Abstract
In a scenario of hydric scarcity, water reuse is essential to minimize make-up; however, increased salinity comes along with recycling streams. Crystallization has been evaluated to remove dissolved ions; nevertheless, contaminants affect its efficiency. In this context, the removal of residual organics from the saline concentrate from electrodialysis applied for water reuse in a Brazilian petroleum refinery was investigated. Aiming at establishing a sustainable virtuous circle, bone char, a waste from food industry, 0.5–1.4 mm particle size, was used as adsorbent for refractory organics removal. Equilibrium adsorption time of 4 h was determined from batch experiments (through 72 h, at 25.0 ± 0.1 °C, pH 7.6 ± 0.5 and 200 ± 1 rpm, solid/liquid ratio of 10 g kg−1) in a shaker and a removal of 54% for dissolved chemical oxygen demand (COD) was observed. Adsorption kinetics (pseudo-first and pseudo-second order; Elovich equation) and equilibrium isotherms were evaluated. Pseudo-second order model for kinetics (R2 = 0.9993; k2 = 0.0184 mg−1 min−1; qe = 3.21 mg g−1) and Freundlich isotherm model best fitted to experimental data (R2 = 0.995), suggesting physisorption in multiple layers. The adsorbent thermal regeneration was evaluated (at 300, 500 and 700 °C, for 60, 120 and 180 min). Partial thermal regeneration was achieved at lower temperature and time demands (300 °C and 60 min—dissolved COD removal was around 40% after the first cycle of regeneration). Further investigation, including scaling-up and a cost-benefit analysis, is necessary in order to assess its use as a viable low-cost alternative for this type of effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water demand in petroleum industry is high and, in a scenario of hydric scarcity, reuse is mandatory to minimize make up (WWAP—United Nations World Water Assessment Programme 2016; Mekonnen and Hoekstra 2016; Paranychianakis et al. 2015). In this context, effluents from oil refineries, in order to be discharged into water bodies, go through wastewater treatment plants that comprise primary (oil and suspended-solid elimination) and secondary (organic load and ammonia removal) stages (Metcalf and Eddy 2014; Santiago 2010; Alva-Argáez et al. 2007). Nevertheless, if water is to be reused, advanced (tertiary) treatment techniques are required to remove dissolved organic or inorganic compounds, such as adsorption onto activated carbon, advanced oxidation processes and membrane separation (including reverse osmosis and electrodialysis for salts) (Garcia et al. 2013; Stensel et al. 2013; Diya-uddeen et al. 2011; Santiago 2010). From one point of view, these techniques provide a high quality permeate for reuse. However, as water recirculates, increasing salinity is inevitably observed in concentrate streams. According to Bechelini et al. (2015), disposal in soil, use in aquaculture basins and solar evaporation, crystallization of salts are options for destination of such brines. However, authors emphasize that the increasing environmental restrictions indicate that brine disposal may not be allowed in the near future, so treatment options will be refractory to the previous biological wastewater treatment (Bechelini et al. 2015).
Besides affecting efficiency of processes to remove dissolved ions, toxicity inherent to typical refractory petroleum organics are also of great concern. Indeed, Bechelini et al. (2015), presented typical characterization for electrodyalisis concentrate stream of a Brazilian petroleum refinery (Table 1) and reported a complex mixture of linear 10 to 20 C hydrocarbons, some with ramifications, but without functional groups. Two aromatic compounds, one benzenic and other phenolic, were exceptions to this trend. Thus, the authors simplified the mixture using five typical organics for modelling. The organics considered in the analysis were three linear alkanes (nonane, 5-(2-methylpropyl), hexadecane, heptadecane), one benzenic and one phenolic compounds—benzene, 1,3-bis (1,1-dimethylethyl)) and phenol, 2,4-bis(1,1-dimethylethyl).
Due to their properties of corrosion, toxicity and carcinogenicity, phenolic compounds are considered priority contaminants by the Environmental Protection Agency (EPA) in the US. For these reasons, Brazilian Agencies imposed the maximum limit of phenols of 0.001 mg L−1 in potable water (Brasil 2011b) and 0.5 mg L−1 of total phenols, for wastewater discharge (Brasil 2011a). Also of high danger is benzene, which is considered the most toxic among BTX (benzene, toluene and xylene) compounds, whose carcinogenicity has been confirmed to be responsible for leukemia and tumors in multiple organs. In fact, acute exposure by inhalation or ingestion is well known to cause death. Thus, the Health Brazilian Agency limits the amount of 0.005 mg L−1 for benzene in potable water (Brasil 2011b) and the Environmental Brazilian Agency establishes 1.2 mg L−1 as the limit in wastewater (Brasil 2011a).
Therefore, residual organics present in saline concentrate of membrane wastewater treatment in petroleum refineries are of great concern not only for the performance of the process, affecting its efficiency, but also for their toxicity. Investigation of their removal could bring about these two important achievements for this type of industry.
One of the potential techniques to be applied for these removals is adsorption, a consolidated process, in general efficient and easy to be well operated. Indeed, literature highlights adsorption has low cost, operational simplicity and low area demand if compared to other advanced wastewater treatment processes such as advanced oxidation processes, reverse osmosis, and others (Bhatnagar et al. 2011; Hashemi 2013; Gwala et al. 2011; Jagtap et al. 2012; Brunson and Sabatini 2014).
The most used adsorbent in water/wastewater decontamination is activated carbon. In fact, organics removal by adsorption in carbon charcoal has been carried out, but with high cost and the performance being affected by increasing salinity. Therefore, other low cost alternatives, such as adsorbents produced from wastes, are becoming more attractive. Among them, bone char may be highlighted for water/wastewater applications, being widely researched for several applications in this field, including removal of fluoride, metals, dyes and pharmaceuticals (Nigri et al. 2017, 2016; Medellin-Castillo et al. 2016; Reynel-Avila et al. 2016, 2015; Brunson and Sabatini 2014; Iriarte-Velasco et al. 2014; Liu et al. 2014; Hashemi et al. 2013; Rojas-Mayorga et al. 2013; Tovar-Gomez et al. 2013; Rocha et al. 2011; Rezaee et al. 2011; Ghanizadeh and Asgari 2011; Moreno-Piraján et al. 2010; Chen et al. 2008; Castilho et al. 2007; Choy and McKay 2005; Cheung et al. 2004; Wilson et al. 2003; Dahbi et al. 1999). Indeed, bone char presents peculiar chemical composition (majorly calcium phosphates, mainly present as hydroxyapatite—HAP, low amounts of calcite—CaCO3 and only 10% in weight of carbon), what differs this material from the conventional activated carbons (mainly constituted of C). In addition, its characteristics of moderate specific surface area (around 90–120m2 g−1), as well as the carbonates present in its structure, derived from its preparation process, make it singular for industrial demands of water reuse.
Besides the efficiency for each specific contaminant removal, two other aspects make bone char promising: its origin, as a waste from tannery, food industry and cattle breeding, and, additionally, the possibility to reuse the apatite, precariously crystallized in the bone char, as a phosphate fertilizer for deficient soils (Siebers et al. 2014, 2013; Smiciklas et al. 2008). Thus, the final aim of this work would be to support the implementation of a sustainable virtuous circle, where bone char could be recycled and used as an adsorbent for other contaminants uptake.
