Abstract
Replacing urethral tissue with functional scaffolds has been one of the challenges in the field of urethral reconstruction or repair over the last few decades. Various scaffold materials have been used in animal studies, but clinical studies in the use of scaffolds for urethral repair are scarce. It was found that in both animal and clinical studies, scaffolds seeded with cells were used for repair of large segmental defects of the urethra, such as in tubular urethroplasty. When the defect area was small, cell-free scaffolds were more likely to be applied. A large amount of pre-clinical and limited clinical evidence showed that natural or artificial materials could be used as scaffolds for urethral repair. Urinary tissue engineering is still in the immature stage, and the safety, efficacy, and cost-effectiveness of scaffolds must be evaluated to allow further study.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Urethroplasty
- Animal models
- Clinical studies
- Material/Scaffold
- Tissue engineering/regenerative medicine
- Urethral stricture
1 Introduction
A variety of congenital and acquired urethral pathologies including hypospadias, strictures, fistulae, and straddle injuries can severely impair normal urethral function, necessitating surgical reconstruction [1,2,3,4]. No universally recognized algorithms for the surgical management of these conditions are in place, although some progress in this field has been made [5,6,7]. A urethral stricture is a narrowing of the urethral lumen, arising from iatrogenic (33%), idiopathic (33%), traumatic (19%), and inflammatory (15%) causes, that may lead to lower urinary tract obstruction [3, 8]. Such obstruction may significantly impair patients’ quality of life by causing voiding difficulties; these, in turn, may negatively affect the upper urinary tract, resulting in deterioration of renal function [9]. The mechanism of urethral stricture development involves the processes of fibrosis and cicatrix formation in the urethral mucosa and surrounding connective tissues. Strictures can occur at any portion of the urethra between the meatus and bladder neck [10]. In the context of urethral stricture description, the term “urethra” typically refers to the anterior urethra including the bulbar and penile urethra [11]. However, posterior urethral strictures including those of the membranous and prostatic urethrae are not included in this definition; instead, these are typically termed “urethral contractures” or “urethral stenoses” [11]. It should be noted that urethroplasties are performed not only in patients with urethral strictures, but also in those with hypospadias and other urethral defects (e.g., urethral fistulae). In particular, repair of the anterior urethra is one of the most demanding surgical problems in urology. A multitude of techniques has been used to treat anterior urethral strictures using buccal mucosa or a penile skin flap for reconstruction of the long urethral defect [12,13,14]. However, in addition to potential donor site morbidity, there is an inadequate amount of graft in many cases. Urothelial cell based tissue engineering has shown promise as an alternative to urethral substitution [15,16,17,18,19,20]. In this chapter, we have evaluated currently available studies regarding the potential of tissue engineering in urethral reconstruction, in particular those describing the use of both acellular and recellularized tissue-engineered constructs in animal and human models. Possible future developments in this field are also discussed.
2 Tissue Engineering for Urethroplasty to Treat Urethral Lesions
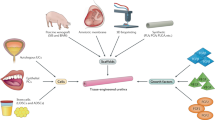
To reconstruct a new tissue, a triad of components is needed: cells, biomaterials to be used as supports or scaffolds, and growth factors [21,22,23]. Various strategies have been proposed over the years for the regeneration of urethral tissue. A woven mesh of polyglycolic acid (PGA) both with and without cells was used to regenerate urethras in various animal models [24– 26]. Naturally derived collagen-based materials such as bladder-derived acellular submucosa, acellular urethral submucosa, and collagen gels have also been tried experimentally in various animal models for urethral reconstruction [27,28,29]. Urethral reconstruction involves the use of different “patches” (which may be called matrices, scaffolds, or substrates) that have to be transplanted into the urethral wall, thus providing a patent lumen. Conventional substitution urethroplasty utilizes different tissues from the patient. Tissue engineered urethroplasty procedures may be performed with both naturally derived and synthetic materials [30, 31].
Many pediatric and adult patients with urethral disease have been successfully treated in an onlay manner using collagen-based matrices. One of the advantages of this method over nongenital tissue grafts used for urethroplasty is that the material is “off the shelf.” This eliminates the necessity of additional surgical procedures for graft harvesting, which may decrease operative time as well as eliminate morbidity from the harvest procedure.
One of the fundamental tasks when developing tissue-engineered “patches” is forming a support matrix that can serve as a substrate for cells (cell-adhesion substrate) and control the configuration of the tissue-engineered structure and the direction of tissue regeneration. Each of the naturally derived and synthetic biocompatible materials utilized for tissue engineering has its advantages and disadvantages.
2.1 Natural Materials
Natural polymers may be divided into natural polymers and acellular matrices [32, 33]. In the quest for an ideal urethral substitute, acellular scaffolds are promising, as they have demonstrated the ability to induce tissue regeneration layer by layer. Following several experimental studies, the use of acellular matrices for urethral reconstruction has become a clinical reality over the last decade. Such biomaterials are prepared via a process leading to the removal of all cells and their components, i.e. decellularization. The remaining product is an acellular matrix, mostly containing collagen that (theoretically) should be non-immunogenic and should cause no allergic reactions. All the above-mentioned matrices are biodegradable and are gradually substituted by the host’s own intercellular matrix.
The bladder submucosal matrix proved to be a suitable graft for repair of urethral defects in rabbits [27]. The neourethras exhibited a normal urothelial luminal lining and organized muscle bundles. These results were confirmed clinically in a series of patients with a history of failed hypospadias reconstruction wherein the urethral defects were repaired with human bladder acellular collagen matrices [34]. The neourethras were created by anastomosing the matrix in an onlay fashion to the urethral plate. The size of the created neourethra ranged from 5 to 15 cm. After a 3-year follow-up, three of the four patients had a successful outcome with regard to cosmetic appearance and function. The acellular collagen-based matrix eliminated the necessity for performing additional surgical procedures for graft harvesting, and both operative time and the potential morbidity from the harvest procedure were decreased. Similar results were obtained in pediatric and adult patients with primary urethral stricture disease using the same collagen matrices [35]. Another study by El-Kassaby et al. of 30 patients with recurrent stricture disease showed that a healthy urethral bed (two or fewer prior urethral surgeries) was needed for successful urethral reconstruction using acellular collagen-based grafts [36]. In their study, the length of the strictures ranged from 2 to 18 cm (average 6.9 cm). All grafts were implanted as ventral onlay patches. The patients were followed for a mean period of 25 months. Where the patients had one or no previous interventions, the success rate between both groups was similar (100% for buccal mucosa vs. 89% for acellular matrix).
Feng et al. compared the mechanical properties and biocompatibility of biomaterials, including bladder submucosa, small intestinal submucosa (SIS), acellular corpus spongiosum matrix, and PGA, to identify the optimal scaffold for urethral tissue engineering [37]. Cytotoxicity, tensile mechanical properties, and pore size were evaluated. Smooth muscle cells were seeded onto the biomaterials to evaluate differences in cell infiltration. They concluded that the acellular corpus spongiosum matrix had better overall performance, indicating that an organ specific matrix may be the most suitable type of scaffold for urethral reconstruction.
A clinical trial using tubularized nonseeded SIS for endoscopic urethroplasty was performed in eight evaluable patients [38]. Two patients with short inflammatory strictures maintained urethral patency. Stricture recurrence developed in the six patients within 3 months of surgery. Authors concluded that endoscopic urethroplasty using an unseeded SIS graft was unsuccessful. On the other hand, various authors usually reported favorable results from SIS urethroplasties in humans. Palminteri et al. observed an 85% success rate with short-term follow-up (mean 21 months) [39]. A dorsal inlay graft was performed in 14 cases, ventral onlay graft in 1, and dorsal inlay plus ventral onlay in 5. The three failures were penile repairs with long strictures (over 5 cm). This group updated their series in 2012, publishing long-term results (71 months) with an overall patency rate of 76% [40].
Sievert et al. performed single-stage urethral reconstruction with homologous acellular urethral matrix grafts [41]. All tissue components were observed in the grafted matrix after 3 months, with further improvement over time; however, the smooth muscle in the matrix was less extensive than in the normal rabbit urethra and was not well oriented.
The value of homologous dermal acellular matrix grafts for urethral reconstruction in humans was investigated by Lin et al. in 2005 [42]. The dermal acellular matrix graft was sutured to a tubular graft and it replaced the defect in the urethra. Following the operation, urethrography revealed the excellent caliber of the reconstructed urethra. Urethroscopic examination showed that the graft was covered by epithelial tissue and grew into the native tissue. They suggested that the homologous dermal acellular matrix graft may serve as an ideal replacement material for complex urethral strictures or defects, without the risk of rejection.
