Abstract
To evaluate the histological characteristics of decellularized human urethra after transplantation into the rat omentum and compare in vivo cell seeding with perfusion-based and cell sheet urethral regeneration. Eight adult human male urethras accompanied with the surrounding corpus spongiosum were obtained. The tissues were decellularized with detergent-based method. The efficacy of decellularization and extracellular matrix preservation was evaluated by several techniques. Decellularized scaffolds were transplanted into the omentum of 12 male rats and located into the scrotum. Biopsies were taken 1, 3, and 6 months postoperatively to assess the natural recellularization. Mesenchymal stem cells obtained from preputial tissue were seeded with perfusion-based and cell sheet techniques as well. Immunohistochemical staining with α-actin, cytokeratin AE1/AE3, synaptophysin, and CD31 antibodies were performed. Removal of nuclear components and preservation of biomechanical properties was confirmed. In-vivo recellularization revealed promising results in progressive angiogenesis and cell seeding of epithelium-like cells in the lining of the urethra as well as smooth muscle cells in the wall structure. In-vitro urethral regeneration revealed that cell sheet engineering was the technique of choice compared to perfusion-based technique. This study may paw the road for clinical application of acellular urethral matrix with the surrounding corpus spongiosum in urological reconstructive surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trauma, infection, inflammation, congenital malformations, and cancer are among the conditions resulting in narrowing or complete blockage of the urethral canal. In addition, renal failure and infertility can affect quality of life and may have devastating consequences following severe urethral stenosis. The treatment of severe urethral stenosis has been considered as a challenge even for skilled urologists over the last decade. Success rate after urethral reconstruction vary from 70 to 90% and most of the patients require multiple procedures.3
Patients with hypospadias and urethral strictures require additional tissue for reconstruction. The application of vascularized skin flaps, buccal mucosa grafts, full-thickness free skin grafts, and classic urethroplasty have been reported recently. However, these techniques are not effective in long and complex strictures and long-term follow-ups demonstrated significant complications and decrease in quality of life.21 In addition, donor site availability and harvesting multiple grafts simultaneously with subsequent high morbidity rate represent principal limitations following the application of buccal mucosa to treat long urethral strictures.2,27 Although different biological tissues have been used for urethral reconstruction, finding the most ideal urethral substitute was always the main goal for reconstructive urology.
The emergence of tissue engineering and regenerative medicine brings new hope for urethral reconstruction.4 Acellular scaffolds have become a clinical reality and considered as ideal urethral substitutes for urethral reconstruction.1 Obtaining acellular matrix is a simple, feasible, and effective process resulting in production of scaffolds containing collagen, elastin, glycosaminoglycans (GAGs), and growth factors. The ideal scaffold for urethral replacement must represent the same mechanical and functional properties as the natural urethra with no immune response, fibrosis or tissue contraction. This scaffold should also provide an appropriate microenvironment for tissue regeneration. Simplicity of production, storage, and transportation are among the advantages of the cell-free matrices as an “off the shelf” material.22 Acellular scaffolds especially from human urethras can provide a reliable microenvironment for the ingrowth of urethral wall cell components. Although cell-seeded matrices represent attractive strategy for tubular implants, heterologous seeding or even homologous cells will induce tissue rejection.23 In addition, only autologous cells have been used in humans for urethral reconstruction.25
The aim of the current study was to evaluate the efficacy, feasibility, and safety of in vivo and in vitro approaches for urethral regeneration using human decellularized urethral scaffold. The superiority of perfusion-based technique or cell sheet engineering was also assessed.
Materials and Methods
Scaffold Preparation
After institutional review board approval and obtaining informed consent from February 2014 to May 2015, human urethral scaffolds accompanied with the surrounding corpus spongiosum were aseptically obtained from 12 transgender patients undergoing sex reassignment surgery (SRS). Urethral tissues were first placed in deionized water and gentamicin at 4 °C for 3 h. Urethral tissues were subsequently placed in Triton X-100 diluted 1:100 in PBS for 4 h. In the next step, they were placed in 3% sodium dodecyl sulfate (SDS) at 37 °C for 3 h. The duration of SDS application was attentively controlled in order to avoid tissue damage. All solutions were changed with 1 h intervals. All the above mentioned steps were conducted under continuous shaking. The rotator was regulated to rotate 70 rpm. All the solutions mentioned above were filtered with a filter of 0.02 µ and were stored out of light in 4 °C. Decellularized Urethras were repeatedly washed with phosphate buffer solution (PBS) several times and were stored in PBS and a cocktail containing penicillin, amphotericin and streptomycin in 4 °C for further use. Urethral tissues were decellularized while left tubular. However, some of them were occasionally cut open longitudinally after decellularization process.