Nevertheless, literature is scarce in applications of organic compounds removal by bone char, applied to real systems. Hashemi et al. (2013) confirmed this investigating the removal of the surfactant sodium dodecyl sulphate from water using bone char. Brunson and Sabatini (2014) analyzed the competition among naturally available organics and fluoride removal from contaminated water using bone char. It was observed no interference of such compounds on fluoride uptake. Rezaee et al. (2011) studied the adsorption of Escherichia coli from air onto bone char, in places subjected to possible contamination, such as hospitals. They reported a removal efficiency up to 99.99%, bringing about an interesting perspective of accessible environment decontamination. Rezaee et al. (2013) also investigated formaldehyde contamination in the air, using acetic acid pre-treated bone char. Acid treatment improved the adsorbent performance, compared to fresh (untreated) bone char. Rezaee et al. (2014) reported a maximum efficiency of 73% for formaldehyde removal by bone char covered with ZnO nanoparticles through photocatalysis decomposition. Another work investigating endotoxins removal from water solutions using bone char showed that 98% of maximum removal, at 40 g L−1 solid/liquid ratio, was achieved (Rezaee et al. 2009). Immersion calorimetry was used by Murillo-Acevedo et al. (2010) to study 2,4-dinitrophenol adsorption by bone char and the amount of dinitrophenol adsorbed depended on the initial concentration, being 30 mg L−1 the solution that exhibited the greatest interaction enthalpy. Other studies, involving dyes removal, have been found in literature, including applications for both anionic or cationic dyes, due to the typical surface charge of bone char (Asgari et al. 2015; Ghanizadeh and Asgari 2011, Ip et al. 2010; Al-Sarawy et al. 2005). Reynel-Avila et al. (2016) tested the adsorption of 3 anionic dyes (acid blue 25, acid blue 74 and reactive blue 4) observing that steric factors might govern dye uptake by bone char, mainly when dynamic is evaluated, suggesting a mechanism of electrostatic adsorbent–solute interactions. Reynel-Avila et al. (2015) assessed the adsorption of naproxen, a pharmaceutical and emerging water pollutant, by bone char in batch conditions. The authors observed it was a feasible and effective process, probably involving electrostatic and non-electrostatic interactions, including a complexation process via phosphate and naproxen, hydrogen bonding and the possibility of hydrophobic interactions via π–π electron. Terasaka et al. (2014) used methylene blue as a model to investigate the removal of organics by bone char produced from thermal treatment of porcine bone, what was possible in the conditions used.
Thus, from the best of present knowledge, no previous investigation of bone char for removal of refractory organics present in real concentrate streams from electrodyalisis in petroleum industry was carried out. Moreover, many aspects need further research, such as the kinetics and mechanisms of organics removal, as well as those concerning bone char regeneration.
In this paper, bone char was evaluated as the adsorbent for the treatment of a concentrate stream from electrodialysis used in tertiary aqueous effluent treatment of a Brazilian petroleum unit, aiming at the reduction of the content of organics in the saline feed to the crystallization unit. Equilibrium and kinetics data were obtained to interpret the possible mechanisms. Different conditions for thermal regeneration of bone char were tested, so that the best methodology, among the investigated conditions, could be suggested. This work is a new contribution for the integration of adsorption, membrane separation process (electrodialysis) and wastewater treatment operations, thus leading to water reuse increase in refining industries. This kind of association is essential for a proper saline waste disposal and for an optimized process sustainability.
2 Experiments
2.1 Preparation of adsorbent and samples
2.1.1 Bone char
Bone char, the adsorbent used in the present work, was supplied by Bonechar Carvão Ativado do Brasil—Maringá—Brazil. The material was produced at temperature of 700 to 750 °C, in a furnace, for 8 h of calcination (Nigri et al. 2017). Sample reduction was carried out according to Brazilian technical standards (ABNT NBR NM 27:2001) and particle size analysis was carried out by sieving, in vibrating system (Bertel Indústria Metalúrgica—Ltda), for 15 min at 5 rpm. Particles of 12–32 mesh size (0.5–1.4 mm) were selected to the adsorption experiments and washed 4 times using distilled water (500 g of bone char to 2 L water) to remove tiny particles that could be adhered to the surface of the bone char. Finally, bone char was dried in a furnace (Sterilifer SX1.1 DTME, series 0057) at 120 °C for 2 h (Ribeiro 2011).
2.1.2 Electrodialysis concentrate effluent (C-EDR)
The real effluent was sampled from the saline concentrate line of electrodialysis, the last step of advanced wastewater treatment in the real refinery. It was stored in 10 to 20 L plastic flasks, after being appropriately cleaned, washed and rinsed.
The characterization of the effluent was carried out according to Standard Methods for Analysis of Water and Wastewater (APHA 2012), for the parameters: filtered Chemical Oxygen Demand (COD) (closed reflux colorimetric method, low range of concentration), conductivity, turbidity, redox potential (Eh), Total Dissolved Solids (TDS), Temperature (°C), pH, Dissolved Oxygen (DO), Salinity (instrumental analysis, Horiba series U-50 multiparameter), Chlorides (argentometric method) and Ca, Sr, Mg, Na (Atomic Absorption, GBC, Avanta model spectrometer). The same parameters were measured after 4 h of adsorption batch experiments (see next section) at (25.0 ± 0.1)°C, pH=(7.6 ± 0.5), (200 ± 1) rpm, solid/liquid ratio of 10 g kg−1.
2.1.3 Dissolved organics determination
Dissolved organics were quantified using Closed Reflux Colorimetric Methodology for low concentration range of Chemical Oxygen Demand (COD) as described in Standard Methods (APHA 2012). Previously to COD tests, samples were passed through 8 µm quantitative filter paper, and then, through 0.22 µm disposable membranes (Millipore Millex GV. Hydrophilic PVDF 0.22 µm), so that bone char residuals and other suspended solids could be eliminated and only dissolved organics would be quantified. Dissolved COD percentual removal was calculated as shown in Eq. (1).
where ER is the percentual dissolved chemical oxygen demand removal, CODin is inlet dissolved chemical oxygen demand and CODout is theoutput chemical oxygen demand.
Refractory organics qualitative characterization for C-EDR effluent before and after adsorption equilibrium study (4 h adsorption batch experiments at 25.0 ± 0.1 °C, pH 7.6 ± 0.5, 200 ± 1 rpm, solid/liquid ratio of 10 g kg−1) was carried out at University of São Paulo (USP). It was used gas chromatography/mass spectrometry (GC/MS Shimadzu QP 2010 plus; capillary column Agilent DB-5MS 30 mm × 0.25 mm; injection volume 1 µL; heat flow rate 7 °C min−1, from 40 to 310 °C).