Fossum et al. surgically treated six boys aged 14–44 months with severe hypospadias with autologous urothelial cell constructs [43]. A two-staged procedure starting with repair of the chordee was performed in all cases. Urothelial cells were harvested via bladder lavage during the first operation and were seeded onto acellular dermis. The seeded scaffold was implanted in a second operation in an onlay fashion. These children were followed up for 3.5–5 years. One patient developed a partial stricture, which was treated conservatively, and another developed an obstruction in the proximal anastomosis that was managed successfully with internal urethrotomy. Two other children developed fistulae requiring surgical correction.
2.2 Synthetic Polymers
Synthetic polymers of naturally occurring α-hydroxy acids are widely used in the field of regenerative medicine. Some of these have been approved by the US Federal Drug Administration (FDA) for use in a number of applications, including sutures [44]. Of interest for urethral tissue engineering are biodegradable matrices (both synthetic and natural). The degradation products of synthetic biodegradable polymers based on α-hydroxy acids are carbon dioxide and water. As these polymers are thermoplastics, they can easily be shaped into a three-dimensional (3D) scaffold with the required porosity and size.
Synthetic polymer scaffolds designed for cell transplantation were reproducibly made on a large scale and studied with respect to biocompatibility, structure, and biodegradation rate [45]. The scaffold induced chondrocyte differentiation with respect to morphology and phenotype and represented a model cell culture substrate that may be useful for a variety of tissue engineering applications [45]. Micos et al. suggested that biodegradable foams of hydrophobic polymers can be efficiently wet by a two-step immersion in ethanol and water, which overcomes the hindered entry of water into air-filled pores [46]. In their study, ethanol readily enters into the porous polymer, after which it is diluted and replaced by water. This method was evaluated for porous disks of poly(l-lactic acid) (PLLA) and poly(dl-lactic-co-glycolic acid) (PLGA) foams of copolymer ratios 85:15 and 50:50. Furthermore, water entry even after 1 h was very close to the plateau value for all pre-wet polymers tested.
Harris et al. established open pore biodegradable matrices, formed using a gas foaming method developed for fabricating matrices without the use of organic solvents and/or elevated temperatures [47]. Disks comprised of polymer and NaCl particles were compression molded at room temperature and subsequently allowed to equilibrate with high pressure CO2 gas (800 psi). Creation of a thermodynamic instability led to the nucleation and growth of gas pores in the polymer particles, resulting in the expansion of the polymer particles. The polymer particles fused to form a continuous matrix with entrapped salt particles. The NaCl particles subsequently were leached to yield macropores within the polymer matrix. The overall porosity and extent of pore connectivity were regulated by the ratio of polymer/salt particles and the size of the salt particles. The utility of these matrices was demonstrated by engineering smooth muscle tissue in vitro. This process, a combination of high-pressure gas foaming and particulate leaching techniques, allows the fabrication of matrices with well-controlled porosity and pore structure.
The fabrication technique of electrospinning allows the fast production of high-porosity scaffolds with defined shapes and architectures. Han and Gouma developed electrospun bioscaffolds that mimic the topology of extracellular matrix (ECM) [48]. ECM is a natural scaffold for cell, tissue, and organ growth. Its topology plays an important role in cell differentiation. The design challenge is to fabricate biomaterials that mimic the ECM’s three-dimensional (3D) structures with defined shapes and complex porous architecture. The urinary bladder matrix (UBM) is used in this work as the model system of the ECM architecture. Cellulose acetate (CA) is the biomaterial of choice for building UBM-mimicking scaffolds. Electrospinning is the fabrication method used to form complex, porous, 3D structures with specific designs in a single-step process. Lee et al. used thermal treatments to enhance the mechanical properties of electrospun poly-(epsilon-caprolactone) (PCL) scaffolds [49]. The biomechanical properties of the thermally bonded electrospun PCL scaffolds were significantly increased without any gross observable and ultrastructural changes when compared to untreated PCL scaffolds. They suggested that the introduction of thermal fiber bonding to electrospun PCL scaffolds improved the biomechanical properties of these scaffolds, making them more suitable for tissue engineering applications.
PGA polymers were used as tissue-engineered autologous urethras for patients who required reconstruction [50]. In this study, epithelial cells were expanded and seeded onto tubularized polyglycolic acid:poly(lactide-co-glycolide acid) scaffolds. Patients then underwent urethral reconstruction with the tissue-engineered tubularized urethras. Urethral biopsies showed that the engineered grafts had developed apparently normal architecture by 3 months after implantation. Tubularized urethras can be engineered and can remain functional in a clinical setting for up to 6 years. Kanatami et al. reported fabrication of an optimal urethral graft using collagen-sponge tubes reinforced with Copoly(l-lactide/epsilon-caprolactone) [P(LA/CL)] fabric [51]. The tubes were dipped in aqueous collagen solution and lyophilyzed to prepare the P(LA/CL)-collagen sponge graft. The grafts were applied to a 1.5 cm rabbit urethral defect (n = 14 for each condition), and tissue repair was evaluated using urethrographical, urethroscopical, and histological examination 1, 3, and 6 months after surgery. For the mesh style graft, all urethras were patent, without fistulae or stenoses, over the entire observation period. Histologically, urethral structure was disorganized for the stent style graft, whereas the urethral tissue on the mesh style graft was slightly fibrotic but completely epithelialized and supported by a regenerated smooth muscle layer at 6 months. These findings suggest that creation of a scaffold suitable for urethral tissue regeneration depends not only on biomaterial composition, but also on the fabrication technique.
2.3 Hybrid or Composite Scaffolds
Hybrid or composite scaffolds are constructed from a synthetic polymer combined with a corresponding natural matrix [52, 53]. Such scaffolds were developed by researchers in the quest for an ideal tissue-engineered material to be used in urology. Hybrid scaffolds are constructed by electrospinning PLGA microfibers onto the abluminal surface of a bladder acellular matrix [53].
There are two types of matrix used in tissue-engineered urethral substitution procedures: acellular matrices and autologous cell–seeded matrices. Acellular matrices have been utilized for a long period of time. An acellular matrix transfer procedure will be successful if: (a) the entire abluminal surface is infiltrated by host epithelial cells; (b) the defect being substituted is short; and (c) there is a good vascular urethral bed. Therefore, procedures using acellular matrices are expected to fail in patients with recurring strictures, marked spongiofibrosis, or long strictures. An autologous cell-seeded matrix is a tissue-engineered construct containing an acellular matrix populated (ex vivo) with autologous cells. A biopsy is obtained from the patient. In specialized sterile laboratories, cells of the desired type are harvested from the biopsy and grown in a culture. Depending on the type of cells and culture method used, this process may take 4–12 days to 3–6 weeks [50, 54, 55]. As soon as the required number of cells is obtained, they are seeded onto a matrix and, 1–7 days later, are implanted into a patient or animal. Frequently used autologous cells are as follows: urothelial cells from the bladder, urethra, or ureter; buccal mucosa epitheliocytes; keratinocytes; fibroblasts; and smooth muscle cells [50, 54, 56,57,58].
De Filippo et al. showed that acellular collagen matrices derived from bladder submucosa seeded with cells from normal urethral tissue can be used for tubularized replacement [56]. They suggested that the collagen matrices seeded with cells may offer a useful alternative in the future for patients requiring a tubularized urethral segment replacement. Li et al. investigated the feasibility of urethral reconstruction using oral keratinocyte (OK)-seeded bladder acellular matrix grafts [57]. In their study, OKs had good biocompatibility with bladder acellular matrix grafts, and urethral reconstruction with these grafts was better than that with bladder acellular matrix grafts alone. Fu et al. evaluated alterations in foreskin epidermal cells, which were used to reconstruct male rabbit anterior urethras in combination with acellular collagen matrices. They concluded that epidermal cells seeded onto acellular collagen matrices can be successfully used to reconstruct urethras that have defects, and are transformed to transitional epithelial cells [58]. Autologous tissue-engineered buccal mucosa was confirmed as a clinically useful autologous urethral replacement tissue in a group of patients with lichen sclerosis urethral stricture. However, it was not without complications, namely fibrosis and contraction [54]. Raya-Rivera et al. constructed engineered urethras with autologous cells and implanted them into patients with urethral defects. A tissue biopsy was taken from each patient, and the muscle and epithelial cells were expanded and seeded onto tubularized polyglycolic acid:poly (lactide-co-glycolide acid) scaffolds. Patients then underwent urethral reconstruction with the tissue-engineered tubularized urethras. Tubularized urethras can be engineered and remain functional in a clinical setting for up to 6 years. These engineered urethras can be used in patients who need complex urethral reconstruction [50].