Scanning Electron Microscopy (SEM)
In order to verify the efficacy of our decellularization procedure in cell removal and preservation of ECM, several images with different magnifications were obtained from both native and decellularized urethral tissues (luminal and abluminal sides). For SEM, all specimens (1 × 1 cm pieces) that were separated from the same regions of both native and decellularized urethras were placed in 2.5% glutaraldehyde for 1.5 h at 4 °C. Afterward, they were washed with PBS for 1.5 h at 4 °C. The PBS was changed with 30 min intervals. Subsequently, the tissues were dehydrated through a graded ethanol series for 9 h and coated with a thin (approximately 4 nm) layer of gold to obtain a specimen suitable for SEM evaluations. A high resolution ion beam coater (model 681, Gatan Inc., America) was used for coating the specimens.
DNA Quantification
DNA isolation was achieved by disruption of cell walls/membranes according to the method described by Laird et al. 14 Briefly, 1 mg of the urethral scaffold was homogenized in 0.25% trypsin and 1 mM EDTA in deionized water. Incubation of the homogenate was performed with invariable stirring for 3 h at 37 °C. Then, the cell lysis was continued with 2% SDS, 5 mM EDTA, 200 mM NaCl, and 100 mM Tris–HCl, (pH 8.5) for 24 h at 55 °C. The DNA extraction was performed in isopropanol and later dissolved in 10 mM Tris–HCl and 0.1 mM EDTA (pH 7.5). Determining the amount of DNA was performed with spectrophotometry at 260 nm.
Biomechanical Properties
Considering the fact that scaffolds must be able to endure intense mechanical loads in native organs, decellularized and native urethras were tested via a tensile-test device (Zwick/Roell, Model: Hct 400/25, Germany) in order to evaluate the tensile strength. Considering the fact that our previous studies revealed that transitional and longitudinal fibers have different tensile strength, these fibers were analyzed in both decellularized scaffolds and natural urethras in two separated tests. Samples were cut into strips in the size of 4 cm × 0.5 cm for estimating the physical property and the maximum elongation of longitudinal fibers. The constant elongation rate was 0.1 mm/s (6 mm/min) at room temperature. After clenching the specimens in sample holders, the tissues were subjected to mounting uniaxial tensile testing. The experiment was continued until urethral tearing and disappearance of load demonstrated by the device. The curve of strength–stress was drawn and stiffness (N/m), elastic modulus and maximum force (N) were calculated by the device.
Surgical Technique
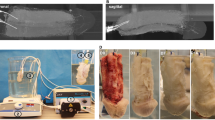
The Animal Ethics Committee approved the experimental protocol. All the rats were treated in compliance with The National Institutes of Health (NIH) guidelines. Transplantation of human decellularized urethral scaffolds with the surrounding corpus spongiosum was performed under general anesthesia by intramuscular injection of Ketamine (150 mg/kg) and Xylazin (15 mg/kg) in 12 male Sprague Dawley rats at 14–16 weeks of age weighting 200–250 g. The peritoneal cavity was exposed through a lower midline laparotomy incision. The omentum was exposed through the incision and decellularized urethra in the size of 5 × 5 mm2 was implanted in the peritoneal cavity using Prolene 3-0. The majority of urethral surface contact was with the peritoneum. In order to avoid the migration of urethral implant, the peritoneum was placed in the scrotum and fixed. These rats underwent another surgery after 1, 3, and 6 months and biopsies were taken for further histopathological evaluations (Fig. 1). In order to prevent the scarification of the animals, the peritoneal cavity was again exposed through the same lower midline laparotomy incision and the previously implanted scaffold that was fixed in the scrotum was removed. The incision was cautiously sutured and each animal was observed through the postoperative period.