2.2 Adsorption experiments
Adsorption pH was monitored (HANNA pH 21), rather than controlled, since buffer capacity of bone char led the system to approximately neutral pH in preliminary tests. Equilibrium adsorption time and kinetics adsorption were determined from batch experiments at three different temperatures, 30.0 ± 0.1, 25.0 ± 0.1 and 20.0 ± 0.1 °C, and pH 7.7 ± 0.5, 7.6 ± 0.5 and 7.9 ± 0.1, respectively. Kinetics experiments lasted for 72 h, at a stirring rate of 200 ± 1 rpm, solid/liquid ratio 10 g kg−1, in a controlled temperature shaker (TECNAL-TE-421). Batch adsorption tests were also carried out in the same shaker, by varying solid/liquid ratios (0.5, 1.0, 2.0, 4.0, 8.0, 10.0, 12.0, 14.0, 16.0, 32.0 and 64.0 g of bone char/kg of effluent). Langmuir and Freundlich isotherm models were evaluated to fit experimental data. Adsorption kinetics was evaluated according to pseudo-first and pseudo-second order models, as well as the Elovich equation.
2.3 Thermodynamics description
Thermodynamics was evaluated from the calculation of entropy, enthalpy and Gibbs free energy variation from the results of the kinetic studies at three different temperatures (30.0 ± 0.1, 25.0 ± 0.1 and 20.0 ± 0.1 °C), pH 7.7 ± 0.5, 7.6 ± 0.5 and 7.9 ± 0.1, respectively, at a stirring rate of 200 ± 1 rpm, solid/liquid ratio of 10 g kg−1).
The entropy and enthalpy were obtained from the plot of ln kc versus \(\frac{1}{T}\), according to Eq. 2.
where R is the gases constant (8.314 J mol−1 K−1), T is the temperature (K), and kc is the adsorption equilibrium constant, as shown in Eq. 3.
where c0 is the initial COD concentration (mg L−1), ce is the equilibrium adsorbate concentration (mg L−1), ρ is the solution density (g L−1), V is the effluent volume (L), m is the adsorbent mass (g), qe is the concentration of organics in the solid phase, expressed as COD (mg g−1).
Gibbs free energy variation (ΔG) was estimated as shown in Eq. (4).
2.4 Bone char regeneration
Bone char regeneration was carried out thermically, using a furnace (SP Labor, SP-1200; serie 286), varying temperature and time. The conditions were chosen based on a 22 factorial design (Statistics®). The results and errors were statistically analyzed (F-test was conducted). Response surface analysis was carried out to outline favorable conditions of regeneration. Table 2 summarizes central and cubic points used in experiments.
After regeneration, one cycle of adsorption using the regenerated material was conducted, so that efficiencies of removal could be compared to the new bone char and the best methodology, among the tested conditions, could be pointed out.
2.5 Characterization
Samples of new bone char (BC-N), saturated bone char (BC-S), thermically regenerated bone char at 300 °C and 60 min (BC-R) and thermically regenerated bone char at 700 °C and 180 min (BC-R*) were characterized. X-ray diffraction was carried out for identification of crystalline phases (powder method or Debye Scherrer in an X-ray diffractometer Panalytical, model Empyrean, using a copper tube and a 2θ scan). X-ray fluorescence was used to determine the presence of chemical elements (PHILIPS PW-2400 model). Fourier transformed infrared spectroscopy, FTIR (Perkin Elmer FTIR 1760X) was also used to access the surface groups on bone char samples prepared in a KBr anidre disk. Surface area and porosity were determined by BET multipoints analysis (Nova 1200E), using N2, preparation temperature of 80 °C, in the vacuum. Thermogravimetric (TG) analyses were carried out (Perkin-Elmer STA 6000 analyzer) to evaluate mass loss and were performed from 30 to 900 °C, applying a heating rate of 10 ºC min−1, using N2 gas (flow of 20 mL min−1). Particles of bone char were observed at scanning electron microscope—SEM (JEOL LSM 6360LV).
3 Results and discussion
3.1 C-EDR effluent characterization and refractory organics
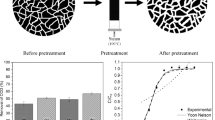
C-EDR had diverse composition, as it was a real process stream. Thus, the average value was obtained from two different lots of effluent to compose its description. The same quality parameters were measured for the treated effluent that had been submitted to bone char adsorptive treatment in a shaker, for 4 h, at (25.0 ± 0.1)°C, pH (7.6 ± 0.5), (200 ± 1)rpm, solid–liquid ratio 10 g kg−1 (Fig. 1).
The results for pH, Cl− e Sr2+ were similar to the mean value characterized by Bechelini et al. (2015). However, this was not observed for conductivity, Na+ e Mg2+. As it is a real process effluent, its characteristics may vary significantly, depending on the process conditions on the day of sampling.
Comparing C-EDR before and after adsorption (Fig. 1), bone char did not seem to interfere significantly in pH, conductivity, total dissolved solids, salinity and turbidity.
The buffer capacity provided by the carbonates and phosphates of the bone char in contact with the effluent was responsible for pH stability. As far as salinity and conductivity are concerned, as both are related to total dissolved solids and no significant change was observed after adsorption, it was concluded that bone char acted preferably in order to remove the organics. On the other hand, for chloride, concentrations raised in 56% after bone char adsorption, suggesting chloride was released into the effluent, what was confirmed by GC/MS (formation of dodecanoyl chloride). The same trend was observed for magnesium (from 25.0 to 56.0 mg L−1, before and after adsorption, respectively).
About qualitative GC/MS characterization, the non-treated C-EDR was a mixture of long chain hydrocarbons, mostly alkanes and alkenes. Indeed, alkanes are not very reactive and have low biological activity, the reason why they are present in the concentrate stream of electrodyalisis. Furthermore, aromatics, carboxylic acids, amines and amides had also been identified. The most prominent peaks were: (Z)-9-octadecenamide, 1,22-dibromo-docosane, hexadecane, 2-deoxy-bis(thioheptyl)-d-ribose, 2,3-bis[(trimethylsilyl)oxy]propyl ester eicosanoic acid, octadecanoic acid, 3-oxo-, methyl ester, 2,4-bis(1,1-dimethylethyl)-phenol, 4-ethyl-undecane, 1-hexadecyl-2,3-dihydro-1H-indene.
After adsorption, it was observed that, from the 70 refractory organics in the influent, 25 were not identified in the effluent any longer; therefore, they had been retained onto the bone char: 10-methylnonadecane, N,N-dipropyl-buten-1-amine, 1-hexadecyl-2,3-dihydro-1H-indene, 4-methyl-1-undecene, 7-methyl-1-undecene, 2-(1-methylethyl)-3-methyl ester (2R-cis)-3,4-oxazolidinedicarboxylic acid, 4-methyl-decane, DEHP (diethylhexylphtalate), 2,6,11-trimethyl-dodecane, 2-methyl-heptadecane, tetradecane, tetrapentacontane, tetratetracontane, tetratriacontane, 1-[[[(2-aminoethoxy)hydroxyphosphinyl]oxy]methyl]-1,2-ethanediyl ester hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester-hexadecanoic acid, 1-methyl-naphthalene, Nonacosane, 5-methyl-5-propyl-nonane, octacosane, 2-hydroxy-1-(hydroxymethyl)ethyl ester octadecanoic acid, 6-ethyl-2-methyl-octane, 4-ethyl-undecane 1,1,1-trimethyl-N,N-bis[2-[(trimethylsilyl)oxy]ethyl]-silanamine.