Tissue-engineered autologous cells can be divided into two types: tissue-engineered constructs developed using a monoculture; and those using co-culture. To develop tissue-engineered constructs using a monoculture, autologous cells are seeded onto one of the surfaces of the support matrix and, as a rule, a certain type of epitheliocyte is used [55]. Constructs using a co-culture are more complex, as the abluminal surface is seeded with epitheliocytes, while the opposite side is seeded with either fibroblasts or smooth muscle cells [50, 59].
According to macroscopic parameters, tissue-engineered constructs can be divided into patch constructs and tubularized constructs. The stricture length to be reconstructed with patch constructs grafts is usually limited, as it is in cases of conventional substitution procedures. Tubularized grafts are usually developed using a co-culture and may be utilized to reconstruct entire urethras [50]. Figure 9.1 overviews matrices and cells used for tissue-engineered urethral reconstruction.
2.3.1 Acellular Matrices Utilized in Animal Models
Kropp et al. suggested using SIS as an acellular urethroplasty matrix [60]. In one of the control groups, full thickness preputial skin from the host rabbit was used. The success rates were the same in both groups (100%), yet preputial graft procedures resulted in the formation of urethral diverticulum in all eight animals.
Chen et al. utilized BAMG (obtained and processed from porcine bladder submucosa) for ventral onlay urethroplasties in 10 rabbits [27]. Complete graft epithelialization was achieved after 2 months; the migration of organized muscle bundles was detected 6 months after implantation. No strictures or complications were observed.
In a study conducted by Nuininga et al., rabbits were divided into four groups, with six animals in each, according to the type of biomatrix used: in group 1, the rabbits underwent partial urethral replacement, which was replaced with one layer of SIS; in group 2, four-layer SIS patch grafts were utilized; group 3 was treated with collagen-based matrices produced from bovine tendons; and in group 4, the animals underwent a sham control operation (urethras were incised ventrally and then sutured) [61]. In groups 1 and 3, complete epithelialization was observed at 1 month after implantation, whereas in group 2, it was achieved at 3 months. One rabbit from group 3 developed a stricture and one rabbit from group 2 developed a fistula near the operation site.
Yang et al. suggested using an acellular corpus spongiosum matrix substitute as a patch [62]. These kinds of matrices were obtained from rabbit urethras and transplanted to repair urethral defects of 10–15 mm in length. Complete epithelialization of the extracellular matrix was achieved at 3 weeks post operation. Well-formed smooth muscle cells were observed after 6 weeks. No strictures or complications were reported.
Huang et al. explored the potential of SIS for urethral reconstruction in rabbits [63]. Tubular SIS grafts were applied in group 1 (n = 6), while a ventral onlay graft was used as a patch in group 2 (n = 6). The epithelium covered the grafts fully after 6 weeks.
An interesting study was published by Kanatani et al., wherein two types of tubularized graft based on type I collagen sponge, reinforced with co-poly(Lactide/ε-caprolactone) (CLLC) fabric, were created [51]. In group 1, the P(LA/CL) fibers were knitted into a vascular stent style; in group 2, the P(LA/CL) fibers were weaved into a mesh style. Each of the grafts was applied to a 15-mm urethral defect. Numerous complications (stenoses, fistulae, or stone formation) were observed in group 1, whereas no complications were seen in group 2. The authors concluded that creation of a scaffold suitable for urethral tissue regeneration depends not only on the biomaterial composition, but also on the fabrication technique.
In a very important study, Dorin et al. determined the maximum potential distance of normal native tissue regeneration when using tubularized unseeded matrices [64]. Twelve rabbits were divided into four groups. In each group, urethral defects of varying length (0.5, 1.0, 2.0, and 3.0 cm) were created. At week 4, only the 0.5 cm group had a normal layer of epithelium surrounded by a layer of smooth muscle within the urethral lumen. In all groups with longer defects, strictures developed by 4 weeks. Therefore, this study proved that acellular matrices in tubularized grafts may be used successfully only to repair defects limited in size.
Villoldo et al. assessed the efficacy of onlay urethroplasty with SIS in rabbits [65]. A total of 30 animals included in the study had 15 mm of the ventral wall of the penile urethra excised. One month later, the rabbits were divided into two groups: group 1 was the control group; in group 2, where the created defect was patched with an SIS onlay graft, epithelialization was achieved after 15 days and smooth muscle bundles were detected at 6 months follow-up. The fact that urethral strictures were created 1 month before urethroplasties were performed brings this study a step closer to clinical reality.
Chung et al. studied the potential of using acellular matrices derived from silk fibroin for urethral repair [66]. The results of urethroplasty with silk fibroin matrices (SFMs) were compared to those with SIS. The follow-up period was 3 months, with no intermediate time points. The success rates of SFM and SIS were found to be the same. No strictures or complications were reported. However, the silk fibroin scaffolds showed reduced immunogenicity.
Kajbafzadeh et al. explored the role of preputial acellular matrix (PAM) for urethral reconstruction [67]. Prepuces were obtained from circumcised boys. The prepuces were decellularized and the produced matrices were used as patches in ventral onlay urethroplasty procedures: PAM in group 1; and PAM plus fibrin sealant in group 2. The effectiveness of repair was evaluated in both groups at a 9 month follow-up. The authors noted that satisfactory vascularity and smooth muscle layer formation were more significant in group 2 (PAM + fibrin sealant).
In all the above-mentioned studies, only male rabbits were used as animal models. In most studies, a longitudinal urethral defect was created, varying in length (10–20 mm); in some studies it was a tubular defect 5–30 mm long. SIS was used as a urethroplasty matrix in four of ten studies, with BAMG being the second most frequently utilized type of scaffold (applied in three of ten studies). One should note that no episodes of graft rejection were reported. Acellular matrices were found to be highly effective in animal models, yet their application range was limited by the size of urethral defect. Complete formation of the urothelial layer on the inner surface of the graft was achieved 4–12 weeks after implantation; either single smooth muscle cells or a regenerated smooth muscle layer was observed 2–12 months after implantation.
2.3.2 Acellular Matrices Utilized in Human Models
The feasibility of applying a bladder submucosal, collagen-based inert matrix as a free graft substitute for urethral repair in patients with hypospadias was explored [34]. All four patients had had a history of hypospadia repair procedures and required yet another repair. The neourethras were created from BAMGs in the size range 5–15 cm. Postoperatively, only one patient developed a fistula. Histological evaluation showed typical urethral stratified epithelium at the site of surgery.
Mantovani et al. were the first to utilize an SIS graft for substitution urethroplasty in a 72 year-old patient with a long stricture of the penile and bulbar urethra [68]. The dorsal onlay technique was used. At a 16 month follow-up, the maximum urine flow rate was 14 ml/s. No complications were observed intraoperatively or postoperatively. In the same year, El-Kassaby et al. used a BAMG matrix for urethral stricture repair [35]. A total of 28 patients with urethral strictures of varied length (1.5–16 cm) underwent reconstructive surgery wherein the ventral onlay technique was used. The success rate was 86%, as one patient developed a urethrocutaneous fistula that closed spontaneously 1 year after repair.
Lin et al. suggested using an acellular dermal matrix (ADM) graft for urethral reconstruction [42]. Homologous ADM was applied as a tubularized graft in 16 patients with urethral strictures and hypospadias. No episodes of rejection were observed. During the 46 month follow-up period, only four patients needed periodical urethral dilatation.
Le Roux et al. evaluated SIS as a substitute in endoscopic urethroplasty [38]. Nine patients were enrolled. Optical urethrotomy was performed prior to the SIS graft implantation. Subsequently, a tubularized SIS graft was implanted into the stricture site. Only two patients had patent lumen at 1 and 2 years follow-up, respectively; all the other patients developed recurring strictures. The authors concluded that endoscopic urethroplasty with unseeded SIS grafts is not to be recommended.
Donkov et al. assessed the success rates of SIS grafts used for dorsal onlay substitution urethroplasty [69]. The graft was fixed using a modified Barbagli technique. The success rate was 89% (eight of nine patients) at 18 months follow-up.
While Hauser et al. also chose SIS for dorsal onlay substitution urethroplasty, the success rate was much lower: only one of five patients did not have stricture recurrence at a 12 month follow-up [70]. The complications observed postoperatively were extravasation, severe urethritis, and urinary tract infection.
Palminteri et al. evaluated the role of SIS grafts for substitution urethroplasties [39]. Three techniques were employed: a dorsal inlay graft was performed in 14 patients, ventral onlay in one patient, and dorsal onlay plus ventral onlay in five patients; 21 month follow-up data demonstrated an average success rate of 85%. The average stricture length was 3 cm. No complications were noted. The three failures were in penile and penile–bulbar urethral strictures with an average length of 5.7 cm.
