitat (e.g., isolation, sedimentation, eutrophication). Unfortunately, information addressing the adverse effect of oil palm expansion on stream invertebrate diversity in Malaysia is scarce. Azrina et al. (2006) found low diversity of aquatic invertebrates in two stations of Langat River (Malaysia) in vicinity of oil palm plantations and their industrial facilities and suggested that it was the consequence of effluents from oil palm mills and agricultural pesticides.
Converting the natural forests to oil palm plantations is also associated with reduction in the natural canopy cover and habitat integrity (Wilcove and Koh 2010). The canopy cover is a very important component of aquatic ecosystem providing continuous allochthonous food source to the aquatic insects especially shredders (Boyero et al. 2011) and also serves as a shelter to adult insects to rest or mate (Hamid and Rawi 2011). Hence, elimination and logging the natural forests for agriculture use would eventually have a negative effect on the biodiversity of aquatic insects.
We found that streams in the isolated local habitats of Gunung Angsi and Hulu Gombak had lower taxonomic richness compared to those in the connected habitat of Berembun. This finding is consistent with previous studies suggesting negative effects of habitat isolation on taxonomic richness (e.g., Gonzalez et al. 1998; Chase 2003; Ostman et al. 2006). Eikaas et al. (2005) found that higher richness of banded kokopu fish in streams of natural forest compared to those in open grassland with obvious effect of distance from the forest margin.
Our results were also in agreement with experimental findings on aquatic organisms (rotifers and protists) reared in artificial habitats of petri dishes by Ostman et al. (2006) who concluded that isolated habitats generally had fewer species at local scales compared to connected habitats. Thus, taxonomic richness was suggested to decrease with increasing isolation at local scales because of reduction in the rescue effects probability (see Brown and Kodric-Brown 1977) and decreased immigration that can improve negative population growth rates (Mouquet and Loreau 2003).
In the present study, we found nested patterns (Monte Carlo test, P < 0.01) of aquatic insects communities in Hulu Gombak and Gunung Angsi where low-diversity streams are subset of those with high diversity. In other words, the common species can be found in all assemblages but the rare species occurs only in the high-diversity assemblages. Such patterns could be found in streams under isolation and local effects (Heino 2009, 2011; Heino et al. 2010). Differential colonization was suggested as a possible mechanism generating nested patterns due to variation in the degree of habitat isolation and ecological requirements of the species. In addition, local habitat conditions may produce nested patterns when there is variability in species tolerance of environmental factors (Heino et al. 2010). Thus, we suggested that presence of nested patterns in Hulu Gombak and Gunung Angsi was driven by isolation and alteration in the local habitat characteristics.
Although we have to be cautious in drawing a general conclusion regarding the effects of forest fragmentation on diversity of aquatic insects because of some difficulties (e.g., streams accessibility, sample size and species identification), we may suggest that habitat isolation and loss of stream connectivity have noticeable effects on diversity and community structure of aquatic insects. We also found that local habitat alteration was the determinant factor shaping the community structure of aquatic insects among streams in both unfragmented and fragmented forests resulting in remarkable disparity in taxonomic richness, ecological groups (i.e., FFGs) and nestedness patterns. Another limitation of the study was the number of samples collected. Hence, it was impossible to generalize the effect of oil palm and road network on diversity of stream insects. Furthermore, it was hard to examine the dispersal patterns and dynamics of aquatic insects in relation to fragmentation and habitat isolation.
From the conservational point of view, maintaining natural habitat minimally impacted is likely the best conservation plan. However, with high rate of deforestation and fast civil and industrial developments, the planning is sometimes beyond control. Therefore, the conservation plans of aquatic ecosystems in tropical forests including those in Malaysia should be established immediately. In order to maintain the high dispersal rate and to minimize the effect of fragmentation, the conservational bridges should be retained among the habitats to maintain natural diversity. The scheduled conservational monitoring programs should be developed by the local government and carried out frequently. Furthermore, intensive ecological research to investigate drivers regulating the community structure of aquatic insects in Southeast Asian streams is recommended.