Literature reports that, since the main phase present in bone char is hydroxyapatite, most surface groups (phosphates, hydroxyls and also calcium carbonate) are negative at pH above 5 (Rocha et al. 2011; Nigri et al. 2016), which was the case in this investigation. Therefore, at the beginning, possible interactions with the organic compounds would occur through hydrogen bonds between electronegative heteroatoms such as oxygen, present in the phosphate, carbonate and hydroxyl groups of bone char, and the hydrogen from O–H and N–H of the carboxylic acids, phenols, amines and amides contained in the effluent. Then, weak interactions, such as van der Waals, among long chains of hydrocarbons adhered to the solid, could be present. Reynel-Avila et al. (2015) also justified naproxen uptake by bone char as a result electrostatic and non-electrostatic interactions, including a complexation process via phosphate and naproxen, hydrogen bonding and the possibility of hydrophobic interactions via π–π electron.
3.2 Equilibrium study
C-EDR organics adsorption onto bone char was monitored throughout 72 h of experiments. At room temperature (25.0 ± 0.1 °C), equilibrium was achieved within 4 h, time after which a variation inferior to 5% was noticed. Thus, adsorption tests were carried out for 4 h, at different solid–liquid ratios, pH of 7.6 ± 0.5, at stirring rate of 200 ± 1 rpm. Langmuir and Freundlich models were fitted to experimental data as shown in Fig. 2 and Table 3. Although both models showed very high determination coefficients (R2 > 0.99) and all of them at a level of significance of 5% (p values), the fundamental Freundlich model seemed to better describe the system, under the tested conditions, taking into account the parameters obtained from the models (Table 3). In fact, the Langmuir parameters were negative, what is possible to occur for adsorption evaluation in aqueous systems, which was the case, suggesting that such a model is not suitable for describing the system C-EDR oganics-bone char. Indeed, the Langmuir hypothesis of monolayer chemical adsorption is certainly far from the real system, since residual organic matter in the effluent is mostly comprised of long chain hydrocarbons (C10 or greater), as was discussed previously. On the other hand, according to Worch (2012), the Freundlich isotherm is widely used to describe adsorption in water treatment processes with activated carbon, and is even used for predictive models for multicomponent adsorption. In addition, Ciola (1981) and McKay (1996); Nascimento et al. (2014) reinforce its application to non-ideal systems, in heterogeneous surfaces and adsorption in multilayer.
Furthermore, considering that the range of organics concentration in the C-EDR is around 30 mg L−1 in terms of dissolved organic carbon and approximately 60 mg L−1, in terms of COD, the Freundlich model is also adequate. It describes well the mean range of concentrations, depicting neither the linear range, occurring at very low concentrations, nor the saturation effect, present at very high concentrations (Worch 2012). Thus, it is assumed the heterogeneity of the solid, applying an exponential distribution to characterize the various types of adsorption sites, of different adsorptive energies (Freundlich 1906; Febrianto et al. 2009). Yet, according to Thommes et al. (2015), Freundlich model indicates physisorption mechanism. Thus, temperature and thermodynamics were evaluated to assess if this was the case and will be discussed in the following section.
Maximum organics removal (64% in terms of COD) was achieved in a solid–liquid ratio of 64 g kg−1 (Fig. 3). Nevertheless, 10 g kg−1 was chosen as the basis of solid–liquid ratio in kinetics experiments, since under such a condition, a removal of 45% COD was possible (representing more than 70% of the maximum removal percentage) applying six times less adsorbent, what may represent a strong advantage concerning future industrial applications.
3.3 Temperature and thermodynamics
As temperature increased, adsorption capacity decreased, showing that an exothermic process had occurred, suggesting physisorption mechanism of uptake of residual organics by bone char. Calculation of thermodynamics parameters, reported in Table 4, confirmed exothermic adsorption in the system (ΔH = − 54.30 kJ mol−1).
As ΔH < 0, according to Marczewski et al. (2016), the process is enthalpy favored and, although entropy has low but negative value, partially negating the enthalpy effect, spontaneity is confirmed with negative Gibbs free energy. ΔH value was neither so low as 40 kJ mol−1, in terms of energy, what is admitted by literature as a high limit for physisorption, nor so high as 80 to 450 kJ mol−1, energy values reported by literature for chemisorption to take place, compared to chemical reactions energy values (Wang et al. 2010). However, gathering the information previously described and considering this refers to a real system, where more than seventy long chains organic compounds are present, hydrogen bonds between bone char and carboxylic acids, or amines, or amides, may happen. Indeed, the high value of ΔH might suggest that not only Van der Waals interactions are present, but also electrostatic ones (Monash and Pugazhenthi 2009).
Rocha et al. (2011) studied the zeta potential of bone char for pH > 5.0 and, as the main phase present in the bone charcoal is hydroxyapatite, most of the surface groups (phosphates, hydroxyl and also carbonate from calcite) are negative at pH higher than 5, which was the case. Nevertheless, as cations are also present (calcium sites) the uptake of anions is still possible. Thus, the few possible electrostatic interactions with organics—between anionic groups of bone char and H+ of phenolic compounds, or carboxylic acids, amines or amides, for example, or between Ca2+ and phenolic OH−, might take place first. Then, weaker interactions, such as van der Waals ones, among long chains hydrocarbons adhered to the solid, might be present.
The type of BET characterization isotherm obtained for the bone char suggests type 5, which is typical of relatively weak adsorbent-adsorbate interactions (Thommes et al. 2015). Another important aspect to reinforce physisorption assumptions was that thermal regeneration, that will be described later on, was possible.
Gibb’s energies show spontaneous nature and feasibility of residual organics sorption by bone char, being the process more energetically favored as temperature decreases. The negative ∆S values suggest the decrease in disorder, what may be due to the decrease of adsorbate concentration in solid-solution interface, indicating thereby the increase in adsorbate concentration onto the solid phase (Rincón-Silva et al. 2016; Goswami and Ghosh 2005). Indeed, according to Goswami and Ghosh (2005), this is a normal consequence of the physical adsorption phenomenon, which takes place through electrostatic interactions as suggested earlier.
3.4 Kinetics of residual organics removal by bone char
C-EDR residual organics adsorption onto bone char was monitored throughout 72 h of experiments. For room temperature (25.0 ± 0.1 °C) it could be noticed an acute removal within only 2 h of experiments and equilibrium was reached after 4 h, achieving 54% in terms of dissolved COD removal (C/Co = 0.46), as shown in Fig. 4. The initial rapid adsorption may be attributed to the occupation of active sites by large molecules of hydrocarbons and less apolar ones, as described previously (see Sect. 3.1). Once these sites are full, slow pore diffusion of the solute into the adsorbent happens, due to steric hindrance. As multilayers may possibly be formed, each molecule would be a new adsorption site to the others.