Fiala et al. used SIS grafts for urethroplasties in 50 patients [71]. The success rate averaged 80%, with a mean follow-up time of 31 months. Recurring strictures developed in one of 10 bulbar, five of 31 bulbopenile, and four of nine penile strictures; these occurred during the first 6 months postoperatively. Farahat et al. placed SIS grafts endoscopically [72]. Only two cases of 10 exhibited stricture recurrence at a mean follow-up time of 14 months; there were no complications.
El-Kassaby et al. conducted a comparative study between BAMGs and buccal mucosal grafts in anterior urethral strictures [36]. The patients were followed for 25 months on average. The success rate for BAMG was 53%, and that of the buccal mucosal graft was 100%. The authors divided patients from the two groups into subgroups: (a) those with a healthy urethral bed (less than two prior surgeries), where BAMG surgeries were successful in 89% of cases and buccal mucosa surgeries in 100%; and (b) those with an unhealthy urethral bed (more than two prior surgeries), where the success rates were 33% (BAMG) and 100% (buccal mucosa).
Palminteri et al. reported the longest (as of today) mean follow-up period (71 months), having worked with patients who had bulbar urethral strictures and underwent urethroplasties using SIS [40]. The success rate was 76%. It should be noted that the failure rate was 14% for strictures <4 cm and 100% for strictures >4 cm.
In the studies mentioned above, the most frequently used type of matrix was SIS, which was, in most cases, used as a material for patch grafts. Tubularized grafts were applied twice: in endoscopic urethroplasties (with a success rate of 22%) [38] and in conventional urethroplasties (with a success rate of 88%); however, when describing the cases of conventional urethroplastic surgery, the authors did not mention the lengths of the urethral stricture [42]. No episodes of graft rejection were reported. Biopsies taken in five studies out of 13 showed normal urethral tissue characteristics at the site of surgery. Almost all studies using acellular matrices reported success rates of 75% or more. Failures were more common in patients with long urethral strictures (>4 cm), in those who had had previous urethroplasties (unhealthy urethral bed, unsatisfactory vascularity), and in those with penile or penile–bulbar strictures. The two studies that used endoscopic approaches exhibited different success rates: 22% vs 80% [38, 72]. The key differences among the groups were in the way the SIS grafts were secured: Le Roux et al. used tubularized grafts [38], while Farahat et al. chose patches [72]. Additionally, the average stricture length in the former study was 2.2 cm, as opposed to 1.47 cm in the latter study. Apparently, the difference in the success rates can be attributed to these two factors.
In addition, Donkov et al. and Hauser et al. both applied SIS in a similar technique (dorsal onlay substitution), but the success rates were completely different (89% vs 20%, respectively) [69, 70]. The difference can be explained by the difference in the way SIS was secured: in the study by Donkov et al. [69], the patch was spread-fixed onto the tunica albuginea, while the mucosa was sutured to the graft margins; in the study by Hauser et al. [70], SIS was anastomosed only to the incised urethra, without being fixed onto the tunica albuginea. Moreover, the strictures in the former study were shorter than those in the latter (4–6 cm vs 3.5–10 cm).
2.3.3 Cellularized Matrices Utilized in Animal Models (Monoculture)
De Filippo et al. were the first to use a tubularized tissue-engineered construct derived from bladder submucosa and seeded with autologous urothelial bladder cells for urethroplasty in rabbits [56]. The cells were obtained via an open biopsy. Urethroplasties were performed to repair 10 mm-long defects. In the control group, similar defects were repaired with unseeded tubularized BAMGs. After 6 months of follow-up, there were no strictures in the former group, whereas the control group exhibited strictures at the site of surgery.
A similar study was conducted by Li et al., although instead of urothelial bladder cells they used OKs [57]. The ventral onlay graft procedure was used to repair 20 mm-long defects. Rabbits in the experimental group (BAMG plus OKs) developed no strictures or complications; in the control group, two rabbits died of infection, two had fistulae, and the remaining eight developed strictures.
Fu et al. used tubularized BAMGs seeded with autologous preputial keratinocytes to repair 15 mm-long defects in three rabbits; there was no control group in the study [58]. At a 2 month follow-up, urethrography showed a wide urethral caliber in all three animals, and histological evaluation showed complete epithelialization at the site of surgery.
Of particular interest is the paper published by Gu et al. [73] They implanted silastic tubes into the peritoneal cavities of nine rabbits. After 2 weeks, the authors harvested the tubes covered by tissue, histological analysis of which revealed myofibroblasts embedded in collagen bundles covered by an outer layer of mesothelium. The tissue was everted and used as a tubularized graft to repair 15 mm-long urethral defects. At 1 month post operation, histological and immunohistochemical analyses showed normal urethral architecture.
In their study, Micol et al. used high-density collagen gel tubes as grafts that were seeded with autologous bladder smooth muscle cells [29]. It took the authors approximately 24 h to produce each tubularized graft. They did not place catheters postoperatively. Seven of sixteen rabbits developed fistulae. In both groups (with eight animals in each), equal numbers of smooth muscle cells were observed after 1 month.
Gu et al. isolated mesothelial cells via omentum biopsy and seeded them onto BAMGs [74]. Fifteen mm-long defects were substituted by tubularized matrices seeded with cells (in nine rabbits) and by those without cells (in nine rabbits from the control group). In the latter, all animals developed strictures, while no stricture formation was observed in the experimental group. At 6 months after implantation, the neo-urethras could not be histologically distinguished from the native urethras.
Xie et al. prepared electrospun silk fibroin matrices and seeded them with urothelial bladder cells that had been isolated and expanded [18]. Dorsal urethral defects 30 mm long were created in female dogs. At 6 months after implantation, the neo-urethras could not be histologically distinguished from the native urethras. It should be noted that the artificial defects involved only the mucosa, not the smooth muscle layer.
Li et al. seeded BAMGs with either epithelial differentiated or undifferentiated rabbit adipose-derived stem cells [75]. BAMGs were labelled with 5-bromo-2′-deoxyuridine. Thirty-six rabbits were divided into three groups (12 animals/group); 20 mm ventral urethral defects were created. In group A, defects were substituted only with BAMGs; in group B with BAMGs plus TGFβ1 siRNA-transfected fibroblasts (Und-rASCs); and in group C with BAMGs plus epithelial-differentiated rabbit adipose-derived stem cells (Epith-rASCs). Only one stricture was observed in group C, whereas almost all animals developed strictures in the first two groups. Complete epithelialization was achieved only in group C.
Wang et al. used a denuded human amniotic scaffold (dHAS); 5 × 10 mm defects were created on the ventral wall of the urethra and were repaired with either dHAS alone (group 1, n = 6) or dHAS + urethral urothelial cells (group 2, n = 6). In group 1, one animal developed an infection and one had a fistula; group 2 exhibited no complications or strictures (the efficacy rate was 100%). Mild inflammatory infiltration was observed in cell-seeded dHAS grafts, as revealed by less pronounced accumulation of CD4+ and CD8+ cells (or neutrophils or other immune cells). Histopathological analysis identified a mild immune response in the cell-seeded dHAS group (p < 0.05). Urethral defects in group 2 were completely resolved in 1 month. At 3 months after surgery, the formation of a smooth muscular layer and rich blood vessels was apparent [76].
In a very recently published Korean study by Chun and Kim et al., they evaluated the combined effect of acellular bladder submucosa matrix (BSM) and autologous urethral tissue for the treatment of long segment urethral stricture in a rabbit model [20]. To prepare the BSM, porcine bladder submucosa was processed, decellularized, configured into a sheet-like shape, and sterilized (Fig. 9.2). Twenty rabbits were randomly divided into groups: normal control, urethral stricture, urethroplasty using BSM only, or BSM/autologous urethral tissue (n = 5 per group). Brief urethroplasty methods are represented in Fig. 9.3. The width of the penile urethra was measured by postoperative urethrograms at 4, 8, and 12 weeks. The control urethrogram showed a wide urethral caliber. Both graft groups showed a similar width to those of the normal group, and the stricture group revealed stenosis. The mean urethral width of the control, stricture, BSM, and BSM/autologous urethral tissue groups at week 12 was 10.3 ± 0.80, 3.8 ± 1.35, 8.8 ± 0.84, and 9.1 ± 1.14 mm, respectively. Although the difference in the width of the BSM and BSM/tissue graft urethroplasty groups was not statistically significant, the BSM/tissue graft group diameter was approximately 0.567 mm wider (Fig. 9.4). The histopathology study revealed that the BSM/autologous urethral tissue graft had a normal urethral lumen area, compact muscular layers, complete epithelialization, and progressive infiltration by vessels in the regenerated urethra. In contrast, the BSM grafts revealed keratinized epithelium, abundant collagenized fibrous connective tissue, and were devoid of bundles of circular smooth muscle (Fig. 9.5). According to these results, the BSM/tissue graft had well-organized integration with the recipient tissues, and the cells within embedded autologous tissue and BSM can synchronistically act as biological activators for incorporation, promoting ingrowth of surrounding urethral tissue into the scaffold. This indicates that autologous urethral tissue at the time of implantation is effective for urethral reconstruction. A BSM graft embedded with autologous urethral tissue can be applied for clinical treatment of strictures requiring partially resected urethra replacements.