Isolation and Culture of Preputial Tissue
Human preputial tissue was obtained during circumcision in children under aseptic condition after receiving written informed consent from their parents. Foreskin is believed to be a reservoir of regenerative cells. Considering the therapeutic value and the functional competences of classical fibroblasts, stromal, progenitor and different types of stem cells, we decided to apply these cells by using tissue explant technique.18 Briefly, samples were washed with sterile PBS, cut into 2-mm2 sections with dissection scissors, and treated with type 1 collagenase solution (0.1 mg/mL) for 1 h at 37 °C. The solution was filtered with a 70-μm mesh filter (falcon). After centrifuging the cells, they were cultured in Dulbecco’s Modified Eagle’s Medium with low glucose (DMEM-LG) supplemented with 10% Fetal bovine serum (FBS), 2 mmol/L l-glutamine, 10 ng/mL bFGF, and 50 U/mL penicillin/streptomycin. Cultured cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. Non-adherent cells were washed away and plastic-adherent cells (MSCs) were cultured for isolation of MSCs on the third day with the same medium (DMEM + FBS). The medium was changed with 3 days intervals. After primary culture, cells were treated with 0.025% Trypsin/EDTA for 2 min in the incubator and continued to be cultured until third passage. Afterward, 6 × 106 cells were seeded on 35 mm temperature-responsive culture dishes (Cellseed, Tokyo, Japan). After 6 days, dishes were incubated at 20 °C for 60 min to obtain cell sheets. By reducing the culture temperature to below the critical solution temperature, cultured cells were spontaneously detached from the temperature-responsive culture dishes surface as an intact sheet. In order to fabricate three-layer sheets, the sheets were transferred onto another sheet and this construct was immediately transferred over the decellularized urethral scaffold (Fig. 2).
Cell sheet technique for in vitro urethral regeneration by MSCs from preputial tissue: reducing the culture temperature to below the critical solution temperature, detachment of cultured cells from the temperature-responsive culture dishes, fabrication of three-layer sheets, and transferring the fabricated cell-sheet on the luminal surface of decellularized urethral scaffold.
Identity of Isolated Stem Cells
Flow Cytometry Analysis
Accutas Enzyme Cell Detachment Medium was applied to detach cells from culture dish. After placing cells into a conical centrifuge tube, cell count and viability analysis was performed. The final cell concentration in the Flow cytometry Staining Buffer was 2 × 107/mL. Harvested cells of the third passage were incubated with CD44, CD45, CD90, CD34, CD73, and CD105 antibodies as MCSs markers (Dako, Trappes, France) for 60 min at 28 °C. Then, the labeled cells were analyzed by flow cytometry.
In Vitro Recellularization
Two different in vitro recellularization techniques were applied in this study. In the static-based procedure, the cell sheets were transferred over the luminal surface of decellularized urethral scaffold (N = 4) and the whole structure was placed in the growth media (DMEM-LG + FBS). The media was changed every 3 days. This process was continued for 2 weeks and the sample was evaluated with histopathological techniques.
In the perfusion-based technique, decellularized urethras were transferred to a closed-system bioreactor that was placed in an incubator for urethral cell seeding. A 100-mL glass bottle was connected to a pump via two holds on the cap of the bottle that were served for the cannula and medium circulation. The scaffolds were remained in a hanging position in the bottle by applying a 12 gauge needle. In order to avoid cell adherence to the container, the bottle was placed on a shaker during the perfusion-based cell seeding process. The circulation time was 1 min with a consequent resting time of 59 min in order to avoid urethral ECM disturbance and enhance the cell adherence to the scaffold. The flow rate was 5 mL/min at 37 °C in 5% CO2. During the first 3 days, 50 mL medium (DMEM-LG + FBS) with an approximate amount of 8.5 × 106 MSCs were circulated. On the fourth day, the medium was centrifuged and the remained cells were added to another 8.5 × 106 cells. The perfusion was continued for the next 17 days; changing the medium with 3 days intervals. The previous medium was centrifuged every time and the remained cells were added to the fresh medium. Afterward, urethras were disconnected from the bioreactor for histopathological evaluations.