References
Ali AB, Ahmad M (1988) Water quality in rice fields and sump ponds and its relationship to phytoplankton growth in rice field fish culture system. Trop Ecol 29:63–70
Al-Shami SA, Md Rawi CS, Ahmad AH, Abdul Hamid S, Mohd Nor SA (2011) Influence of agricultural, industrial, and anthropogenic stresses on the distribution and diversity of macroinvertebrates in Juru River Basin, Penang, Malaysia. Ecotox Environ Safe 74:1195–1202
Andren H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Atmar W, Patterson BD (1993) The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96:373–382
Azhar B, Lindenmayer DB, Wood J, Fischer J, Manning A, McElhinny C, Zakaria M (2011) The conservation value of oil palm plantation estates, smallholdings and logged peat swamp forest for birds. Forest Ecol Manag 262:2306–2315
Azrina M, Yap C, Rahim Ismail A, Ismail A, Tan S (2006) Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Langat River, Peninsular Malaysia. Ecotox Environ Safe 64:337–347
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, 2nd edn. EPA 841-B-99-002. USEPA, Office of Water, Washington, DC
Boyero L, Pearson RG, Camacho R (2006) Leaf breakdown in Australian tropical streams: the role of different species in ecosystem functioning. Arch Hydrobiol 166:453–466
Boyero L, Ramirez A, Dudgeon D, Pearson RG (2009) Are tropical streams really different? J N Am Benthol Soc 28:397–403
Boyero L, Pearson RG, Dudgeon D, Ferreira V, Graça MAS, Gessner MO, Boulton AJ, Chauvet E, Yule CM, Albariño RJ, Ramírez A, Helson JE, Callisto M, Arunachalam M, Chará J, Figueroa R, Mathooko JM, Gonçalves JF Jr, Moretti MS, Chará-Serna AM, Davies JN, Encalada A, Lamothe S, Buria LM, Castela J, Cornejo A, Li AOY, M’Erimba C, Villanueva VD, del Carmen Zúñiga M, Swan CM, Barmuta LA (2011) Global patterns of stream detritivore distribution: implications for biodiversity loss in changing climates. Global Ecol Biogeogr 21:134–141
Brown JH, Kodric-Brown A (1977) Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58(2):445–449
Chase JM (2003) Community assembly: when should history matter? Oecologia 136:489–498
Corley RHV (2009) How much palm oil do we need? Environ Sci Policy 12:134–139
Delong MD, Brusven MA (1998) Macroinvertebrate community structure along the longitudinal gradient of an agriculturally impacted stream. Environ Manage 22:445–457
Douglas I, Spencer T, Greer T, Bidin K, Sinun W, Meng WW (1992) The impact of selective commercial logging on stream hydrology, chemistry and sediment loads in the Ulu Segama rain-forest, Sabah, Malaysia. Philos T R Soc B 335:397–406
Dudgeon D (2006) The impacts of human disturbance on stream benthic invertebrates and their drift in North Sulawesi, Indonesia. Freshw Biol 51:1710–1729
Dudgeon D (2008) Tropical streams ecology. Elsevier, North Holland
Dudgeon D (2010) Prospects for sustaining freshwater biodiversity in the 21st century: linking ecosystem structure and function. Curr Opin Environ Sustain 2:422–430
Eikaas HS, Harding JS, Kliskey AD, Mcintosh AR (2005) The effect of terrestrial habitat fragmentation on fish populations in small streams: a case study from New Zealand. Nor Geogr Tidsskr 59:269–275
Elliott JM (1971) Some methods for the statistical analysis of samples of benthic invertebrates. Freshwater Biological Association, Ambleside
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fitzherbert EB, Struebig MJ, Morel A, Danielsen F, Bruhl CA, Donald PF, Phalan B (2008) How will oil palm expansion affect biodiversity? Trends Ecol Evol 23:538–545
Forbes AE, Chase JM (2002) The role of habitat connectivity and landscape geometry in experimental zooplankton metacommunities. Oikos 96:433–440
Gonzalez A, Lawton J, Gilbert F, Blackburn T, Evans-Freke I (1998) Metapopulation dynamics, abundance, and distribution in a microecosystem. Science 281:2045–2047
Gopal B (2005) Does inland aquatic biodiversity have a future in Asian developing countries? Hydrobiologia 542:69–75
Gucker B, Boechat IG, Giani A (2009) Impacts of agricultural land use on ecosystem structure and whole-stream metabolism of tropical Cerrado streams. Freshw Biol 54:2069–2085
Hamid SA, Rawi CSM (2011) Influence of substrate embeddedness and canopy cover on the distribution of Ephemeroptera, Plecoptera and Trichoptera (EPT) in tropical rivers. Aquat Insects 33:281–292
Heino J (2005) Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshw Biol 50:1578–1587
Heino J (2009) Biodiversity of aquatic insects: spatial gradients and environmental correlates of assemblage-level measures at large scales. Freshw Rev 2:1–29
Heino J (2011) A macroecological perspective of diversity patterns in the freshwater realm. Freshw Biol 56:1703–1722
Heino J, Muotka T (2005) Highly nested snail and clam assemblages in boreal lake littorals: roles of isolation, area, and habitat suitability. Ecoscience 12:141–146
Heino J, Soininen J (2007) Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biol Conserv 137:78–89
Heino J, Ilmonen J, Kotanen J, Mykra H, Paasivirta L, Soininen J, Virtanen R (2009) Surveying biodiversity in protected and managed areas: algae, macrophytes and macroinvertebrates in boreal forest streams. Ecol Indic 9:1179–1187
Heino J, MykrÄ H, Rintala J (2010) Assessing patterns of nestedness in stream insect assemblages along environmental gradients. Ecoscience 17:345–355
Hoyle M, Gilbert F (2004) Species richness of moss landscapes unaffected by short-term fragmentation. Oikos 105:359–367