Preparation of bladder submucosa matrix (BSM). (a) Bladder extraction from pig. (b) Separation of submucosa. (c) Gross image of acellular BSM, characterized by a thin sheet structure, acellular composition, multidirectional tensile strength, and lack of chemical cross-links. (d) Lyophilized acellular BSM sheet
Process of nontransected ventral urethral stricture model generation and urethroplasty. (a) Urethral defect created by urethrectomy and suturing. (b) Excision of healthy urethral muscle and endothelial tissue from normal urethra. (c) Embedded urethral tissue on the BSM with fibrin glue (shown below the magnified figure). Prepared combined graft with BSM and autologous urethral tissue. (d) Completed onlay graft. Scale bar, 10 μm
Width of the penile urethra. (a) Retrograde urethrography (representative images taken at week 12) shows complete tubularization of both grafted urethras similar to the control group throughout the study period. The stricture group shows stenosis. (b) Urethral diameter measured at 4, 8, and 12 weeks post operation. The symbols on the top of the bars indicate significant differences at P < 0.05. P values were obtained with analysis of variance and Tukey’s test. BSM, urethrotomy, and onlay urethroplasty with an acellular BSM scaffold graft; BSM/tissue, urethrotomy and onlay urethroplasty with a graft composed of autologous urethral tissue and an acellular BSM scaffold
Histological, Masson’s trichrome, and immunohistochemical analysis of harvested urethra. (a–d) Low-magnification images of H&E-stained sections (40×); both graft groups show thick circular smooth muscle and a similar area of the urethral lumen as observed in the control group. The stricture group shows loose muscular layers and narrow lumen. (e–h) High-magnification images of H&E-stained sections (200×); the BSM/tissue grafts show compressed columnar epithelium, newly formed capillaries, and abundant circular bundles of smooth muscle, similar to controls. BSM grafts show fibrosis-like morphology and a simple smooth muscle layer as observed in the stricture group. (i–l) BSM/tissue grafts reveal scattered, variably sized circular bundles of smooth muscle. BSM grafts show few muscle fibers, extensive collagen deposition, and keratinized squamous cell epithelium. (m–p) BSM/tissue grafts express stratified columnar urothelium and stratified squamous cells. BSM grafts have a thin and irregular urothelial layer (400×)
Male rabbits were used in ten of the eleven studies reviewed above. Female dogs were used as animal models in one study. Despite the fact that preclinical and clinical studies demonstrated better success rates with SIS, six of the eleven reviewed studies utilized BAMGs. SIS grafts were not applied at all. In most studies, matrices were seeded with epithelial cells. Also, mesothelial cells, bladder smooth muscle cells, bone marrow mesenchymal stem cells, and rabbit adipose-derived stem cells were used. The authors did not mention any problems with expanding the cells or seeding them onto different matrices; 50% of the surgeries were performed with tubularized constructs; and 8 of 11 studies had control groups, where similar matrices without cells were used. All 11 studies showed significantly better results with cellularized matrices. Epithelial cells and smooth muscle cells at the site of surgery were observed at a mean follow-up time of 4 weeks.
2.3.4 Cellularized Matrices Utilized in Human Models (Monoculture)
Fossum et al. obtained urothelial cells via bladder washing [55]. The cells were cultured and seeded onto decellularized dermal matrices that were used to surgically treat six boys with scrotal or perineal hypospadias. The mean follow-up time was 87 months. Only one boy had to undergo an optical urethrotomy to improve voiding.
2.3.5 Recellularized Matrices Utilized in Animal Models (Co-Culture)
Feng et al. seeded two types of autologous cells (lingual keratinocytes and corporal smooth muscle cells) onto both sides of porcine acellular corpus spongiosum matrices (ACSMs) [59]. The best results were achieved in the group in which urethral defects were repaired with matrices containing both types of cells. Animals in the other two groups (ACSMs alone, and ACSMs plus OKs) developed strictures.
De Filippo et al. substituted 30 mm defects with either tubularized BAMGs alone, or BAMGs seeded with two types of cells (BAMGs plus BUCs plus BSMCs) [77]. The success rate in the group wherein cell-seeded matrices were utilized was 100%.
Mikami et al. formed tubularized tissue engineered urethras comprised of collagen mesh matrices and autologous cells of two types—keratinocytes and buccal mucosa smooth muscle cells [78]. These grafts were used to repair urethral defects in the experimental group (10 dogs) and were not applied to defects in the control group (10 dogs). The authors reported statistically significant differences in the number of complications between the two groups. The control dogs developed fistulae and strictures.
Orabi et al. used the same types of matrix in male dogs; 6 cm perineal urethra segments were removed and substituted with either tubularized matrices with two types of cells (BAMGs plus BUCs and BSMCs) or BAMGs alone (control group) [19]. In the control group, 100% of animals developed strictures and fistulae, whereas in the experimental group there were no strictures at the 12 month follow-up.
Li et al. hypothesized that TGFβ1 plays a very important role in the formation of fibrosis. Using OK- and TGFβ1 siRNA transfected fibroblast-seeded BAMGs, the authors were able to minimize the activity of TGFβ1 and avoid the formation of fibrosis [79]. In three groups of rabbits (nine animals/group) the urethral defects were repaired with: (a) BAMGs alone; (b) BAMGs plus OKs; and (c) BAMGs plus OKs plus TGF-β1-siRNA transfected fibroblasts. Rabbits in group (a) developed strictures, while those in groups (b) and (c) had none. However, in group (c) the formation of capillaries in the epithelial lower layer was observed at 6 months after implantation.
Xie et al. seeded autologous OKs and fibroblasts onto silk fibroin matrices; 5 cm urethral mucosal defects were repaired using matrices alone and matrices with two cell types (in five female dogs in each case) [80]. No strictures or complications developed in the group with cell-seeded matrices.
Tissue-engineered constructs with different cell types in a co-culture system are structurally more similar to the native urethra than other constructs. In three of the six studies reviewed above, BAMGs were utilized. The inner surface of the matrix was seeded with OKs (in four of six studies) or bladder urothelial cells (in two of six studies), while the outer surface of the matrix was seeded with smooth muscle cells or fibroblasts (in two of six studies). Each study included a control group in which matrices without cells were used. The authors did not mention any problems regarding culturing autologous cells or creating tissue-engineered constructs with two types of cells. The animal models were male rabbits (in three of six studies), male dogs (in two of six studies), and female dogs (in one study). Tubularized constructs were applied in 50% of studies. The authors used tissue-engineered constructs containing different cell types with 100% success in five of six studies and with 70% success in one study.
2.3.6 Recellularized Matrices Utilized in Human Models (Co-Culture)
Bhargava et al. were the first to use a tissue-engineered construct for urethral repair in clinical patients [54]. Sterilized donor de-epidermized dermis was utilized as a matrix, onto which buccal mucosa keratinocytes and fibroblasts were seeded. Five patients with urethral stricture secondary to lichen sclerosis participated in the study. Three patients underwent a two-stage procedure and two patients a one-stage procedure. Postoperatively, one patient required complete graft excision because of marked fibrosis, and one had partial graft excision due to hyperproliferation of tissue. Three patients required some form of instrumentation because of recurring strictures. At present, the mean follow-up time is 111.8 months [81]. All patients who underwent urethroplasties are able to void independently. All four patients with tissue-engineered buccal mucosa (TEBM) void spontaneously. Three of five patients used intermittent self-calibration.
Raya-Rivera et al. used a PGA matrix, of which the inner surface was seeded with expanded bladder epithelial cells and the outer surface with bladder smooth muscle cells [50]. All patients had post-traumatic obliteration of membranous urethra. They had scar tissues excised and tissue-engineered urethras implanted. The success rate was 100% at a 71 month follow-up. Biopsies showed that the grafts had developed a normal-appearing architecture by 3 months after implantation.