The same equilibrium time was observed at 35.0 ± 0.1 °C. Nevertheless, removal of organics by bone char decreased (around 31% of removal). According to Marczewski et al. (2016) the effect of temperature on organics adsorption for water applications is of great relevance, once it depends on the species solubility in water, which is directly affected by temperature. In other words, despite the fact that equilibrium time was the same, adsorption of residual organics by bone char was less favored at higher temperature, which is typical when exothermic adsorption takes place. Indeed, this was the case when the thermodynamics parameters were calculated, as presented in Table 4.
On the other hand, studying the system at 20.0 ± 0.1 °C, final removal was similar to that at ambient temperature (around 50%); however, equilibrium was reached only after 18 h of experiment, suggesting that lower temperatures could diminish organics uptake rate by bone char. According to Al-Qodah (2000), changes in temperature are known to influence the adsorbent capacity in equilibrium for a given adsorbate, which seemed to be the case. Actually, increasing temperature leads to enhancing diffusion rate of adsorbed molecules through external and internal pore layers, due to decrease in viscosity of the solution.
Kinetics modelling was carried out and pseudo-second order model best fitted experimental data as shown in Table 5. Parameters for different conditions of temperature and pH are also reported. Pseudo-second order model is based on adsorption capacity of the solid phase (Crini and Badot 2008). It is assumed that the difference between solid phase concentration at any time and at equilibrium is the driving force of adsorption and adsorption global rate is the square of the driving force (Ho and McKay 1999). The model previewed, at ambient temperature, a qe value of 3.205 mg g−1, which is very close to the real value (qe = 3.225 mg g−1).
3.5 Thermal regeneration of bone char
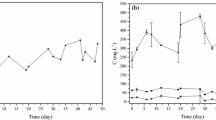
Economic feasibility depends directly on the regeneration capacity of an adsorbent. In this context, thermal regeneration of bone char saturated with residual C-EDR organics was carried out in a furnace, in different operational conditions of temperature and time, according to a 22 factorial design, and both factors were found to interfere on the thermal regeneration process, as shown in the Pareto chart of Fig. 5a. F test was conducted and calculated F value (44.37) was more than ten times the table F value (4.26) for a level of significance of 5%, indicating that there are evidences of significant difference among the conditions, related to COD percentual removal.
Response surface study for percentual chemical oxygen demand (COD) removal after first cycle of thermal regeneration of bone char, a pareto chart, for intervenient factors furnace temperature and time; b contour surface, showing favorable regeneration condition at 300 °C and 60 min. (Color figure online)
Curiously, better results were obtained at lower temperature and time conditions (300 °C and 60 min), what represents an attractive gain from the point of view of process, as costs with energy may be optimized, compared to the other conditions, more time consuming or more energetically demanding. After the first cycle of the regeneration process, adsorption tests showed COD removal by regenerated bone char around 40%, as can be seen in Fig. 5b. As an example, the five model residual organic compounds present in C-EDR, presented by Bechelini et al. (2015), which were nonane,5-(2-methylpropyl); hexadecane, heptadecane, benzene, 1,3-bis (1,1-dimethylethyl)) and phenol, 2,4-bis(1,1-dimethylethyl), have boiling point inferior to 300 °C, so that it is assumed they had enough energy to escape from the substrate. In fact, as van der Waals interactions are weak, an energy amount in the order of magnitude of vaporization-condensation of such compounds would be sufficient to promote desorption. On the other hand, high temperatures and times in the furnace led to decrease in adsorption capacity, and bone char effiency for COD removal vanished.
Possible explanations for this observation would be changes in the bone char crystalline structure or the decrease of surface area by raising calcination temperature, as observed and reported by Medellin-Castillo et al. (2016), studying fluoride uptake by bone char. Indeed, these authors reported a linear decrease in the adsorption capacity of bone char as calcination temperature raised from 400 to 800 °C and a difference in crystalline structure of calcium phosphates present in bone char.
It is important to highlight that the color of bone char had completely changed in this condition of regeneration (the black natural aspect of the bone char changed completely into a white powder). Indeed, Rocha et al. (2011), in thermogravimetric analysis (TGA) of similar bone char, observed that the CO2 loss for calcite starts at 675 °C. Thus, some characterization techniques were applied in order to elucidate about possible adsorption–desorption mechanisms.
3.6 Characterization of bone char
New bone char particles (0.5–1.4 mm) presented surface area of 90 m2/g and mesoporous structure (porous diameter of 5.7 nm and total porous volume of 0.2513 cm3 g−1, determined at 80 °C by BET multiple points method. Surface area was inferior from those reported by Nigri et al. (2016), 139 m2 g−1, and Ribeiro (2011), 119 m2 g−1, using bone char from the same supplier, to remove fluoride from aqueous solutions. Tovar-Gòmez et al. (2013) also obtained higher surface area studying bone char from two different suppliers (104 and 129 m2 g−1).
For porosity, mesoporous structure corroborate results reported by literature (Nigri et al. 2016; Rojas-Mayorga et al. 2015; Tovar-Gòmez et al. 2013; Ribeiro 2011), in spite of presenting thinner porous length. Sample density was 1 g cm−3 while liquid density was estimated at 0.808 g cm−3.
Taking into account the hysteresis loop obtained by BET analysis, shown in Fig. 6, bone char N2 isotherm would be classified into Type V, according to Thommes et al. (2015), in their IUPAC Technical Report, concerning new proposed updated classification of physisorption isotherms. According to the authors, for Type V isotherms shape, for low range of p/p0, relatively weak micro or mesoporous adsorbent-adsorbate interactions are present, what makes it, in this region, very similar to Type III isotherms; as p/p0 increases, “molecular clustering is followed by pore filling”.
The shape of hysteresis loop is very close to type H3, according to IUPAC Technical Report (Thommes et al. 2015), suggesting that the bone char could be constituted by non-rigid aggregates of plate-like particles or macropores of the pore network that are not completely filled.
Indeed, X-ray diffraction (XRD) analysis showed a relatively amorphous material and few peaks could be distinguished in the analysis. However, minor amounts of calcite could also be identified by the XRD (main peak around 3.0 Å). In fact, for new bone char, saturated bone char and regenerated bone char at the lowest temperature (300 °C), the crystallographic planes corresponding to the hydroxyapatite (HAP) were not well defined (Fig. 7).
In other words, a proper crystalline structure of HAP could not be achieved either during bone char production or during its regeneration at 300 °C and 60 min. Medellin-Castillo et al. (2016), while studying the influence of calcination temperature on crystalline phases, crystallite size, obtained similar results and contents of hydroxyapatite, monetite, and other calcium phosphates, present in their synthesized bone char. The authors also observed that for 400 °C of calcination temperature, HAP in bone char did not present a proper crystalline structure. On the other hand, for 800 °C of calcination temperature, the authors had reported all characteristic planes of hydroxyapatite. Furthermore, it is interesting to highlight that thermal regeneration at higher temperature and time in the current work (700 °C, 180 min) also seemed to provide a more crystalline structure to the HAP present in bone char used in this study (Fig. 7d), as the peaks at 2θ = 22.1, 27.0, 39.5 and 41.8 became more prominent. In addition, two other peaks that were not observed in Fig. 7a–c, could be outlined: at 2θ = 27.9 and 33.7. Nevertheless, this represented a loss in adsorption capacity, as previously discussed.