Histology and Immunohistochemistry
To assess the efficiency of the decellularization procedure in the maintenance of ECM and removal of cell components, processed urethras (3 mm × 3 mm) were fixed in formalin for 24 h, rinsed in distilled water (dH2O), dehydrated in graded ethanol, embedded in paraffin, sectioned at 5 μm and stained with Hematoxylin–Eosin (H&E) and trichrome. Two pathologists who were blind to the study analyzed the specimens.
Nuclear staining was performed with blue-fluorescent 4′,6‐diamidino‐2‐phenylindole (DAPI) for visualization of dsDNA in normal and decellularized scaffolds. The solution was diluted with PBS to 30 nM and pipetted on both specimens. During incubation in a dark room for 30 min, fluorescence enhancement occurs as a consequence of water displacement in DAPI and minor groove of the nuclei. Afterward, the samples were rinsed with dH2O, and evaluated under fluorescence microscope.
After washing the specimens in 0.01 N hydrochloric acid for 2 min, the tissue-engineered sections were stained in 0.1% sirius red solution in saturated picric acid in order to identify the type of collagen in Picrosirius red staining.
Differentiation of collagen, elastin, muscle, mucin, and fibrin were also used with Russell–Movat’s (pentachrome) staining. In this method five colors are detected for identification of different constituents of connective tissue in a single stained slide. In brief, nuclei and elastic fibers are stained in black, collagen and reticular fibers are stained in yellow, mucin is stained in blue, Fibrin is stained with bright red and muscles is stained with red color.
The biopsies were also stained with H&E and incubated with α smooth muscle actin, cytokeratin AE1/AE3, synaptophysin, and CD31 antibodies to show cell seeding in decellularized urethras. All antibodies were purchased from Dako (Trappes, France). In order to ameliorate penetration of the antibody for the purpose of immunohistochemical (IHC) staining, samples were adequately fixed in 4% Paraformaldehyde (8 min) and immersed in 0.2% Triton X-100 (10 min) diluted 1:100 in PBS. Improper or extended fixation was avoided to enhance antibody binding capability. Sections were incubated with primary antibodies, after being blocked with 1% BSA/PBS. At each time point, harvested tissues were evaluated with image analysis using Photoshop 10.0 software (Adobe Systems, Inc., Mountain View, CA, USA), and Image Pro (Image Pro Inc., Boston, MA, USA). Five photomicrographs (X100) of each sample were used for scoring. The means of the obtained scores were used as final values for analysis. The inner (luminal) surface of the urethral tissues was examined.
Statistical Analysis
Statistical Package of Social Science software (version 19 SPSS) was used for statistical analysis. Data are expressed as mean ± SE. Independent sample t test was used for the results of the IHC staining. A p value <0.05 was considered as statistically significant.
Results
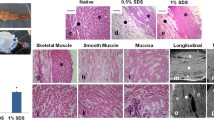
By the end of decellularization process, urethral tissues became whitish and translucent in appearance. The gross appearance of decellularized scaffolds was maintained and H&E staining showed no cellular or nuclear remnants in decellularized urethras. While histological evaluations showed that ECM was satisfactorily preserved, DAPI staining revealed that complete cell removal was achieved. Preservation of collagen in urethral ECM was also confirmed by pentachrome and picrosirius red staining. Muscle fibers and elastin were also present after decellularization process as confirmed by pentachrome staining. Picrosirius red staining showed that the majority of collagens in urethra are type I and were stained in red color. Moreover, the result of trichrome staining were in compatible with pentachrome and picrosirius red staining and confirmed the efficacy of decellularization process in preservation of ECM in decellularized urethras (Fig. 3).
The result of SEM demonstrated that decellularization process did not have any effect in disarrangement of the matrix or collagen degradation. Well-organized pores that were appropriate for further cell seeding were also detected. These results were similar in both luminal and abluminal sides of the urethral tissues. DNA quantification of decellularized urethral matrice showed 1.1 ng dsDNA per mg dry weight of ECM which was <4% in the decellularized scaffold compared to control.