To sum up, the authors applied tissue-engineered constructs with two types of cells to manage very complicated cases of urethral stricture disease: lichen sclerosis in Bhargava et al. and post-traumatic membranous urethral obliteration in Raya-Rivera et al. [50, 54] In the former, additional interventions were needed, while in the latter, the use of tubularized synthetic matrices seeded with urothelial and smooth muscle cells obtained from bladder demonstrated 100% success at long-term follow-up. Histological evaluation showed that the engineered grafts had developed apparently normal architecture by 3 months after implantation. All adverse fibrotic reactions were observed within the first year after implantation. The results have been durable for three out of five patients for 9 years, although all of them required re-interventions [81].
3 Current Challenges and Future Perspectives
Urethral tissue engineering provides fertile ground for further research, with many problems remaining unresolved. One of the questions to be answered is, “What are the potential indications for substitution urethroplasty procedures using tissue-engineered constructs?” At present, clinicians are successfully utilizing oral (buccal) mucosa to repair complex urethral strictures and hypospadias. Its use has yielded good results, not only in patients with primary urethral strictures but also in those with recurring strictures and lichen sclerosis, with most authors reporting low donor-site morbidity (sometimes extremely low, especially when mucosa is harvested from the cheek or the ventral surface of the tongue). Moreover, the procedure of harvesting is simple, reproducible, and well tolerated by the majority of patients. The view that oral mucosa may not provide enough tissue for urethral repair seems to be groundless. Mucosa from both cheeks as well as from the ventral surface of the tongue may provide enough material to create a 16 cm graft.
While buccal mucosa possesses many advantages, its use is associated with a number of problems that have to be addressed. First, any graft harvesting procedure entails some degree of donor site morbidity, which, no matter how minimal, still has a negative impact on the patients. The larger the amount of tissue removed from the donor site, the higher the negative impact. Second, long strictures may require harvesting of long grafts from several donor sites. This increases surgical duration as well as intraoperative and postoperative complication rates [82]. Third, in cases of recurrent strictures where a secondary urethroplasty is needed, the lack of oral mucosa may drive the need to find other substitution tissues. A similar problem may arise during the first stage of a two-stage reconstruction that often requires additional epithelialization of the urethral bed. Thus, selecting and designing an ideal substitution material remains a great challenge. Tissue-engineering options may help address this challenge. The development of a perfect tissue-engineered construct (matrix + cells obtained using a non-invasive technique, possibly from urine) will eliminate the problems of donor site morbidity and limited amount of available substitution tissue [83, 84], even in patients with long strictures and in those who have had previous urethroplasties. It will also decrease operative time and the risks of intraoperative and postoperative complications.
Among the problems our analysis of the reviewed preclinical studies brings to light is the problem of developing animal models for urethral repair. Only one preclinical study reviewed above created a model of urethral stricture: its authors excised a segment of the urethra 1 month before urethroplasties were performed, thus simulating the conditions under which grafts normally take place [65]. In most of the reviewed preclinical trials, a urethral defect was created intraoperatively, to be immediately repaired with a matrix. However, the process of graft take in an injured urethra may substantially differ from that in a normal urethral bed. What is more, only four out of twenty-eight preclinical studies used large animals (dogs) [18, 19, 78, 80], and only two of them used male dogs [19, 78]. While we are aware that, anatomically, the female and male urethras are different, and that spongiofibrosis, being the major cause of strictures in males, never occurs in females, we still decided to include the studies conducted on female animals in our review. The reason for doing so was that the authors of the studies introduced a novel technique to prepare matrices by electrospinning, and suggested using silk fibroin matrices [18, 80]. As for developing animal models for urethral repair, Sievert et al. introduced three methods of stricture induction in large animals (minipigs): urethrotomy, ligation, and thermocoagulation [85]. Stricture severity was found to be higher in the model that used thermocoagulation. From these results, we may assume that it is best to conduct experiments on large animals and develop a model of urethral stricture to mimic the conditions of graft take in humans.
It should be noted that SIS grafts were the most frequently applied matrices in the preclinical and clinical studies reviewed above. They were used in five of eleven preclinical studies and in nine of thirteen clinical studies. Synthetic matrices were not used at all, with the exception of one study that utilized a hybrid matrix [CLLC plus collagen sponge tubes (CST)]. All the matrices took well and caused no immunogenic and/or proinflammatory reactions. All of them (except CLLC plus CST, and SFM) were homogeneous or heterogeneous acellular grafts that contained mostly collagen and were devoid of any native cellular components, thus having reduced immunogenicity and bioactivity. The success of acellular matrices is known to be contingent on the vascularization of the graft and regeneration of native mucosa at the implantation site. Moreover, an ideal matrix must be biodegradable, its breakdown products must be non-toxic, and it must be substituted by the host’s own intercellular matrix [86, 87]. Also, an ideal matrix should provide an environment in which adhesion, proliferation, migration, and differentiation of cells can occur to enable functional tissue formation [30, 31]. Therefore, when an acellular matrix is used for urethral reconstruction, the following processes should take place: (a) the matrix becomes covered with the native urothelium; (b) it completely degrades; and (c) it becomes substituted by the host’s extracellular matrix (ideally, with smooth muscle fibers being formed).
Another issue to be touched upon is the efficacy of acellular matrices in both preclinical and clinical studies. As was shown by Dorin et al. [64], urothelial regeneration has some limitations, which is why acellular matrices are less successful in patients with long urethral strictures and in those who have had previous repairs (unsatisfactory vascularity). Hence, it is not advisable to use acellular matrices to repair strictures longer than 2 cm. As for short strictures (<2 cm), which, in theory, can be reconstructed with acellular matrices, they are successfully managed using either primary anastomosis or a non-transecting anastomotic technique [7, 88]. In cases of longer strictures, where urethroplasty is indicated [6], the use of acellular matrices does not seem to be expedient. Patients with an unhealthy urethral bed (severe spongiofibrosis, recurring strictures, or previous urethroplasties) have higher risks of poor graft vascularization, graft contraction, and stricture recurrence, as was shown in several clinical trials [21, 39, 40, 70]. To sum up, the applications for acellular matrices in the treatment of urethral strictures are limited.
Problems with acellular matrices made researchers look for other options. This is why they turned to designing tissue-engineered constructs, containing not only a matrix but also autologous cells. Interestingly, the cells seeded onto the surface of a matrix (when cultured in vitro and immediately after implantation onto a patient) survive by molecular diffusion. Later, the seeded cells enter a hypoxic state, thus stimulating the endogenous release of angiogenic growth factors [89, 90]. If adequate angiogenesis is achieved, the cells expand and new tissue is formed. In the absence of adequate angiogenesis, the cells die.
It is noteworthy that in the experimental studies that compared the efficacy of acellular and cellularized tissue engineered constructs, failure rates when using acellular matrices were significantly higher than were those in the studies that utilized only acellular matrices. This may be explained either by some degree of bias or by differences in study designs—the lengths of the urethral defects in the studies presented in the animal models using acellular matrices were 5–20 mm, whereas the lengths of the urethral defects in the studies presented in the animal models using cellularized matrices varied from 10 to 30 mm.
While SIS has been successfully utilized as a material for grafts in earlier trials, it was not used in the preclinical and clinical studies reviewed here. Most preclinical studies applied BAMGs, while two clinical studies used MukoCell® matrices and ADMs. Furthermore, the authors seeded matrices with different types of autologous epithelial cells, smooth muscle cells, and stem cells. Preclinical studies showed better success with cellularized matrices than with acellular ones. Fossum et al. obtained urothelial cells via bladder washing [55]. Urethroplasties with this type of cell were rather successful (83%) at a mean follow-up time of 87 months. We believe bladder washing is a promising technique, as it is minimally invasive and simple. Recently, some authors have reported that stem cells can also be obtained from urine [83, 84, 91], which makes this approach even more attractive.
Since the native urethra is comprised of the inner epithelial lining and corpus spongiosum that contain endothelial and smooth muscle cells, the next step taken by some researchers was to create a tissue-engineered construct that would contain a matrix and two types of cell. As a rule, in such cases, the inner surface is seeded with epithelial cells, while the outer surface is seeded with smooth muscle cells. Preclinical studies mostly applied BAMGs, whereas two clinical studies used ADMs and PGA matrices (thus, in the reviewed studies, a synthetic matrix was used only once). The inner surface was seeded either with bladder epithelial cells or with buccal mucosa cells. The use of bladder urothelial cells seems to be preferable; however, the main drawback is that they are obtained via an open bladder biopsy. Therefore, in this respect, buccal mucosa cells appear to be a better choice, as the technique employed to obtain them is less invasive.