In thermogravimetric analysis, the three typical mass loss areas for bone char were present (humidity, organic carbon loss and decarbonation from the calcite or dehydroxylation from hydroxyapatite) for the samples of new bone char, saturated bone char and regenerated bone char at 300 °C, 60 min (Fig. 8) (Nigri et al. 2017; Rojas-Mayorga et al. 2013; Rocha et al. 2011).
However, higher temperature of regeneration (700 °C) seemed to cause some structural modification in the bone char, as the mass loss was extremely different for this sample (Fig. 8d). In fact, it was neither possible to clearly distinguish the typical three regions of mass loss (Nigri et al. 2017; Rojas-Mayorga et al. 2013; Rocha et al. 2011), nor to attribute the typical percentages of each one, as the total weight loss did not even reach 3%.
Taking into account that carbonates from calcite and hydroxyls from hydroxyapatite are active sites for the refractory organics, as previously discussed, higher temperatures, leading to CO2 release, would represent another important cause of lowering organics removal. On the other hand, for the regenerated bone char at lower temperature and time (300 °C and 60 min) the thermogravimetric analysis curve was very close to that obtained for new bone char. In other words, from the point of view of thermal degradation, nothing happened and the structure of the adsorbent was maintained. Hence, high recovery of COD removal efficiency was observed.
For the saturated bone char, TGA clearly showed the presence of the adsorbed organic matter, evidenced by the pronounced mass loss observed up to 200 °C (about 9.0%, against 4.8% in the same region, observed in new bone char thermogram).
Scanning Electronic Microscopy (SEM) shows high porosity and irregularities on the bone char surface, as shown in Fig. 9. Rocha et al. (2011) identified chemical species on the particle surface of bone char by SEM and the authors observed high contents of calcium and phosphorous, as expected, being these the major elements, in accordance with the origin of the solid (cow bones, constituted of hydroxyapatite, which is the bearing phase of phosphorus).
X-ray fluorescence diagrams (suppressed) showed presence of P, S, Ca, Si, Al, Mg, Na, O, Fe, Ca and K for samples of new, saturated and regenerated bone char, corroborating literature reports (Nigri et al. 2016; Rojas-Mayorga et al. 2013; Ribeiro 2011). Indeed, Rocha et al. (2011) reported presence of CaO, TiO2, P2O5, Na2O, S, K2O, SiO2, MnO, Al2O3,Fe2O3, MgO.
FTIR analysis for (a) C-EDR effluent, (b) new bone char, (c) saturated bone char, (d) bone char regenerated at 300 °C and 60 min and (e) bone char regenerated at 700 °C and 180 min are shown in Fig. 10. Effluent curve (Fig. 10a) shows water peaks. However, besides being possible to attribute peak of 1638 to water (wavenumber around 1620 cm−1 shows H–O–H deformation, Oliveira et al. 2010), aromatic C=C are also around 1600. Thus, this FTIR was not a clarifying methodology for effluent composition determination.
Back to water in bone char samples, a small band of 1620 cm−1 is present in new, saturated and lower temperature regenerated bone char. On the other hand, this is not present in the bone char that has been regenerated at high temperature (700 °C, 180 min), indicating that, although all the samples had been previously dried, some traces of humidity could be present in samples b, c and d. Another point to consider is that peak 3400 (OH from water) disappeared for high temperature regenerated bone char and a new peak at 3571 cm−1 emerged. This can be attributed to OH of hydroxyl, what reinforces the assumption of structural changes after high temperature regeneration.
As expected, carbonate group is present in all samples of bone char (1463 and 884 cm−1) as well as bands located at 1026 cm−1, at 603 cm−1 and 565 cm−1 which are due to the absorption by the phosphate group (Rocha et al. 2011; Purevsuren et al. 2004; Nakamoto 1977; Farmer 1974).
4 Conclusions
Residual organics present in real concentrate effluent of electrodyalisis of an oil refinery could be adsorbed by bone char in batch laboratory tests and the process was spontaneous and exothermic (ΔG25°C = − 11.36 kJ mol−1 and ΔH = − 54.30 kJ mol−1). Equilibrium adsorption time at ambient temperature was 4 h, for 10 g bone char kg−1effluent and a removal of 54% of dissolved COD was achieved. Kinetics could be described by pseudo-second order model and Freundlich isotherm model best fitted equilibrium experimental data (R2 = 0.995), suggesting that molecules were adsorbed in multiple layers, probably due to the presence of long chains organic compounds, in the saline stream. The use of bone char is still considerable (achieving 64% for 64 g bone char kg−1effluent) since partial regeneration was possible. Thermal regeneration was achieved at not very high temperature and time demands (300 °C and 60 min), conditions under which adsorption capacity was well recovered (COD removal around 40% after the first cycle of regeneration). Thus, bone char used in lab scale tests removed, with considerable efficiency, refractory organics from saline streams of electrodyalisis of petroleum refineries. Further investigation, including scaling-up and a cost-benefit analysis, is necessary in order to assess its use as a viable low-cost alternative for this type of effluent.