The results of mechanical properties were in accordance with SEM and Histological evaluations. Accordingly, no significant difference was detected in the tensile strength of decellularized and normal urethras. Decellularized tissue showed the following mechanical properties: maximal longitudinal strength = 0.006 MPa, Young’s modulus = 0.010 MPa, and transitional strength = 0.64 N/mm. However, the biophysical properties of the native tissue were as follow: maximal longitudinal strength = 0.008 MPa, Young’s modulus = 0.013 MPa, and transitional strength = 0.74 N/mm) (Fig. 4).
Flowcytometry outcomes demonstrated that the expression of CD44, CD45, CD90, CD34, CD73, and CD105 was 91.5, 0.059, 98.5, 0.594, 93.2, and 91% respectively. The results confirmed the exhibition of MSCs properties in the majority of cultured cells (Fig. 5).
None of the animals demonstrated any adverse effect, and all survived until the time of biopsy without any noticeable complication. At the time of biopsy, grafted scaffolds persisted at the same position and no significant shrinkage, reactive or infectious changes were detected in macroscopic view. However, microscopic evaluation of grafts revealed variable grades of in vivo recellularization. In spite of the fact that pre-surgical histological evaluation confirmed complete acellularization of the implants, host cell infiltration and angiogenesis was confirmed histologically 1 month after surgery. No infiltration of mononuclear cells was detected during all the follow-ups. There was no evidence of fibrosis or scarring in the urethras at any of the retrieval periods. Angiogenesis was increased during the entire duration of the study. H&E and IHC staining demonstrated visible vessels in all the follow-ups with larger vessels 6 month after implantation indicating the evidence of angiogenesis in implanted urethras. Less cell seeding and angiogenesis was detected in the biopsies taken after 1 month compared with the implanted urethras 3, and 6 months after surgery. Anti α-actin staining demonstrated the formation of organized muscle bundles 6 months after grafting. The presence of a confluent transitional cell layer was confirmed by pan cytokeratin staining both in short and long-term follow-ups. However, Synaptophysin staining was negative in all the follow-ups.
In spite of the fact that our results revealed that in vivo cell seeding was superior to in vitro techniques, histopathological evaluation showed promising results in cell seeding process both in cell sheet engineering and perfusion-based technique. However, the results of cell sheet application were more satisfactory with noticeably similar structure to the natural urethra (Figs. 6 and 7). The quantitative data are presented in the Table 1. Briefly, statistical analysis of in vivo technique showed that the mean ± SD expression of all markers increased significantly 3 months after implantation (p < 0.0001). In addition, comparison of markers between the last two follow-ups revealed a significant increase in expression of α-actin, CD31, and cytokeratin (p = 0.01, p = 0.03, and p = 0.005, respectively). In spite of the fact that IHC markers were positive in the specimens obtained from in vitro techniques, cell sheet method was superior in expressing α-actin, CD31, and cytokeratin compared to perfusion-based technique (p = 0.04, p = 0.03, and p = 0.04, respectively).
Discussion
To the best of our knowledge this is the first study demonstrating urethral regeneration using both in vivo and in vitro techniques. Considering the fact that the ECM was almost intact after decellularization procedure, the scaffolds may maintain the structure after transplantation and more satisfactory results may be obtained in regards of cell seeding process. Our data suggested that in vivo recellularization of urethra with the surrounding corpus spongiosum is technically feasible and can be more effective with promising results compared to in vitro recellularization. Nevertheless, the results of the in vitro urethral regeneration showed that cell sheet approach was more effective regarding recellularization process compared to perfusion-based method.
The importance of urethral regeneration in urology has evoked many efforts to improve surgical technique and use cell-seeded and non-seeded tissue engineering approaches.30 Tissue engineering is considered as an alternative technology to improve survival rate and quality of life in patients with damaged urethras. Matrices used for urethral tissue engineering should provide mechanical robustness, plasticity, low immunogenicity, and biodegradability. In our study, we developed a naturally derived collagen-based tissue substitute of acellular human urethral matrix and evaluated its potentials for urethral regeneration with both in vivo and in vitro techniques.