Hulten E (1937) Outline of the history of arctic and boreal biota during the quaternary period. Thule, Stockholm
Ims RA, Petter Leinaas H, Coulson S (2004) Spatial and temporal variation in patch occupancy and population density in a model system of an arctic Collembola species assemblage. Oikos 105:89–100
Iwata T, Nakano S, Inoue M (2003) Impacts of past riparian deforestation on stream communities in a tropical rain forest in Borneo. Ecol Appl 13:461–473
Kasangaki A, Chapman LJ, Balirwa J (2008) Land use and the ecology of benthic macroinvertebrate assemblages of high-altitude rainforest streams in Uganda. Freshw Biol 53:681–697
Kneitel JM, Miller TE (2003) Dispersal rates affect species composition in metacommunities of Sarracenia purpurea inquilines. Am Nat 162:165–171
Koh LP, Wilcove DS (2008) Is oil palm agriculture really destroying tropical biodiversity? Conserv Lett 1:60–64
Larsen S, Ormerod SJ (2010) Combined effects of habitat modification on trait composition and species nestedness in river invertebrates. Biol Conserv 143:2638–2646
Lorion CM, Kennedy BP (2009) Relationships between deforestation, riparian forest buffers and benthic macroinvertebrates in neotropical headwater streams. Freshw Biol 54:165–180
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Malmqvist B, Zhang Y, Adler PH (1999) Diversity, distribution and larval habitats of North Swedish blackflies (Diptera: Simuliidae). Freshw Biol 42:301–314
Mantel N (1967) The detection of disease clustering and generalized regression approach. Cancer Res 27:209–220
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software, Oregon
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America. Kendall Hunt, Dubuque
Monaghan MT, Robinson CT, Spaak P, Ward JV (2005) Macroinvertebrate diversity in fragmented Alpine streams: implications for freshwater conservation. Aquat Sci 67:454–464
Mori T, Murakami M, Saitoh T (2010) Latitudinal gradients in stream invertebrate assemblages at a regional scale on Hokkaido Island, Japan. Freshw Biol 55:1520–1532
Morse JC, Yang L, Tian L (1994) Aquatic insects of China useful for monitoring water quality. Hohai University Press, Nanjing
Mouquet N, Loreau M (2003) Community patterns in source-sink metacommunities. Am Nat 162:544–557
Negishi JN, Sidle RC, Noguchi S, Nik AR, Stanforth R (2006) Ecological roles of roadside fern (Dicranopteris curranii) on logging road recovery in Peninsular Malaysia: preliminary results. For Ecol Manag 224:176–186
Ostman Ö, Kneitel JM, Chase JM (2006) Disturbance alters habitat isolation’s effect on biodiversity in aquatic microcosms. Oikos 114:360–366
Patterson BD, Atmar W (1986) Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol J Linn Soc 28:65–82
Seaby RM, Henderson PA (2006) Species diversity and richness version 4. Pisces Conservation Ltd., Lymington
Sodhi NS, Brook BW (2006) Southeast Asian biodiversity in crisis. Cambridge University Press, Cambridge
Song MY, Leprieur F, Thomas A, Lek-Ang S, Chon TS, Lek S (2009) Impact of agricultural land use on aquatic insect assemblages in the Garonne river catchment (SW France). Aquat Ecol 43:999–1009
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358
Urban MC (2004) Disturbance heterogeneity determines freshwater metacommunity structure. Ecology 85:2971–2978
Wantzen KM (2006) Physical pollution: effects of gully erosion on benthic invertebrates in a tropical clear-water stream. Aquat Conserv 16:733–749
Whittaker RH (1960) Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monogr 30:280–338
Wilcove DS, Koh LP (2010) Addressing the threats to biodiversity from oil-palm agriculture. Biodivers Conserv 19:999–1007
Yule C, Yong H (2004) Freshwater invertebrates of the Malaysian region. Akademi Sains Malaysia, Kuala Lumpur
Acknowledgments
We express our heartfelt gratitude to various people involved in this study; Hazdri Abdullah, Mohd Shukri, Hamzah, Siti Khatijah, Yahya Tahir, Wan Zaki, Kalimuthu for their tireless help in the field. To many others who are directly or indirectly helping us during this study, we are deemed indebted. We are grateful to the Dean, School of Biological Sciences, Universiti Sains Malaysia in Penang, for providing field and laboratory facilities to conduct this research. To Forest Research Institute Malaysia counterparts headed by Dr Christine Fletcher and Dr Abdul Rahman Kassim, we thank them for their financial support, help and understanding. The Conservation of Biodiversity (CBioD) Project is a national project executed by the Ministry of Natural Resources and the Environment and implemented by the Forest Research Institute Malaysia. The CBioD Project is co-funded by the UNDP-GEF (MAL/04/G3) and ITTO [PD 165 02 Rev.3 (F)]. Key partners to the CBioD Project are Perak ITC S/B, Perak SEDC, Forestry Headquarters and State Forestry Departments of Peninsular Malaysia. The project is a joint effort with the University of Miami, Duke University and Harvard University. Thanks for three anonymous reviewers and the handling editor for their constructive comments and suggestions which improve the paper significantly.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael T. Monaghan
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Md Rawi, C.S., Al-Shami, S.A., Madrus, M.R. et al. Local effects of forest fragmentation on diversity of aquatic insects in tropical forest streams: implications for biological conservation. Aquat Ecol 47, 75–85 (2013). https://doi.org/10.1007/s10452-012-9426-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-012-9426-8