Abstract
Although the hemocompatibility of left-ventricular assist devices (LVADs) has continuously improved, assessment of hemolysis remains mandatory in pre-clinical testing. The ASTM-F1841 has standardized this assessment since 1997. However, the recommended usage of fresh, non-pooled human blood is hardly feasible with the test loop volume specified therein, when testing the device under test versus a predicate device as required by the international standard 10993-4. In this study, we compared ASTM-conforming (ASTM) and downscaled (mini) test loops with a one-third priming volume for the assessment of blood damage at the ASTM operating point. Blood damage was assessed for HeartMate 3 and BPX-80 in 6 experiments with heparinized porcine slaughterhouse blood for 6 h. We analyzed plasma free hemoglobin (pfHb), von Willebrand factor (vWF) concentration and collagen-binding functionality and calculated indices of hemolysis and vWF-ratios. The mini test loops provided significantly higher pfHb increase and consistently stronger vWF-ratio decrease and yielded a significantly better differentiation of the pumps. Interestingly, indices of hemolysis were generally lower in the mini set-up, indicating less adverse effects by the mini loop itself. Thus, we propose our mini test loop as suitable tool for clinically relevant standardized assessment of blood damage by future LVADs with single-donation human blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although patients today still suffer hemorrhagic and ischemic adverse events, hemocompatibility-related properties of left-ventricular assist devices (LVADs) have continuously improved over the years.16,30 Optimized design features such as the intrinsic artificial pulse, wide blood-flow gaps and the fully magnetically levitated rotor of the recently certified HeartMate 3™ (HM3, Abbott, USA) generate less shear-dependent von Willebrand factor (vWF) degradation and hemolysis and significantly reduce pump thrombosis in LVAD patients.3,11,17,20 However, sub-lethal red blood cell damage, lower degrees of hemolysis and degradation of vWF still occur in modern LVADs and can cumulatively contribute to activate multiple pathways that lead to adverse events.3,25,27,30 Thus, assessment of blood damage remains a key requisite in pre-clinical testing of future LVADs, yet the focus is shifting towards more sensitive markers and better differentiation of the pumps.
Since 1997, the current in vitro assessment of hemolysis in continuous-flow LVADs has been regulated in the American Society of Testing and Materials (ASTM) standard F1841-97 (2017)2 to ensure equal testing and comparable results by means of a specifically characterized test loop and procedure. ASTM set values for temperature (37 ± 1 °C), pressure head (100 ± 3 mmHg) and flow rate (5 ± 0.25 L min−1) define an operating point with clinical relevance for the in vitro verification of LVADs. Although testing may be performed with bovine or porcine blood, usage of fresh, non-pooled human blood is recommended. However, with one single-donation of human blood, it is hardly feasible to comply with both the set volume of 450 ± 45 mL of the ASTM test loop and the direct comparison with a predicate device as required by the international standard 10993-4:2017.9
Recently, volume-reduced test loops with human blood were used to compare the blood damage between two centrifugal LVADs with optimal or equal settings of the pump speed. However, neither pressure head nor flow rate were set to reflect a clinically relevant or at least equal operating point, which impedes the conclusions and comparison of those results.33
To our knowledge, our study is the first to introduce a downscaled test loop set-up that would allow for testing of at least a static reference and one device under test versus a predicate device with single-donation human blood while maintaining the ASTM standard operating point. Moreover, we investigated if accelerated blood damage due to a higher number of pump-passages yields better differentiation of the pumps.
In addition, we examine the supplementary assessment of vWF as a more sensitive marker for LVAD-related shear-dependent blood damage in vitro, utilizing the same set-up as for hemolysis assessment.
Materials and Methods
In n = 6 independent experiments, we systematically compared ASTM-conforming (ASTM) and downscaled (mini) test loops for the assessment of LVAD-related blood damage of the implantable continuous-flow LVAD HM3 and the extracorporeal BPX-80 Bio-Pump® (BPX-80, Medtronic, Ireland). The HM3 pumps were explanted devices, that had been thoroughly cleaned and disinfected prior to this study with cleansing agents for medical devices (Sekusept™ aktiv, ECOLAB Healthcare, Germany) according to manufacturers’ instructions, and we received the pumps clean and dry. All pumps were reused throughout the study and interchanged between the ASTM and mini set-ups to diminish individual pump-related effects.