A biological scaffold which can preserve elastin content after its decellularization process may improve tissue regeneration compared to synthetic scaffolds.28 In this study, the entire decellularized scaffolds contained elastin confirmed by pentachrome staining. In addition, preserved mechanical property of decellularized scaffold suggests the maintenance of ECM which is crucial for further cell seeding.
Regarding urethral reconstruction techniques, porcine small intestinal submucosa (SIS) was used in the study of Kropp et al. in a rabbit model the results of which demonstrated the regeneration of normal epithelium with vascularized collagen and smooth muscle backing.13 In another study in rabbit model, porcine acellular bladder submucosa matrix grafting was used for urethral reconstruction the results of which showed a normal cellular organization with a patent and functioning urethra.5 The first experimental study was reported by Sievert et al. using a homologous tubular urethral scaffold. The results demonstrated that although all the urethral components were observed in the grafted scaffolds after 3 months, smooth muscles were not well oriented compared to normal rabbit urethra.26 In spite of the fact that the urethral scaffold was not implanted in the original place, the results of the present study revealed the expression of α-actin both in in vitro and in vivo techniques.
Bi-layer silk scaffold with superior urethral biocompatibility has been considered as a promising new option in comparison to SIS for urethral reconstruction.7 In another study in 2015, it was demonstrated that collagen scaffolds modified with collagen-binding VEGF (CBD-VEGF) could enhance the collagen performance and provide an alternative strategy in reconstruction of large urethral defects.10 In our previous study in rabbit model, we used tissue-engineered preputial matrix and fibrin sealant for repairing segmental urethral defects with promising results.11 While in the current study the urethral scaffold was not placed in the original place for urethral reconstruction, promising results were obtained in terms of urethral regeneration for further studies.
In the study of Fu et al., penile urethral mucosal defect was induced in the anterior urethra of rabbit model and repaired with either tubular grafts seeded with autologous foreskin epidermal cells or cell-free tubular matrices. The results demonstrated better regeneration pattern in the cell-seeded group.9 In a recent study, a tissue-engineered sheet graft was constructed using bone marrow-derived MSC and smooth muscle cell-seeded bladder acellular matrix for urethral reconstruction the result of which was promising.16 In one study in 2013, unseeded and pre-seeded collagen-based tubular matrices with autologous bladder epithelial and smooth muscle cells were used for reconstruction of long urethral defects. The results demonstrated that poor tissue development and strictures in scaffolds without cells.19 In another study, urethroplasties were performed with unseeded and seeded tubularized matrices with autologous bladder epithelial and smooth muscle cells. The results showed that application of seeded matrixes is more successful for long segmental penile urethra replacement.8 Although previous studies revealed the superiority of pre-seeded scaffolds compared to cell-free scaffolds, these two techniques were not compared in the current study. However, the results of non pre-seeded matrices in the scrotal region were promising in terms of tissue regeneration.
According to the result of another study, although decellularized rabbit thoracic aorta were seeded with urothelial cells and successfully replaced in the excised pendulous urethras of rabbits with regeneration of all implanted matrices, the pre-seeding of the scaffolds does not seem to be compulsory before the implant.20 The results of the current study demonstrated that grafting of human acellular urethral tissue in rat model is feasible and safe. Application of this technique and further implantation of recellularized urethral tissue may pave the road for further urethral reconstructive approaches. In addition, all animals demonstrated a luminal epithelialization and rapid angiogenesis verified by histological analyses without the evidence of graft rejection or fistula in any of the experimental animals.
In regards of clinical studies, an acellular collagen matrix with adequate urothelial-cell epithelialization and urethral tissue regeneration was developed in the study of Chen et al. 6 In the follow-up, no clinical changes were demonstrated in patients who underwent urethral reconstruction with the acellular matrix. In a clinical trial, tubularized non-seeded SIS was used for urethral stricture repair in 8 patients. Urethral patency was maintained in 2 patients with short inflammatory strictures and stricture recurrence developed in the other 6 patients after 3 months of operation.15 The results of the current study suggest that some tissue specific effects may be observed that make urethral tissue for urethral regeneration more successful than SIS.