References
Al-Qodah Z.: Adsorption of dyes using shale oil ash. Water Res. 17, 4295–4303 (2000)
Al-Sarawy, A., Rashed, I.G., Hanna, M.A., Wali, F.K.M.: Removal of some 4-pyrazolone dyes from aqueous solutions by adsorption onto different types of carbon. Desalination 186(1), 129–153 (2005)
Alva-Argáez, A., Kokossis, A.C., Smith R.: The design of water-using systems in petroleum refining using a water-pinch decomposition. Chem. Eng. J. 128, 33–46 (2007)
APHA—American Public Health Association. Standard methods for examination of water and wastewater. APHA, Washington, DC (2012)
Asgari, G., Almas, H., Fardmal, J., Ghanbari, F., Darai, Z., Akbari, S.: Optimization of catalytic ozonation process for removal of reactive black 5 dye using bone char ash modified by magnesium oxide and applying taguchi design. J. Mazandaran Univ. Med. Sci. 24(122), 252–264 (2015)
Brasil—Ministério do Meio Ambiente. Resolução CONAMA n° 430, de 13 de maio de 2011. Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução nº 357, de 17 de março de 2005, do Conselho Nacional do Meio Ambiente-CONAMA. Diário Oficial da República Federativa do Brasil, Poder Executivo, Brasília (2011a)
Brasil—Ministério da Saúde. Portaria nº 2.914, de 12 de dezembro de 2011. Dispõe sobre os procedimentos de controle e de vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Diário Oficial da República Federativa do Brasil, Poder Executivo, Brasília (2011b)
Bechelini, E.M.A., Borba, R.P., Seckler, M.M., Rocha, S.D.F.: Water recovery from saline streams produced by electrodialysis. Environ. Technol. J. 36(3), 386–394 (2015)
Bhatnagar, A., Kumar, E., Sillanpaa, M.: Fluoride removal from water by adsorption: a review. Chem. Eng. J. 171, 811–840 (2011)
Brunson, L.R., Sabatini, D.A.: Practical considerations, column studies and natural organic material competition for fluoride removal with bone char and aluminum amended materials in the main Ethiopian Rift valley. Sci. Total Environ. 488–489, 580–587 (2014)
Castilho, N.A.M., Ramos, R.L., Perez, R.O., Cruz, R.F.G., Pina, A.A., Rosales, J.M.M., Coronado, R.M.G., Rubio, L.F.: Adsorption of fluoride from water solution on bone char. Ind. Eng. Chem. Res. 46, 9205–9212 (2007)
Chen, Y.-N., Chai, L.-Y., Shu, Y.-D.: Study of arsenic(V) adsorption on bone char from aqueous solution. J. Hazard. Mat. 160, 168–172 (2008)
Cheung, C.W., Choy, K.K.H., Ko, D.C.K., Mckay, G., Porter, J.F.: Sorption equilibria of metal ions on bone char. Chemosphere. 54(3), 273–281 (2004)
Choy, K.K.H., Mckay, G.: Sorption of cadmium, cooper, and zinc ions onto bone char using Crank diffusion model. Chemosphere. 60(8), 1141–1150 (2005)
Ciola, R.: Fundamentos da catálise. [Fundamentals of catalysis]. Moderna, São Paulo (1981)
Crini, G., Badot, P.M.: Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption process using batch studies: a review of recent literature. Progr. Polym. Sci. 33(4), 399–447 (2008)
Dahbi, S., Azzi, M., De La Guardia, M.: Removal of hexavalent chromium from wastewaters by bone charcoal. Fresenius’ J. Anal. Chem. 363, 404–407 (1999)
Diya-Uddeen, B.H., Daud W., AbduL Aziz, A.R.: Treatment technologies for petroleum refinery effluents: a review. Process. Saf. Environ. Prot. 89, 95–105. (2011) (2011)
Farmer, V.C.: The infrared spectra of minerals. Mineralogical Society, London (1974)
Febrianto, J., Kosasih, A.N., Sunarso, J., Ju, Y.H., Indraswati, N., Ismadji, S.: Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater. 162(2–3):616–645 (2009)
Freundlich, H.M.: Over the adsorption in solution. J. Phys. Chem. A 57, 385–470 (1906)
Garcia, N., Moreno, J., Cartmell, E., Rodriguez-Roda, I., Judd, S.: The application of microfiltration-reverse osmosis/nanofiltration to trace organics removal for municipal wastewater reuse. Environ. Technol. 34, 3183–3189 (2013)
Ghanizadeh, G.H., Asgari, G.: Adsorption kinetics and isotherm of methylene blue and its removal from aqueous solution using bone charcoal. React. Kinet. Mech. Cat. 102, 127–142 (2011)
Goswami, S., Ghosh, U.C.: Studies on adsorption behaviour of Cr (VI) onto synthetic hydrous stannic oxide. Water 31 (4) (2005)
Gwala, P., Andey, S., Mhaisalkar, V., Labhasetwar, P., Pimpalkar, S., Kshirsagar, C.: Lab scale study on electrocoagulation defluoridation process optimization along with aluminium leaching in the process and comparison with full scale plant operation. Water Sci. Technol. 63, 2788 (2011)
Hashemi, S., Rezaee, A., Nikodel, M., Ganjidost, H., Mousavi, S.M.: Equilibrium and kinetic studies on the adsorption of sodium dodecyl sulfate from aqueous solution using bone char. React Kinet Mech Cat. 109, 443–446 (2013)
Ho, Y.S., McKay, G.: Pseudo-second order model for sorption processes. Proc. Biochem. 34(5), 451–465 (1999)
Ip, A.W.M., Barford, J.P., McKay, G.: Biodegradation of reactive black 5 and bioregeneration in upflow fixed bed bioreactors packed with different adsorbents. J. Chem. Technol. Biotechnol. 85, 658–667 (2010)
Iriarte-Velasco, U., Ayastuy, J.L., Zudaire, L., Sierra, I.: An insight into the reactions occurring during the chemical activation of bone char. Chem. Eng. J. 251, 217–227 (2014)
Jagtap, S., Yenkie, M.K., Labhsetwar, N., Rayalu, S.: Fluoride in drinking water and defluoridation of water. Chem. Rev. 112(4), 2454–2466 (2012)
Liu, J., Huang, X., Liu, J., Wang, W., Zhang, W., Dong, F.: Adsorption of arsenic(V) on bone char: batch, column and modeling studies. Environ. Earth Sci. 72, 2082–2090 (2014)
Marczewski, A.W., Seczkowska, M., Deryło-Marczewska, A., Blachnio, M.: Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon: effect of temperature. Adsorption 22, 777–790 (2016)
McKay, G.: Use of adsorbents for the removal of pollutants from wastewaters. CRC Press, Boca Raton (1996)
Medellin-Castillo, N.A., Padilla-Ortega, E., Tovar-García, L.D., et al.: Removal of fluoride from aqueous solution using acid and thermally treated bone char. Adsorption 22, 951 (2016)
Mekonnen, M.M., Hoekstra, A.Y.: Four billion people facing severe water scarcity. Sci. Adv. 2, e1500323 (2016)
Metcalf & Eddy, Tchobanoglous, G., Stensel, H.D., Burton, F.: Wastewater Engineering: Treatment and Resource Recovery, 5ed. McGraw-Hill Education, New York (2014)
Monash, P., Pugazhenthi, G.: Adsorption of crystal violet dye from aqueous solution using mesoporous materials synthesized at room temperature. Adsorption 15, 390–405 (2009)
Moreno-Piraján, J.C., Gómez, R., Giraldo, L.: Removal of Mn, Fe, Ni and Cu ions from wastewater using cow bone charcoal. Materials 3, 452–466 (2010)
Murillo-Acevedo, Y.S., Giraldo, L., Moreno-Piraján, J.C.: Characterization of the adsorption of 2,4- dinitrophenol from aqueous solution onto bovine bone char by immersion calorimetry. Adsorp. Sci. Technol. 28 (8/9) (2010)
Nakamoto, K.: Infrared and Raman Spectra of Inorganic and Coordination Compounds. Wiley, New York (1977)