Test Loop Set-Up
ASTM loops were set up according to F1841-97 (2017).2 In brief, a test loop consisted of 2 m of 3/8″-diameter polyvinyl-chloride-(PVC)-tubing (Bloodline ECC tubing, Raumedic, Germany), a 0.8 L blood reservoir (MEDOS, Germany), a throttle to yield the pressure head of 100 mmHg and mounted sensors for pressure (Xtrans, CODAN, Germany), temperature (Medos, Germany) and flow (Transonic, USA). To accommodate the size of the HM3 in- and outlet, 4 cm of tubing at each position were replaced with 1/2″-diameter PVC-tubing. To maintain constant temperature of 37 ± 1° C, the test loop was fixed in a water-bath (Lauda, Germany).
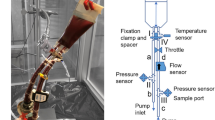
The mini test loops were downscaled to a one-third ASTM priming volume of 160 mL and 36 cm of the same tubing and mounted with the same sensors and throttle as performed for the ASTM loops (Fig. 1). To prevent bending of the tubing at the inlet and outlet of the mini test loop reservoir (#51494, 100 mL, Qosina, USA), connectors were fixed with a spacer and a clamp. Temperature of the mini test loops was controlled with a heating-hood (HM3) or omitted due to heat development by the pump itself (BPX-80). A detailed description and schematics of both test loops (Figs. S1 and S2) are given in the supplementary information.
Blood Collection and Preparation
For each of the 6 test days, three 1-L-bottles of porcine slaughterhouse blood (15,000 international units (IU) sodium heparin (B. Braun, Germany), 0.09% (w/v) glucose, 5.0% (v/v) isotonic saline solution, 0.016 g L−1 gentamycin) were freshly collected and inspected for hematocrit, red and white blood cell and platelet count (hematology analyzer Sysmex XT2000i vet, Sysmex, Germany) and hemoglobin and lactate concentration (blood gas analyzer ABL 825 Flex, Radiometer, Germany). Blood was pooled in a 2 L mixing bag for parenteral nutrition (Baxter, Ireland) and base excess was adjusted to 0 ± 5 mM with sodium hydrogen carbonate (8.4% (w/v), Fresenius, Germany). Adjustment for hematocrit of 30 ± 2% was performed during priming and filling of each test loop according to the respective ratios of isotonic saline solution and pooled blood.
Test Loop Handling
All test loops were primed with isotonic saline solution and de-aired before being filled with blood. To yield a hematocrit of 30 ± 2%, excess saline solution was drained and replaced with blood. The total volume was 160 ± 5 and 480 ± 15 mL for the mini and ASTM test loops, respectively, with 1% accepted deviation between each HM3 and BPX-80 set-up. After a second de-airing, all test loops were run for 5 min to mix completely before verification and adjustment of hematocrit and base excess. For similar pre-pumping conditions, the corresponding flow rate of 2.5 ± 0.06 L min−1 of the limiting pump’s minimal speed was set for all test loops. After pre-pumping sampling, the flow rate and pressure head were adjusted to the ASTM operating point (5 ± 0.25 L min−1 and 100 ± 3 mmHg, respectively) and timers were started for further sampling.
A static reference reservoir was similarly prepared and kept at static conditions in either the heating-hood or water bath.
Blood Sampling
After each 1 and 0.5 mL discard for ASTM and mini test loops, respectively, seven 2.5-mL-samples were taken before starting the operating point (pre-pumping sample) and then every 60 min for 6 h onwards. Samples were directly assessed for blood count, blood gas and activated clotting time and processed to platelet-poor plasma in 3.2-%-tri-sodium-citrate tubes (Sarstedt, Germany). Plasma samples were stored at − 20 to − 80 °C according to standard clinical practice and manufacturer’s instructions until further protein analysis.
Cleaning Procedure
Re-usage of blood pumps requires thorough cleaning of all parts in contact with blood. To ensure removal of cells and proteins, all pumps were first rinsed thoroughly with tap water until effluent was visually clear. The pumps were then set up in a cleaning-in-place (CIP) loop consisting of a simple PVC-tubing loop with sample ports for filling and de-airing. CIP loops were filled with a pepsin/citrate solution and run for 1 h followed by 30 min rinsing with de-ionized water. Pumps and CIP loops were then dried overnight by filtered compressed air and boxed until the next experiment.