In the study of Fu et al., the biomechanical properties of different biomaterials including bladder submucosa, polyglycolic acid (PGA), SIS, and acellular corpus spongiosum matrix was assessed for urethral tissue engineering the result of which verified that acellular corpus spongiosum matrix was the most effective type of scaffold.9 One of the roles of the corpus spongiosum in erection is preventing the urethra from pinching closed. So, the urethra is able to maintain as a viable channel for ejaculation.12 Due to the supporting role of urethral bulb (corpus spongiosum) for urethra tissue, its help in increasing the zone of coaptation within the sphincteric (membranous) urethra, and its active role in male urinary continence,24 in the current study we decellularized human urethra with the surrounding corpus spongiosum and used the whole scaffold during our in vitro and in vivo recellularization techniques. By this technique, we can avoid the stricture of the urethra in further clinical investigations for whole urethral reconstruction in hypospadias repair.
One of the approaches in tissue engineering is the injection of isolated cell suspensions and the use of biodegradable scaffolds such as such as PGA, polylactic acid (PLA), and polylactic coglycolic acid (PLGA). Cell sheet engineering, as used in the current study, can avoid the limitations of the mentioned tissue engineering approaches. The surface of temperature-responsive culture dishes allow satisfactory attachment, spread, and proliferation of various cells. In addition, loss of differentiated form and function of cells as well as the preservation of cell-to-cell and cell-to-extracellular matrix connections are among the advantages following the application of cell sheet-based technology.17 In addition, only few percent of cultured cells are transplanted to target tissues, which significantly decrease the predictable therapeutic effects. It should be also mentioned that cell sheet transplantation has several advantages over direct injection of cell suspensions or using biodegradable scaffolds for regenerative therapy. Cell sheets can be implanted to the host tissue directly and without usage of suture with the intact deposited ECM. In addition, cell sheet engineering can overcome the limitations of the application of biodegradable scaffolds due to the fact that no support material is used for implantation of cell sheets to the host tissue.29
To the best of our knowledge, this is the first study in which the surrounding human corpus spongiosum was not detached from the urethra and the whole structure was engineered using both in vitro and in vivo techniques. However, not tracking the MSCs can be considered as one of the limitations of the current study. It should be also mentioned that the size of grafts used in this experimental study was small. So, we cannot state that replacement by natural host tissue would occur rapidly in the size of graft essential for clinical purposes which is another limitation of the current study. We aimed to compare these techniques in this preliminary experiment to find the best method for future studies. The results revealed that in vivo technique was superior to in vitro methods. Implanting a pre-seeded scaffold seems to result in more satisfactory results in a short period after implantation. However, not implanting a pre-seeded scaffold as a group in the animal model is one of the limitations of the current study Despite of very promising preliminary results of this study, further studies are required to consolidate the technique before this approach can be used in clinical practice for urethral reconstruction.
Conclusion
The acellular urethral scaffold matrix appears to be a useful material for urethral regeneration in the rat model. Due to the fact that this regenerated urethral tissue is histologically similar to natural urethra, these collagen matrices may be used as a useful substitute for patients requiring a tubularized urethral segment replacement. Although we obtained satisfactory results in in vivo and in vitro procedures, further experience and longer follow-up are needed to validate these findings and confirm the superiority of this scaffold to be used as an “off the shelf material”.
Abbreviations
- SEM:
-
Scanning electron microscopy
- ECM:
-
Extracellular matrix
References
Atala, A., et al. The potential role of tissue-engineered urethral substitution: clinical and preclinical studies. J. Tissue Eng. Regen. Med. 2015. doi:10.1002/term.2112.
Barbagli, G., et al. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J. Urol. 174(3): 955–957, 2005; discussion 957–958.
Blaschko, S. D., et al. Repeat urethroplasty after failed urethral reconstruction: outcome analysis of 130 patients. J. Urol. 188(6):2260–2264, 2012.
Brown, B. N., and S. F. Badylak. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 163(4):268–285, 2014.
Chen, F., J. J. Yoo, and A. Atala. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology 54(3):407–410, 1999.
Chen, F., J. J. Yoo, and A. Atala. Experimental and clinical experience using tissue regeneration for urethral reconstruction. World J. Urol. 18(1):67–70, 2000.
Chung, Y. G., et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS ONE 9(3):e91592, 2014.