Another important question regards graft placement. Ventral graft placement is preferred in patients with urethral strictures located in the proximal bulbar urethra [14, 92, 93]. This is because, in the proximal bulbar urethra, the lumen is dorsally eccentric, and the ventral aspect of the surrounding tissues of the corpus spongiosum is rather thick. This thick portion of the bulbar urethra has sufficient blood supply to vascularize the graft. Moving distally, the urethral lumen becomes more centrally placed and the corpus spongiosum becomes thinner, which is why the use of ventral graft location may be associated with higher risks of inadequate vascularization and stricture recurrence. Thus, in patients with strictures located in the distal bulbar urethra, one should opt for dorsal (onlay or inlay) graft placement. This is why we believe dorsal graft placement should be preferred in clinical studies assessing the use of tissue-engineered constructs.
Overall, the evidence presented in the reviewed studies appears to support the view that tissue-engineered constructs with different cell types should be used as grafts of choice in substitution urethroplasty procedures. Tissue-engineered constructs should be utilized in the following groups of patients: in those with long strictures (> 2 cm); in those with multiple strictures; in those with subtotal and total strictures; in those with an unhealthy urethral bed (severe spongiofibrosis, recurring strictures after multiple urethrotomies and dilations, previous urethroplasties); and in those with soft tissue deficit at the site of surgery (hypospadias, failed hypospadia repair). The use of “off-the-shelf” tissue engineered constructs can minimize (or, in cases where non-invasive techniques to obtain autologous cells are employed, even eliminate) donor site morbidity, decrease operative time, and reduce the risks of intraoperative and postoperative complications. However, further investigations are needed to prove this.
Interestingly, despite the fact that there has been much international debate regarding naturally derived vs synthetic scaffolds, almost all the studies reviewed here utilized natural matrices. Only two studies applied a hybrid matrix and a synthetic one. Therefore, more attention should be paid to synthetic matrices and their possible applications in substitution urethroplasty procedures.
Urethral tissue engineering is, without any doubt, a promising field of regenerative medicine. Yet creating a tissue-engineered construct with different types of autologous cells is a complex biotechnological process, requiring clean room laboratories and highly specialized personnel. A lack of the mentioned facilities and specialists, as well as a lack of clinical studies, limit the wide application of tissue engineering to replace urethras. Urethral tissue engineering provides effective treatment options for patients with strictures and congenital anomalies. However, with the development of biotechnologies in this field, we will harness new research results that will enable us to tackle other problems as well, such as various lesions of the bladder and ureter, chordee, or erectile dysfunction.
Conclusions
The studies reviewed in this chapter have described different approaches to the development of matrices, harvesting and expanding autologous cells, and producing tissue-engineered constructs of various complexities (patch grafts and tubularized grafts). More clinical studies are needed to explore these and other problems on a larger scale.
References
Bhatnagar A, Upadhyaya VD, Kumar B. Congenital urethrocutaneous fistula: case report with review of literature. Indian J Plast Surg. 2012;45:563–5.
Elgammal MA. Straddle injuries to the bulbar urethra: management and outcome in 53 patients. Int Braz J Urol. 2009;35:450–8.
Kim BS, Kwon TG. Urethral reconstruction using autologous vein grafts for the management of urethral strictures. Curr Urol Rep. 2015;16:467.
Macedo A Jr, Rondon A, Ortiz V. Hypospadias. Curr Opin Urol. 2012;22:447–52.
Chapple C. Anterior urethral surgery: current concepts and future directions. Eur Urol. 2010;58:42–5.
Chapple C, Andrich D, Atala A, Barbagli G, Cavalcanti A, Kulkarni S, et al. SIU/ICUD consultation on urethral strictures: the management of anterior urethral stricture disease using substitution urethroplasty. Urology. 2014;83:S31–47.
Morey AF, Watkin N, Shenfeld O, Eltahawy E, Giudice C. SIU/ICUD consultation on urethral strictures: anterior urethra--primary anastomosis. Urology. 2014;83:S23–6.
Fenton AS, Morey AF, Aviles R, Garcia CR. Anterior urethral strictures: etiology and characteristics. Urology. 2005;65:1055–8.
Tritschler S, Roosen A, Fullhase C, Stief CG, Rubben H. Urethral stricture: etiology, investigation and treatments. Dtsch Arztebl Int. 2013;110:220–6.
Lumen N, Hoebeke P, Willemsen P, De Troyer B, Pieters R, Oosterlinck W. Etiology of urethral stricture disease in the 21st century. J Urol. 2009;182:983–7.
Chapple C, Barbagli G, Jordan G, Mundy AR, Rodrigues-Netto N, Pansadoro V, et al. Consensus statement on urethral trauma. BJU Int. 2004;93:1195–202.
Bhandari M, Dubey D, Verma BS. Dorsal or ventral placement of the preputial/penile skin onlay flap for anterior urethral strictures:does it make a difference? BJU Int. 2001;88:39–43.
Kozinn SI, Harty NJ, Zinman L, Buckley JC. Management of complex anterior urethral strictures with multistage buccal mucosa graft reconstruction. Urology. 2013;82:718–22.
Barbagli G, Lazzeri M. Penile urethral stricture reconstruction--flap or graft? Graft. J Urol. 2011;186:375–6.
Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterans. Br J Urol. 1998;81:735–7.
Xu YM, Xu QK, Fu Q, Sa YL, Zhang J, Song LJ, et al. Oral complications after lingual mucosal graft harvesting for urethroplasty in 110 cases. BJU Int. 2011;108:140–5.
Cattan V, Bernard G, Rousseau A, Bouhout S, Chabaud S, Auger FA, et al. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur Urol. 2011;60:1291–8.
Xie M, Song L, Wang J, Fan S, Zhang Y, Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J Surg Res. 2013;184:774–81.
Orabi H, AbouShwareb T, Zhang Y, Yoo JJ, Atala A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol. 2013;63:531–8.
Chun SY, Kim BS, Kwon SY, Park SI, Song PH, Yoo ES, et al. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J Korean Med Sci. 2015;30:301–7.
Ahn HH, Kim KS, Lee JH, Lee MS, Song IB, Cho MH, et al. Porcine small intestinal submucosa sheets as a scaffold for human bone marrow stem cells. Int J Biol Macromol. 2007;41:590–6.
Narayanan K, Leck KJ, Gao S, Wan AC. Three-dimensional reconstituted extracellular matrix scaffolds for tissue engineering. Biomaterials. 2009;30:4309–17.
Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32:7571–80.
Bazeed MA, Thuroff JW, Schmidt RA, Tanagho EA. New treatment for urethral strictures. Urology. 1983;21:53–7.
Olsen L, Bowald S, Busch C, Carlsten J, Eriksson I. Urethral reconstruction with a new synthetic absorbable device. An experimental study. Scand J Urol Nephrol. 1992;26:323–6.
Atala A, Vacanti JP, Peters CA, Mandell J, Retik AB, Freeman MR. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J Urol. 1992;148:658–62.
Chen F, Yoo JJ, Atala A. Acellular collagen matrix as a possible "off the shelf" biomaterial for urethral repair. Urology. 1999;54:407–10.
Sievert KD, Tanagho EA. Organ-specific acellular matrix for reconstruction of the urinary tract. World J Urol. 2000;18:19–25.
Micol LA, Arenas da Silva LF, Geutjes PJ, Oosterwijk E, Hubbell JA, Feitz WF, et al. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials. 2012;33:7447–55.
Atala A. Regenerative medicine strategies. J Pediatr Surg. 2012;47:17–28.
Atala A. Tissue engineering of reproductive tissues and organs. Fertil Steril. 2012;98:21–9.
Micol LA, Ananta M, Engelhardt EM, Mudera VC, Brown RA, Hubbell JA, et al. High-density collagen gel tubes as a matrix for primary human bladder smooth muscle cells. Biomaterials. 2011;32:1543–8.
Ribeiro-Filho LA, Sievert KD. Acellular matrix in urethral reconstruction. Adv Drug Deliv Rev. 2015;82-83:38–46.
Atala A, Guzman L, Retik AB. A novel inert collagen matrix for hypospadias repair. J Urol. 1999;162:1148–51.
El-Kassaby AW, Retik AB, Yoo JJ, Atala A. Urethral stricture repair with an off-the-shelf collagen matrix. J Urol. 2003;169:170–3. discussion 3
el-Kassaby A, AbouShwareb T, Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 2008;179:1432–6.
Feng C, Xu YM, Fu Q, Zhu WD, Cui L, Chen J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J Biomed Mater Res A. 2010;94:317–25.
le Roux PJ. Endoscopic urethroplasty with unseeded small intestinal submucosa collagen matrix grafts: a pilot study. J Urol. 2005;173:140–3.
Palminteri E, Berdondini E, Colombo F, Austoni E. Small intestinal submucosa (SIS) graft urethroplasty: short-term results. Eur Urol. 2007;51:1695–701. discussion 701
Palminteri E, Berdondini E, Fusco F, De Nunzio C, Salonia A. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology. 2012;79:695–701.