Nascimento, R.F., Lima, A.C.A., Vidal, C.B., Melo, D.Q., Raulino, G.S.C: Adsorção aspectos teóricos e aplicações ambientais [Adsorption: theoretical aspects and environmental applications]. Imprensa Universitária, Fortaleza (2014) (Portuguese)
Nigri, E.M., Cechine, M.A.P., Mayer, D.A., Mazur, L.P., Loureiro, J.M., Rocha, S.D.F., Vila, V.J.P.: Cow bones char as a green sorbent for fluorides removal from aqueous solutions: batch and fixed-bed studies. Environ. Sci. Pollut. Res. (2016). https://doi.org/10.1007/s11356-016-7816-5
Nigri, E.M., Bhatnagar, A., Rocha, S.D.F.: Thermal regeneration process of bone char used in the fluoride removal from aqueous solution. J. Cleaner Prod. 142, 3558–3570 (2017)
Oliveira, S.V., Cavalcanti, S.N., Rabello, G.P., Araújo, E.M., Fook, M.V.L.: Análise no infravermelho da hidroxiapatita. In: VI National Congress of Mechanical Engineering, Paraíba, Brazil (2010)
Paranychianakis, N.V., Salgot, M., Snyder, S.A., Angelakis, A.N.: Water reuse in EU states: necessity for uniform criteria to mitigate human and environmental risks. Crit. Rev. Environ. Sci. Technol. 45, 1409–1468 (2015)
Purevsuren, B., Avid, B., Narangerel, J., Gerelmaa, T., Davaajav, Y.: Investigation on the pyrolysis products from animal bone. J. Mater. Sci. 9(2), 737–740 (2004)
Reynel-Avila, H.E., Mendoza-Castillo, D.I., Bonilla-Petriciolet, A., Silvestre-Albero, J.: Assessment of naproxen adsorption on bone char in aqueous solutions using batch and fixed-bed processes. J. Mol. Liq. 209, 187–195 (2015)
Reynel-Avila, H.E., Mendoza-Castillo, D.I., Bonilla-Petriciolet, A., Silvestre-Albero, J.: Relevance of anionic dye properties on water decolorization performance using bone char: Adsorption kinetics, isotherms and breakthrough curves. J. Mol. Liq. 219, 425–434 (2016)
Rezaee, A., Ghanizadeh, G.H., Behzadiyannejad, G.H., Yazdanbakhsh, A., Siyadat, S.D.: Adsorption of endotoxin from aqueous solution using bone char. Bull. Environ. Contam. Toxicol. 82, 732–737 (2009)
Rezaee, A., Ramin, M., Niliahmadabadi, A.: Adsorption of Escherichia coli using bone char. J. Appl. Sci. Environ. Manag. 15(1), 57–62 (2011)
Rezaee, A., Rangkooy, H., Jonidi-Jafari, A., Khavanin, A.: Surface modification of bone char for removal of formaldehyde from air. Appl. Surf. Sci. 286, 235–239 (2013)
Rezaee, A., Rangkooy, H., Jonidi-jafari, A., Khavanin, A., Jafari, A.J.: High photocatalytic decomposition of the air pollutant formaldehyde using nano-ZnO on bone char. Environ. Chem. Lett. 12, 353–357 (2014)
Ribeiro, M. V.: Uso de carvão de osso bovino na defluoretação de água para uso em abastecimento público. Dissertação de Mestrado em Engenharia Metalúrgica e de Minas, UFMG, Brasil (2011)
Rincón-Silva, N.G., Moreno-Piraján, J.C., Giraldo, L.: Equilibrium, kinetics and thermodynamics study of phenols adsorption onto activated carbon obtained from lignocellulosic material (Eucalyptus globulus labill seed). Adsorption 22, 33–48 (2016)
Rocha, S. D. F., Ribeiro, M.V., Viana, P.R.M., Mansur, M. B.: Bone char: an alternative for removal of diverse organic and inorganic compounds from industrial wastewater. In: Bhatnagar, A. (ed.) Application of Adsorbents for Water Pollution. Bentham Science Publishers Ltd., Sharjah, v.14.http://www.benthamscience.com/ebooks/forthcomingtitles.htm (2011)
Rojas-Mayorga, C.K., Bonilla-Petriciolet, A., Aguayo-Villarreal, I.A., Hernández-Montoya, V., Moreno-Virgen, M.R., Tovar-Gómez, R., Montes-Morán, M.A.: Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrolysis. 104, 10–18 (2013)
Santiago, V.M.J. Pesquisas e implantação de tecnologias de ponta no tratamento e reuso de efluentes hídricos em refinarias. In: VII Simpósio Internacional de Qualidade Ambiental [Researches and implementation of new technologies for wastewater treatment and reuse in oil refineries. In: VII International Symposium on Environmental Quality], Porto Alegre, RS, Brazil (2010)
Siebers, N., Kruse, J., Leinweber, P.: Speciation of phosphorus and cadmium in a contaminated soil amended with bone char: sequential fractionations and XANES spectroscopy. Water Air Soil Pollut. 224, 1564 (2013)
Siebers, N., Godlinsk, F., Leinweber, P.: Bone char as phosphorus fertilizer involved in cadmium immobilization in lettuce, wheat, and potato cropping. J. Plant Nutr. Soil Sci. 177, 75–83 (2014)
Smiciklas, I., Onjia, A., Raicevi, S., Janackovi, D., Mitric, M.: Factors influencing the removal of divalent cations by hydroxyapatite. J. Hazard. Mat. 152, 876–884 (2008)
Stensel, H.D., Burton, F.L., Tchobanoglous, G.: Wastewater Engineering: Treatment And Reuse, Metcalf & Eddy Inc., New York (2013)
Terasaka, S., Kamitakahara, M., Yokoi, T., Ioku, K.: Effect of preparation temperature on the ability of bonechar to remove fluoride ion and organic contaminants. J. Ceram. Soc. Jpn. 122(1432), 995–999 (2014)
Thommes, M., Kaneko, K., Neimark, A., Olivier, J.P., Reinoso, F.R., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem., 87(9–10), 1051–1069 (2015)
Tovar-Gómez, R., Moreno-Virgen, M.R., Dena-Aguilar, J.A., Hernández-Montoya, V., Bonilla-Petriciolet, A., Montes-Morán, M.A.: Modeling of fixed-bed adsorption of fluoride on bone char using a hybrid neural network approach. Chem. Eng. J. 228, 1098–1109 (2013)
Wang, L., Zhang, J., Zhao, R., Li, C., Li, Y., Zhenf, C.: Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: equilibrium, kinetic and thermodynamic studies. Desalination 254, 68–74 (2010)
Wilson, J.A., Pulford, I.D., Thomas, S.: Sorption of Cu and Zn by bone charcoal. Environ. Geochem. Health 25, 51–56 (2003)
Worch, E.: Adsorption technology in water treatment: fundamentals, processes, and modeling, p. 332. Walter de Gruyter GmbH & Co, Berlin (2012) p
WWAP—United Nations World Water Assessment Programme. The United Nations world water development report 2016: water and jobs. Paris (2016)
Acknowledgements
The authors would like to gratefully acknowledge Brazilian Agencies CNPq (National Council for Scientific and Technological Development), FAPEMIG (Minas Gerais State Research Foundation), and CAPES-PROEX (Foundation for the Coordination and Improvement of Higher Level or Education Personnel-Pro-Rectory of Extension) for financial support. Polytechnic School of Universidade de São Paulo (USP), PPGEM (Post Graduation Program on Metallurgical, Materials and Mining Engineering) from Universidade Federal de Minas Gerais, Bonechar Carvão Ativado do Brasil and Petrobras are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mesquita, P.d., Cruz, M.A.P., Souza, C.R. et al. Removal of refractory organics from saline concentrate produced by electrodialysis in petroleum industry using bone char. Adsorption 23, 983–997 (2017). https://doi.org/10.1007/s10450-017-9917-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-017-9917-y