De Filippo, R. E., et al. Penile urethra replacement with autologous cell-seeded tubularized collagen matrices. J. Tissue Eng. Regen. Med. 9(3):257–264, 2015.
Fu, Q., et al. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 99(5):1162–1165, 2007.
Jia, W., et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 69:45–55, 2015.
Kajbafzadeh, A. M., et al. The application of tissue-engineered preputial matrix and fibrin sealant for urethral reconstruction in rabbit model. Int. Urol. Nephrol. 46(8):1573–1580, 2014.
Korkes, F., et al. Recreational use of PDE5 inhibitors by young healthy men: recognizing this issue among medical students. J. Sex. Med. 5(10):2414–2418, 2008.
Kropp, B. P., et al. Rabbit urethral regeneration using small intestinal submucosa onlay grafts. Urology 52(1):138–142, 1998.
Laird, P. W., et al. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 19(15):4293, 1991.
le Roux, P. J. Endoscopic urethroplasty with unseeded small intestinal submucosa collagen matrix grafts: a pilot study. J. Urol. 173(1):140–143, 2005.
Li, C. L., et al. Urethral reconstruction using bone marrow mesenchymal stem cell- and smooth muscle cell-seeded bladder acellular matrix. Transplant. Proc. 45(9):3402–3407, 2013.
Matsuura, K., et al. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release 190:228–239, 2014.
Najar, M., et al. Mesenchymal stromal cells from the foreskin: tissue isolation, cell characterization and immunobiological properties. Cytotherapy 18(3):320–335, 2016.
Orabi, H., et al. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur. Urol. 63(3):531–538, 2013.
Parnigotto, P. P., et al. Experimental defect in rabbit urethra repaired with acellular aortic matrix. Urol. Res. 28(1):46–51, 2000.
Peterson, A. C., and G. D. Webster. Management of urethral stricture disease: developing options for surgical intervention. BJU Int. 94(7):971–976, 2004.
Qi, N., W. J. Li, and H. Tian. A systematic review of animal and clinical studies on the use of scaffolds for urethral repair. J. Huazhong. Univ. Sci. Technol. Med. Sci. 36(1):111–117, 2016.
Raya-Rivera, A., et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377(9772):1175–1182, 2011.
Rehder, P., et al. Hypothesis that urethral bulb (corpus spongiosum) plays an active role in male urinary continence. Adv. Urol. 2016:6054730, 2016.
Ribeiro-Filho, L. A., and K. D. Sievert. Acellular matrix in urethral reconstruction. Adv. Drug Deliv. Rev. 82–83:38–46, 2015.
Sievert, K. D., and E. A. Tanagho. Organ-specific acellular matrix for reconstruction of the urinary tract. World J. Urol. 18(1):19–25, 2000.
Sinha, R. J., et al. Donor site morbidity in oral mucosa graft urethroplasty: implications of tobacco consumption. BMC Urol. 9:15, 2009.
Sivaraman, B., C. A. Bashur, and A. Ramamurthi. Advances in biomimetic regeneration of elastic matrix structures. Drug Deliv. Transl. Res. 2(5):323–350, 2012.
Tang, Z., and T. Okano. Recent development of temperature-responsive surfaces and their application for cell sheet engineering. Regen. Biomater. 1(1):91–102, 2014.
Xue, J. D., et al. Seeding cell approach for tissue-engineered urethral reconstruction in animal study: a systematic review and meta-analysis. Exp. Biol. Med. (Maywood) 241(13):1416–1428, 2016.
Acknowledgments
We are highly grateful from Mrs. S. Lotfi for her precise final linguistic revision of the manuscript.
Conflict of interest
No conflict of interest exists in relation to the submitted manuscript and there was no source of extra institutional commercial funding or funding received from National Institutes of Health (NIH), Wellcome Trust, Howard Hughes Medical Institute (HHMI) and others.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kajbafzadeh, AM., Abbasioun, R., Sabetkish, S. et al. Future Prospects for Human Tissue Engineered Urethra Transplantation: Decellularization and Recellularization-Based Urethra Regeneration. Ann Biomed Eng 45, 1795–1806 (2017). https://doi.org/10.1007/s10439-017-1857-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1857-x