Analysis of Hemolysis
Analysis of hemolysis was performed according to DIN 58931:2010-088 by means of the cyanmethemoglobin (HiCN) method. In brief, plasma samples were thawed in a water bath at 37° C for 8 min and diluted 1:5 (v/v) with HiCN conversion solution (Hemoglobin FS, DiaSys, Germany) in duplicates in standard micro cuvettes (Brandt, Germany). After incubation, converted plasma free hemoglobin (pfHb) was photometrically detected at 540 nm with 680 nm reference wavelength. Duplicate results were accepted with a coefficient of variation (CV) ≤ 0.12. Hemolysis is presented as ΔpfHb (mg dL−1) and modified and normalized milligram index of hemolysis (MIH and mgNIH, respectively), with
with ΔpfHbt: increase of plasma free hemoglobin (mg L−1) in the sampling interval, Hbpre: pre-pumping total hemoglobin (mg L−1), Hctt: hematocrit (%), Qt: flow rate (L min−1), Vt: test loop volume (L), T: elapsed time (min) and #passages: absolute number of pump-passages
To account for the greater relative influence of sampling on the volume of the mini test loops, the number of pump-passages in Eqs. (2) and (3) is corrected for the change of volume and flow rate over the sampling intervals by means of Eq. (4) instead of assuming a constant Vt and Qt, respectively.
Analysis of vWF Degradation
vWF concentration (vWF:Ag) and collagen-binding functionality (vWF:CB) were analyzed with an in-house-developed enzyme-linked immunosorbent assay (ELISA). In brief, high-binding polystyrene 96-well plates (Microlon600, Greiner, Germany) were coated with either 3.3 µg mL−1 α-vWF antibody (A0082, DAKO, Denmark) in 0.05 M carbonate buffer pH 9.6 overnight at 4 °C or 50 µg mL−1 porcine collagen I/III (95%/5%, Matrix, Germany) in pH 7.4 DPBS for 36 h at room temperature. Samples were thawed in a water bath at 37° C for 15 min, diluted 1:200 and 1:40 in PBS for vWF:Ag and vWF:CB, respectively, and determined in duplicates. Samples were incubated for 60 min and bound vWF was detected with 162.5 ng mL−1 HRP-conjugated α-vWF antibody (P0026, DAKO, Denmark), 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Ultra-TMB, ThermoFisher, Germany) and 2 M sulfuric acid (AppliChem, Germany) at 450 and 620 nm reference wavelength. An in-house-generated porcine plasma pool (n = 8) and pathologic controls were used for generation of the standard curve and verification of assays procedure, respectively. Duplicate results were accepted with a CV ≤ 0.2.
Ratios of vWF:CB and vWF:Ag were calculated for each sample with a cut-off for clinically relevant vWF degradation of 0.8031 and were depicted as absolute ratios and normalized (ΔvWF-) ratios with
with ΔvWF-ratiot: decrease of vWF-ratio in the sampling interval.
Statistical Analysis
Statistical analysis was performed with SPSS Statistics 24 (IBM, USA) and Prism 8 (GraphPad, USA). Results were analyzed for each group, and (log) normal distribution was verified with Shapiro Wilks test. Outliers were identified by means of the ROUT method21 with a maximum false discovery rate of 0.5%. Non-parametric data is depicted as median with interquartile range (IQR), and normally- or log-normally-distributed continuous variables are depicted as mean ± standard deviation (SD) or with 95% confidence interval (CI) and geometric mean with 95% CI, respectively. Comparison within the groups were performed with mixed-effects analysis with Geisser-Greenhouse correction and Dunnett’s correction for multiple comparisons. For comparison between the groups, ANOVA or mixed-effects analysis with Geisser-Greenhouse correction and Tukey’s multiple comparison correction, Kruskal–Wallis test or t test were used, as appropriate. An adjusted exact p-value was considered significant with p ≤ 0.05 (*).
Results
Mean technical and baseline hematologic parameters of ASTM and mini test loops are depicted in Tables 1 and 2. Flow rate and pressure difference did not differ significantly between the test loops. Due to excessive pump-generated heating, the BPX-80 mini test loop was removed from the heating-hood after the first experiment. Thus, its mean temperature at the start of the operating point was significantly lower compared to the other test loops and was not ASTM-conforming, but re-stabilized to 36.8 ± 1.3 °C within the first 60 min. Due to the downscaled volume, the number of pump-passages at 360 min was significantly higher in the mini test loops with a mean factor of 3.12 and 3.13 for BPX-80 and HM3-loops, respectively. Mean baseline hematologic parameters did not differ significantly between all test loops.