Sievert KD, Bakircioglu ME, Nunes L, Tu R, Dahiya R, Tanagho EA. Homologous acellular matrix graft for urethral reconstruction in the rabbit: histological and functional evaluation. J Urol. 2000;163:1958–65.
Lin J, Hao JR, Jin J, Deng SM, Hu J, Na YQ. Homologous dermal acellular matrix graft for urethral reconstruction in man (report of 16 cases). Zhonghua Yi Xue Za Zhi. 2005;85:1057–9.
Fossum M, Svensson J, Kratz G, Nordenskjold A. Autologous in vitro cultured urothelium in hypospadias repair. J Pediatr Urol. 2007;3:10–8.
Williams DF. Biomaterials and biocompatibility. Med Prog Technol. 1976;4:31–42.
Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y). 1994;12:689–93.
Mikos AG, Lyman MD, Freed LE, Langer R. Wetting of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams for tissue culture. Biomaterials. 1994;15:55–8.
Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402.
Han D, Gouma PI. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomedicine. 2006;2:37–41.
Lee SJ, Oh SH, Liu J, Soker S, Atala A, Yoo JJ. The use of thermal treatments to enhance the mechanical properties of electrospun poly(epsilon-caprolactone) scaffolds. Biomaterials. 2008;29:1422–30.
Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–82.
Kanatani I, Kanematsu A, Inatsugu Y, Imamura M, Negoro H, Ito N, et al. Fabrication of an optimal urethral graft using collagen-sponge tubes reinforced with Copoly(L-lactide/epsilon-caprolactone) fabric. Tissue Eng. 2007;13:2933–40.
Fu WJ, Xu YD, Wang ZX, Li G, Shi JG, Cui FZ, et al. New ureteral scaffold constructed with composite poly(L-lactic acid)-collagen and urothelial cells by new centrifugal seeding system. J Biomed Mater Res A. 2012;100:1725–33.
Horst M, Madduri S, Milleret V, Sulser T, Gobet R, Eberli D. A bilayered hybrid microfibrous PLGA--acellular matrix scaffold for hollow organ tissue engineering. Biomaterials. 2013;34:1537–45.
Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol. 2008;53:1263–9.
Fossum M, Skikuniene J, Orrego A, Nordenskjold A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr. 2012;101:755–60.
De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002;168:1789–92. discussion 92-3
Li C, Xu YM, Song LJ, Fu Q, Cui L, Yin S. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J Urol. 2008;180:1538–42.
Fu Q, Deng CL, Song XF, Xu YM. Long-term study of male rabbit urethral mucosa reconstruction using epidermal cell. Asian J Androl. 2008;10:719–22.
Feng C, Xu YM, Fu Q, Zhu WD, Cui L. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng Part A. 2011;17:3011–9.
Kropp BP, Ludlow JK, Spicer D, Rippy MK, Badylak SF, Adams MC, et al. Rabbit urethral regeneration using small intestinal submucosa onlay grafts. Urology. 1998;52:138–42.
Nuininga JE, van Moerkerk H, Hanssen A, Hulsbergen CA, Oosterwijk-Wakka J, Oosterwijk E, et al. Rabbit urethra replacement with a defined biomatrix or small intestinal submucosa. Eur Urol. 2003;44:266–71.
Yang SX, Yao Y, Hu YF, Song C, Wang LL, Jin HM. Reconstruction of rabbit urethra using urethral extracellular matrix. Chin Med J. 2004;117:1786–90.
Huang X, Luo J, Liao Y, Qu Y, Yang Z. Study on small intestinal submucosa as repair materials in urethral reconstruction. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:206–9.
Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol. 2008;26:323–6.
Villoldo GM, Loresi M, Giudice C, Damia O, Moldes JM, DeBadiola F, et al. Histologic changes after urethroplasty using small intestinal submucosa unseeded with cells in rabbits with injured urethra. Urology. 2013;81:1380 e1-5.
Chung YG, Tu D, Franck D, Gil ES, Algarrahi K, Adam RM, et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One. 2014;9:e91592.
Kajbafzadeh AM, Sabetkish S, Tourchi A, Amirizadeh N, Afshar K, Abolghasemi H, et al. The application of tissue-engineered preputial matrix and fibrin sealant for urethral reconstruction in rabbit model. Int Urol Nephrol. 2014;46:1573–80.
Mantovani F, Trinchieri A, Castelnuovo C, Romano AL, Pisani E. Reconstructive urethroplasty using porcine acellular matrix. Eur Urol. 2003;44:600–2.
Donkov II, Bashir A, Elenkov CH, Panchev PK. Dorsal onlay augmentation urethroplasty with small intestinal submucosa: modified Barbagli technique for strictures of the bulbar urethra. Int J Urol. 2006;13:1415–7.
Hauser S, Bastian PJ, Fechner G, Muller SC. Small intestine submucosa in urethral stricture repair in a consecutive series. Urology. 2006;68:263–6.
Fiala R, Vidlar A, Vrtal R, Belej K, Student V. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur Urol. 2007;51:1702–8. discussion 8
Farahat YA, Elbahnasy AM, El-Gamal OM, Ramadan AR, El-Abd SA, Taha MR. Endoscopic urethroplasty using small intestinal submucosal patch in cases of recurrent urethral stricture: a preliminary study. J Endourol. 2009;23:2001–5.
Gu GL, Zhu YJ, Xia SJ, Zhang J, Jiang JT, Hong Y, et al. Peritoneal cavity as bioreactor to grow autologous tubular urethral grafts in a rabbit model. World J Urol. 2010;28:227–32.
Gu GL, Xia SJ, Zhang J, Liu GH, Yan L, Xu ZH, et al. Tubularized urethral replacement using tissue-engineered peritoneum-like tissue in a rabbit model. Urol Int. 2012;89:358–64.
Li H, Xu Y, Xie H, Li C, Song L, Feng C, et al. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng Part A. 2014;20:774–84.
Wang F, Liu T, Yang L, Zhang G, Liu H, Yi X, et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit. 2014;20:2430–8.
De Filippo RE, Kornitzer BS, Yoo JJ, Atala A. Penile urethra replacement with autologous cell-seeded tubularized collagen matrices. J Tissue Eng Regen Med. 2015;9:257–64.
Mikami H, Kuwahara G, Nakamura N, Yamato M, Tanaka M, Kodama S. Two-layer tissue engineered urethra using oral epithelial and muscle derived cells. J Urol. 2012;187:1882–9.
Li C, Xu YM, Liu ZS, Li HB. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology. 2013;81:1075–80.
Xie M, Xu Y, Song L, Wang J, Lv X, Zhang Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J Surg Res. 2014;188:1–7.
Osman NI, Patterson JM, MacNeil S, Chapple CR. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur Urol. 2014;66:790–1.
Kim BD, Ver Halen JP, Grant DW, Kim JY. Anesthesia duration as an independent risk factor for postoperative complications in free flap surgery: a review of 1,305 surgical cases. J Reconstr Microsurg. 2014;30:217–26.
Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–33.
Bharadwaj S, Liu G, Shi Y, Markert C, Andersson KE, Atala A, et al. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng Part A. 2011;17:2123–32.
Sievert KD, Selent-Stier C, Wiedemann J, Greiner TO, Amend B, Stenzl A, et al. Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol. 2012;187:1101–9.
Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224–30.
Silver FH, Pins G. Cell growth on collagen: a review of tissue engineering using scaffolds containing extracellular matrix. J Long-Term Eff Med Implants. 1992;2:67–80.
Andrich DE, Mundy AR. Non-transecting anastomotic bulbar urethroplasty: a preliminary report. BJU Int. 2012;109:1090–4.
Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–75.
Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–41.
Wu S, Wang Z, Bharadwaj S, Hodges SJ, Atala A, Zhang Y. Implantation of autologous urine derived stem cells expressing vascular endothelial growth factor for potential use in genitourinary reconstruction. J Urol. 2011;186:640–7.
Kulkarni S, Barbagli G, Sansalone S, Lazzeri M. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int. 2009;104:1150–5.
Barbagli G, Sansalone S, Djinovic R, Romano G, Lazzeri M. Current controversies in reconstructive surgery of the anterior urethra: a clinical overview. Int Braz J Urol. 2012;38:307–16. discussion 16
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ha, YS., Kim, TH. (2018). Urethra. In: Kim, B. (eds) Clinical Regenerative Medicine in Urology. Springer, Singapore. https://doi.org/10.1007/978-981-10-2723-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-2723-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2722-2
Online ISBN: 978-981-10-2723-9
eBook Packages: MedicineMedicine (R0)