Sample Exclusion
In total, 4 samples of one experiment with the BPX-80 mini test loop and 4 samples of 3 experiments with the HM3 mini test loop were identified as outliers by means of the ROUT method.21 The identified outliers of the BPX-80 mini test loop were most probably caused by the overheating event of the pump that had a large impact on increasing pfHb and moderate influence on decreasing vWF-ratio, and we thus excluded the whole test loop from statistical analysis. The identified outliers of the HM3 mini test loop comprised high values of both pfHb and vWF-ratio (n = 2) or pfHb alone (n = 1). Of note, three of those samples of two HM3 mini test loops also had decreased pH < 7.0. A possible cause might have been the formation of microbubbles due to not fully de-aired test loops with accumulating effects towards the end of the test and/or mistakes during sampling. Respective samples were excluded from statistical analysis. In one experiment, the static reference was not prepared and could thus not be evaluated (n = 5).
Assessment of Hemolysis
Mean pre-pumping pfHb was ≤ 17 mg dL−1 and not significantly different for all test loops and static reference (Table 3). Within all test loops but not the static reference, mean pfHb significantly increased over time (Fig. 2 and Table 4). Between the set-ups, the mini test loops produced significantly more total pfHb than ASTM test loops from 180 min and 300 min onwards, and mean slopes were significantly steeper for BPX-80 and HM3 with a factor of 2.12 and 2.36, respectively (Table 5). Regression coefficient of mean pfHb increase was ≥ 0.94 for all test loops. Compared to the static reference, pfHb in the mini test loops was significantly higher from 60 min onwards, whereas increase in the ASTM loops was not significant before 180 and 300 min for BPX-80 and HM3, respectively.
Longitudinal profiles of ΔpfHb of ASTM and mini test loops are depicted as mean ± SD with mean linear regression and significance of time vs. pre (straight lines) and consistant significance of loop vs. static reference (dashed lines). Significant differences between the slopes (s; right) are depicted within the ASTM (blue) and mini set-up (red) and between the ASTM and mini set-up (red-blue straight and dashed line for BPX-80 and HM3, respectively). The significant difference between the pump differentiation of the ASTM and mini set-up is depicted in black.
Moreover, the mini test loops provided a significantly greater differentiation between the mean pfHb increase of the pumps compared to the ASTM loops (0.052 vs. 0.029, p = 0.005, Fig. 2 and Table 5).
Interestingly, with correction for volume, flow, hematocrit and total hemoglobin, mean MIH (Fig. 3 and Table 6) and mgNIH (Fig. 4 and Table 7) at 360 min were generally lower in the mini test loops than in the ASTM loops. Both set-ups similarly showed a significantly higher total pfHb, MIH and mgNIH for BPX-80 than for HM3.
Assessment of vWF Degradation
Mean pre-pumping vWF-ratios were not significantly different and in normal range of > 0.80 for all test loops and static reference (Fig. 5a and Table 8). Within all test loops but not the static reference, vWF-ratios significantly decreased over time below 0.80 indicating the development of an acquired von Willebrand syndrome (AvWS). Between the set-ups, decrease of mean normalized vWF-ratios was stronger in the mini test loops and yielded a greater differentiation between the pumps (Fig. 5b and Table 9). The strongest mean decrease occurred in the BPX-80 mini test loop, however, this test loop also had the highest standard deviation. Interestingly, we observed an increase of mean vWF-ratios from 120 to 180 min in the ASTM loops, which was only marginal in the mini loops.
Longitudinal profiles of vWF-ratio (a) and ΔvWF-ratio (b) of ASTM (blue) and mini test loops (red) are depicted as mean ± SD with mean regression and significance of time vs. pre (*) and significance of loop vs. static reference (#). The shaded area in (a) marks the AvWS range with pathological vWF-ratios ≤ 0.80.
Discussion
In this study, we systematically compared ASTM-conform and downscaled set-ups for the assessment of LVAD-related hemolysis and vWF degradation at the ASTM operating point.
Exemplarily, the LVAD-related blood damage was assessed for the extracorporeal BPX-80 and the implantable continuous-flow LVAD HM3, which introduces new design-features for less blood damage.
Assessment of Hemolysis
Since the volume of the mini test loops was reduced to one-third of the ASTM test loops, the mini test loops accumulated approximately three times as many pump-passages. This resulted in significantly increased total pfHb due to significantly accelerated hemolysis in the mini test loops by factor 2.12 and 2.36 for BPX-80 and HM3, respectively. Moreover, the mini test loops showed a significantly greater difference of mean pfHb increase between BPX-80 and HM3, thus providing a significantly better differentiation of the pumps compared to the ASTM loops. This is consistent with Mueller et al., who assumed more marked parameter changes for a higher number of pump-passages,22 and might be especially valuable for the differentiation of marginal variations between design versions during the developmental process and LVADs with low hemolytic potential.
Our mgNIH results of the BPX-80 ASTM loop are in the range between 3.35 and 6.6 mg 100 L−1 of current literature of hemolysis assessments also using porcine blood and the same pump, whereas the mgNIH of our mini test loop is slightly lower.14,15,28 Our results of the HM3 loop could only be compared to an ASTM conform set-up with bovine blood that reported higher hemolysis compared to our results.3 Differences between reported data for both pumps could have been influenced by differing test parameters between the studies such as the duration of the testing, temperature and blood characteristics. To overcome some of these issues, Mueller et al. introduced the MIH to correct for both species- and set-up-specific differences regarding total hemoglobin as well as volume and flow rate.22 Since 1997, the MIH has been the recommended index of hemolysis and is most often referred to as “milligram of hemoglobin released into plasma per milligram of total hemoglobin pumped through the loop”.2 In other words, the MIH describes the released fraction of total hemoglobin per pump-passage. Interestingly, besides maintaining the differences between the hemolytic profiles of the pumps, the MIH was generally lower in the mini test loops. This implies an overall better hemocompatibility of our mini test loops compared to the ASTM loops, even though Mueller et al. suspected that “very small” test loop volumes might accumulate adverse effects.22
Our findings of different MIH for the same pump at the same operating point in different set-ups further underlines the impact of the test loop itself and thus the importance of equal test loop set-ups for direct intra- and inter-study comparisons of blood pumps.
Moreover, we recommend a more refined equation for the MIH, that considers changes in flow rate and blood volume over time.
Assessment of vWF Degradation
Compared to pre-pumping values, significant vWF-degradation and development of AvWS occurred in all test loops but not in the static reference. Consistent with the increase of pfHb, decrease of normalized vWF-ratios was stronger and differentiation between the pumps was higher in the mini test loops. The observed increase of mean vWF-ratios at 180 min in the ASTM loops might indicate platelet activation and subsequent secretion of high molecular weight vWF.4 Accordingly, the only slight increase of vWF-ratios in the mini loops might reflect less such platelet activation and thus better hemocompatibility, consistent with the lower indices of hemolysis of the mini loops. Possible causes for the better hemocompatibility might be the reduced surface-to-volume ratio of the mini test loops or a different, e.g., less turbulent, flow regime.
For the in vitro assessment of LVAD-related vWF damage, different model set-ups with plasma5,10 or whole blood26,33 have been used. However, since pre-clinical testing of blood pumps is expensive and time-consuming, integration of vWF and hemolysis assessment is desirable, as is a fast and reliable method of analysis. Moreover, physiological effects might be missing or altered when using plasma instead of whole blood, especially with calcium-complexing anticoagulation, which slows down the vWF refolding process.32
Based on this study, we propose the parallel assessment of vWF degradation by ELISA-based analysis of vWF concentration and collagen-binding functionality in the standardized mini test loops. Besides being faster and less labor-intensive than multimer analysis, in our opinion, directly measuring (loss of) vWF-functionality itself promotes a more meaningful interpretation of the results. Moreover, other than the vWF-platelet-interaction, the collagen-binding functionality is not affected by heparin and pfHb, respectively,1,7,12,29 and is thus better suited for the in vitro testing of LVAD-related blood damage.
Although functional assessment of LVAD-related vWF degradation in vivo has led to controversial results,6,13,18,19,24in vitro, vWF provides a sensitive marker for shear-dependent blood damage that might gain even more relevance regarding the progress in LVAD development.27
Limitations
Our study suffers general limitations of small samples, especially for the 360-min-sample of the HM3 mini test loop. Moreover, few discrepancies from the ASTM standard existed in our set-up: First, our institution uses porcine slaughterhouse blood that is not taken by venipuncture and thus prone to higher stress-dependent pre-activation. Nevertheless, this could be neglected for reliable pfHb assessment, as we did not observe abnormal values of pre-pumping pfHb.22 Second, our institutional heparin dosage is higher than recommended and could have affected platelet activation.12 However, this would affect all test loops and the static reference equally and thus might be of minor impact. Third, isotonic saline solution is used for priming of the test loops instead of phosphate buffered solution (PBS), lacking the buffer capacity comprised by PBS. Although we adjusted the base excess for better blood conservation, the pH was not regulated over the testing period and significantly decreased from pre to 360 min in all test loops. Since both hemolysis and vWF activity as well as degradation are influenced by pH, physiologic pH-adjustment during testing might be considered for a refined standardized testing.2,22,23
Conclusion
Our mini test loop allows for parallel assessment of hemolysis and vWF degradation, and combines the advantages of standardized testing and small priming volumes without accumulating adverse effects. The lower MIH, i.e., less pfHb per pump-passage than generated in the ASTM-conform loop, rather implies an improved hemolysis-relevant hemocompatibility of the mini test loops. General characteristics of the hemolytic pump-profiles were maintained, as reflected by the significantly higher increase of pfHb and MIH of BPX-80 vs. HM3. Moreover, the mini test loop provided significantly better differentiation of the hemolytic pump profiles.
vWF degradation led to clinically relevant loss of functionality and development of AvWS in all test loops. Consistent with the hemolysis results, decrease of normalized vWF-ratios was stronger in the mini test loops and yielded a better differentiation between the pumps.
Thus, our mini test loop is well suited for pre-clinical testing at the ASTM operating point and allows for a more meaningful analysis of LVAD-related blood damage. The mini test loop reduces the required amount of blood for a direct pump comparison from 900 ± 90 mL to only 320 ± 10 mL blood and thus enables standardized in vitro testing of future LVAD with one single-donation of fresh human blood.
References
Adachi, T., T. Matsushita, Z. Dong, A. Katsumi, T. Nakayama, T. Kojima, H. Saito, J. E. Sadler, and T. Naoe. Identification of amino acid residues essential for heparin binding by the A1 domain of human von Willebrand factor. Biochem. Biophys. Res. Commun. 339:1178–1183, 2006. https://doi.org/10.1016/j.bbrc.2005.11.126.
ASTM International. ASTM F1841-97. Standard Practice for Assessment of Hemolysis in Continuous Flow blood Pumps. West Conshohocken, PA: ASTM International, 2017. https://doi.org/10.1520/f1841-97r17.
Bourque, K., C. Cotter, C. Dague, D. Harjes, O. Dur, J. Duhamel, K. Spink, K. Walsh, and E. Burke. Design rationale and preclinical evaluation of the HeartMate 3 left ventricular assist system for hemocompatibility. ASAIO J. 62:375–383, 2016. https://doi.org/10.1097/MAT.0000000000000388.
Bryckaert, M., J.-P. Rosa, C. V. Denis, and P. J. Lenting. Of von Willebrand factor and platelets. Cell. Mol. Life Sci. 72:307–326, 2015. https://doi.org/10.1007/s00018-014-1743-8.
Coghill, P. A., S. Kanchi, Z. J. Azartash-Namin, J. W. Long, and T. A. Snyder. Benchtop von Willebrand factor testing: comparison of commercially available ventricular assist devices and evaluation of variables for a standardized test method. ASAIO J. 2018. https://doi.org/10.1097/MAT.0000000000000849.
Crow, S., D. Chen, C. Milano, W. Thomas, L. Joyce, V. Piacentino, R. Sharma, J. Wu, G. Arepally, D. Bowles, J. Rogers, and N. Villamizar-Ortiz. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann. Thorac. Surg. 90:1263–1269, 2010. https://doi.org/10.1016/j.athoracsur.2010.04.099.
Da, Q., M. Teruya, P. Guchhait, J. Teruya, J. S. Olson, and M. A. Cruz. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: implications for circulatory devices. Blood 126:2338–2341, 2015. https://doi.org/10.1182/blood-2015-05-648030.
DIN 58931:2010-08. Haematology—Determination of Haemoglobin Concentration in Blood—Reference Method. Berlin: Beuth Verlag GmbH, 2010. https://doi.org/10.31030/1623224.
DIN EN ISO 10993-4:2017-12. Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood (ISO 10993-4:2017); German Version EN_ISO_10993-4:2017. Berlin: Beuth Verlag GmbH, 2017. https://doi.org/10.31030/2597308.
Egger, C., J. Maas, T. Hufen, T. Schmitz-Rode, and U. Steinseifer. Establishing a method for in vitro investigation of mechanical parameters causing acquired von Willebrand syndrome in ventricular assist devices. Artif. Organs 37:833–839, 2013. https://doi.org/10.1111/aor.12116.
Farrar, D. J., K. Bourque, C. P. Dague, C. J. Cotter, and V. L. Poirier. Design features, developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J. 53:310–315, 2007. https://doi.org/10.1097/MAT.0b013e3180536694.
Gao, C., B. Boylan, J. Fang, D. A. Wilcox, D. K. Newman, and P. J. Newman. Heparin promotes platelet responsiveness by potentiating αIIbβ3-mediated outside-in signaling. Blood 117:4946–4952, 2011. https://doi.org/10.1182/blood-2010-09-307751.
Geisen, U., K. Brehm, G. Trummer, M. Berchtold-Herz, C. Heilmann, F. Beyersdorf, J. Schelling, A. Schlagenhauf, and B. Zieger. Platelet secretion defects and acquired von Willebrand syndrome in patients with ventricular Assist devices. J. Am. Heart. Assoc. 2018. https://doi.org/10.1161/jaha.117.006519.
Hijikata, W., T. Shinshi, J. Asama, L. Li, H. Hoshi, S. Takatani, and A. Shimokohbe. A magnetically levitated centrifugal blood pump with a simple-structured disposable pump head. Artif. Organs 32:531–540, 2008. https://doi.org/10.1111/j.1525-1594.2008.00576.x.
Hoshi, H., J. Asama, W. Hijikata, C. Hara, T. Shinshi, T. Yasuda, K. Ohuchi, A. Shimokohbe, and S. Takatani. Hemolytic performance of a MagLev disposable rotary blood pump (MedTech Dispo): effects of MagLev gap clearance and surface roughness. Artif. Organs 30:949–954, 2006. https://doi.org/10.1111/j.1525-1594.2006.00332.x.
Kormos, R. L., J. Cowger, F. D. Pagani, J. J. Teuteberg, D. J. Goldstein, J. P. Jacobs, R. S. Higgins, L. W. Stevenson, J. Stehlik, P. Atluri, K. L. Grady, and J. K. Kirklin. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transplant. 38:114–126, 2019. https://doi.org/10.1016/j.healun.2018.11.013.
Mehra, M. R., D. J. Goldstein, N. Uriel, J. C. Cleveland, M. Yuzefpolskaya, C. Salerno, M. N. Walsh, C. A. Milano, C. B. Patel, G. A. Ewald, A. Itoh, D. Dean, A. Krishnamoorthy, W. G. Cotts, A. J. Tatooles, U. P. Jorde, B. A. Bruckner, J. D. Estep, V. Jeevanandam, G. Sayer, D. Horstmanshof, J. W. Long, S. Gulati, E. R. Skipper, J. B. O’Connell, G. Heatley, P. Sood, and Y. Naka. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N. Engl. J. Med. 378:1386–1395, 2018. https://doi.org/10.1056/NEJMoa1800866.
Meyer, A. L., D. Malehsa, C. Bara, U. Budde, M. S. Slaughter, A. Haverich, and M. Strueber. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ. Heart Fail. 3:675–681, 2010. https://doi.org/10.1161/CIRCHEARTFAILURE.109.877597.
Meyer, A. L., D. Malehsa, U. Budde, C. Bara, A. Haverich, and M. Strueber. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC 2:141–145, 2014. https://doi.org/10.1016/j.jchf.2013.10.008.
Moazami, N., K. Fukamachi, M. Kobayashi, N. G. Smedira, K. J. Hoercher, A. Massiello, S. Lee, D. J. Horvath, and R. C. Starling. Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J. Heart Lung Transplant. 32:1–11, 2013. https://doi.org/10.1016/j.healun.2012.10.001.
Motulsky, H. J., and R. E. Brown. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 7:123, 2006. https://doi.org/10.1186/1471-2105-7-123.
Mueller, M. R., H. Schima, H. Engelhardt, A. Salat, D. B. Olsen, U. Losert, and E. Wolner. In vitro hematological testing of rotary blood pumps: remarks on standardization and data interpretation. Artif. Organs 17:103–110, 1993. https://doi.org/10.1111/j.1525-1594.1993.tb00419.x.
Müller, J. P., A. Löf, S. Mielke, T. Obser, L. K. Bruetzel, W. Vanderlinden, J. Lipfert, R. Schneppenheim, and M. Benoit. pH-Dependent Interactions in dimers govern the mechanics and structure of von Willebrand factor. Biophys. J. 111:312–322, 2016. https://doi.org/10.1016/j.bpj.2016.06.022.
Netuka, I., T. Kvasnička, J. Kvasnička, I. Hrachovinová, P. Ivák, F. Mareček, J. Bílková, I. Malíková, M. Jančová, J. Maly, P. Sood, K. S. Sundareswaran, J. M. Connors, and M. R. Mehra. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J. Heart Lung Transplant. 35:860–867, 2016. https://doi.org/10.1016/j.healun.2016.05.019.
Olia, S. E., T. M. Maul, J. F. Antaki, and M. V. Kameneva. Mechanical blood trauma in assisted circulation: sublethal RBC damage preceding hemolysis. Int. J. Artif. Organs 39:150–159, 2016. https://doi.org/10.5301/ijao.5000478.
Restle, D. J., D. M. Zhang, G. Hung, J. L. Howard, F. Kallel, M. A. Acker, P. Atluri, and C. R. Bartoli. Preclinical models for translational investigations of left ventricular assist device-associated von Willebrand factor degradation. Artif. Organs 39:569–575, 2015. https://doi.org/10.1111/aor.12428.
Rosenberg, G., C. A. Siedlecki, C.-S. Jhun, W. J. Weiss, K. Manning, S. Deutsch, and W. Pierce. Acquired von Willebrand syndrome and blood pump design. Artif. Organs 42:1119–1124, 2018. https://doi.org/10.1111/aor.13291.
Sakota, D., R. Sakamoto, H. Sobajima, N. Yokoyama, S. Waguri, K. Ohuchi, and S. Takatani. Mechanical damage of red blood cells by rotary blood pumps: selective destruction of aged red blood cells and subhemolytic trauma. Artif. Organs 32:785–791, 2008. https://doi.org/10.1111/j.1525-1594.2008.00631.x.
Sobel, M., P. M. McNeill, P. L. Carlson, J. C. Kermode, B. Adelman, R. Conroy, and D. Marques. Heparin inhibition of von Willebrand factor-dependent platelet function in vitro and in vivo. J. Clin. Invest. 87:1787–1793, 1991. https://doi.org/10.1172/JCI115198.
Susen, S., A. Rauch, E. van Belle, A. Vincentelli, and P. J. Lenting. Circulatory support devices: fundamental aspects and clinical management of bleeding and thrombosis. J. Thromb. Haemost. 13:1757–1767, 2015. https://doi.org/10.1111/jth.13120.
Tiede, A., J. Priesack, S. Werwitzke, K. Bohlmann, B. Oortwijn, P. Lenting, R. Eisert, A. Ganser, and U. Budde. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. J. Thromb. Haemost. 6:569–576, 2008. https://doi.org/10.1111/j.1538-7836.2008.02909.x.
Xu, A. J., and T. A. Springer. Calcium stabilizes the von Willebrand factor A2 domain by promoting refolding. Proc. Natl. Acad. Sci. USA. 109:3742–3747, 2012. https://doi.org/10.1073/pnas.1121261109.
Zayat, R., A. Moza, O. Grottke, T. Grzanna, T. Fechter, T. Motomura, C. Schmidt-Mewes, T. Breuer, R. Autschbach, R. Rossaint, A. Goetzenich, and C. Bleilevens. In vitro comparison of the hemocompatibility of two centrifugal left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 157:591–599.e4, 2019. https://doi.org/10.1016/j.jtcvs.2018.07.085.
Acknowledgments
The authors thank Thomas Berg from the Department of Thoracic and Cardiovascular Surgery, Medical Faculty, RWTH Aachen University, Aachen, Germany, for the loan of the HM3 and equipment. This study was supported by the Elisabeth and Rudolf Hirsch Foundation for Medical Research (Cologne, Germany) and the European Regional Development Fund (ERDF) of the European Union and North-Rhine Westphalia (Grant Number: EFRE-0800410).
Conflict of interest
The authors declare no conflicts of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ender A Finol oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Woelke, E., Klein, M., Mager, I. et al. Miniaturized Test Loop for the Assessment of Blood Damage by Continuous-Flow Left-Ventricular Assist Devices. Ann Biomed Eng 48, 768–779 (2020). https://doi.org/10.1007/s10439-019-02404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02404-z