Abstract
Purpose
To evaluate the efficacy of indocyanine-green angiography (ICGA)-guided navigated focal laser photocoagulation for diabetic macular edema (DME).
Study design
Prospective, interventional case series.
Methods
Six patients (8 eyes) were enrolled in this study. Fluorescein angiography (FA) and ICGA were performed using the Heidelberg Retina Angiogram 2 (Heidelberg Engineering). Navigated focal laser photocoagulation was delivered to the microaneurysms on ICGA using Navilas® (OD-OS GmbH, Germany). Central retinal thickness (CRT) and macular volume (MV) were measured by Cirrus HD-OCT (Carl Zeiss Meditec). At 6 months, the best-corrected visual acuity (BCVA), CRT and MV were compared to the values measured on day 0. The distances from the center of fovea to the closest microaneurysms (MAs) were measured on the pre-planned Navilas® image.
Results
All eyes had previous treatment history. At 6 months, ICGA-guided navigated focal laser photocoagulation significantly reduced the CRT and the MV (p<0.05), and there was improvement in the BCVA (p<0.05). At 3 months, 5 out of the 8 eyes (63%) underwent additional ICGA-guided navigated focal laser photocoagulation due to remnants of MAs that had been confirmed by ICGA. There was no observed recurrence of edema after the ICGA-guided navigated focal laser photocoagulation during the 6-month follow-up. The mean distance from the center of fovea to the closest MAs was 624.8 ± 377.7 μm (range 336.0–1438.9 μm).
Conclusion
Our data suggest ICGA-guided navigated focal laser photocoagulation may be effective for the treatment of DME.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) is a leading cause of blindness in most industrialized nations [1, 2]. Although the pathogenesis of DME remains unclear, it is thought to be related to the breakdown of the blood-retina barrier, leaking microaneurysms (MAs), and upregulation of cytokines, as well as other factors [3, 4]. Among the several classifications of DME, the terms focal and diffuse DME are frequently used without clear definition [5]. The Early Treatment Diabetic Retinopathy Study (ETDRS), used fluorescein angiograms to classify eyes with majority leakage associated with MAs as focal, and eyes with less leakage associated with MAs as diffuse [6, 7]. In the ETDRS, focal laser photocoagulation was applied for leakage from the MAs, and grid laser photocoagulation was applied to areas of diffuse leakage or thickened retinae [6, 7]; these techniques had become golden standard for treating DME.
After the validation of the enhanced expression of the pro-angiogenic cytokine vascular endothelial growth factor (VEGF) in DME patients [8, 9], anti-VEGF therapy has become the first-line treatment [10,11,12]. Recent clinical studies that compared anti-VEGF therapy and focal/grid laser treatment demonstrate the superiority of the anti-VEGF monotherapy in the treatment of DME [10, 11], yet the efficacy of additional focal/grid laser photocoagulation appeared to be lower than expected. However, since anti-VEGF treatment requires repeated intravitreous injections over an indefinite period, there have been safety concerns regarding the treatment of systemic diseases such as cardiovascular diseases [13]. Moreover, it is also reported that macular edema persists in some patients, despite receiving multiple intravitreous injections of anti-VEGF drugs [14, 15].
Recently, precise focal photocoagulation using navigated laser photocoagulation (Navilas®, OD-OS GmbH) combined with anti-VEGF antibody therapy has been shown to have promising efficacy while reducing the burden of the anti-VEGF agent [16]. This suggests that precise laser photocoagulation of the MAs, which are responsible for the DME, could resolve the edema while reducing the reliance on anti-VEGF agents.
Previously, we reported that indocyanine green angiography (ICGA) was more sensitive in detecting responsible MAs for DME versus FA, and that ICGA-guided laser photocoagulation appeared to be effective in treating refractory DME [17]. In diffuse DME that was originally diagnosed using FA, ICGA imaging often proved to be useful in detecting MAs as well as the focal spots of leakage [17]. However, due to the lack of information on the location of the foveal avascular zone or small retinal vessels during ICGA, it can sometimes be difficult to identify the exact location of ICGA-positive MAs.
Based on these studies, we hypothesized that the use of a combination of ICGA-guided focal laser photocoagulation and navigated laser photocoagulator (Navilas®) might be more useful in the treatment of DME. Therefore, the aim of this study was to evaluate the efficacy of ICGA-guided navigated focal laser photocoagulation in the treatment of refractory DME. here, the definition of focal laser photocoagulation is the same as in ETDRS, i.e. direct laser photocoagulation on MAs.
Methods
Patients and study design
This study was a 6-month, prospective, interventional case series. After each patient was informed about the risks and benefits of laser photocoagulation, written, informed consent was obtained. This study was approved by the Institutional Review Board (IRB) of the Nagoya City University Graduate School of Medical Science, conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki, and registered at UMIN (identification number; UMIN 000012470).
Between October 3, 2013 and March 20, 2014, 6 patients with DME in a total of 8 eyes were enrolled in this study and underwent ICGA-guided navigated focal laser photocoagulation. The inclusion criteria were: (1) clinical presentation with diffuse DME with obvious leaking MA; (2) older than 20 years of age; and (3) eligible for FA/ICGA. The major exclusion criteria included: (1) previous ocular surgery or sub-Tenon’s injection of triamcinolone acetonide within 3 months; (2) retinal photocoagulation (including focal/grid laser photocoagulation) within 6 months; (3) presence of media opacity; (4) renal dysfunction; or (5) presence of epiretinal membrane or vitreomacular traction syndrome. We did not exclude glaucoma if the intraocular pressure (IOP) was well controlled by eye drops.
All patients underwent comprehensive ophthalmological examinations, including measurement of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy and indirect ophthalmoscopy. BCVA was measured using a Japanese standard decimal visual acuity chart, with the decimal BCVA calculated using the logarithm of the minimum angle of resolution (LogMAR) scale. FA and ICGA were performed using the Heidelberg Retina Angiogram 2 (Heidelberg Engineering) before and at 3 months after treatment.
Cirrus HD-OCT (Carl Zeiss Meditec) was used to evaluate the central retinal thickness (CRT) and the macular volume (MV) before, and at 3 and 6 months after laser photocoagulation. In this study, DME type was classified as focal or diffuse based on the features stated below. The characteristics of focal macular edema are: (1) location outside the foveal center with or without center involvement; (2) asymmetric increases in retinal thickness on OCT -scan; and (3) accumulation of pin-point leakage in early phase of FA. The characteristics of diffuse macular edema are: (1) increased retinal thickness with center involvement on the OCT macular thickness map; (2) symmetrically increased retinal thickness on B-scan OCT; (3) fluorescein leakage starting from early phase and continuously increasing to late phase [4].
Prior to the laser photocoagulation, DME was classified into the following three types: serous retinal detachment (SRD), with detachment of the sensory retina from the retinal pigment epithelium but no cystoid spaces at the presumed fovea; cystoid macular edema (CME), which presented predominantly as cystoid spaces within the area; and sponge-like retinal swelling with no SRD or CME [18].
Evaluating the density of MAs in FA and ICGA
In each eye, we overlaid the retinal thickness topographic map (6 x 6 mm) obtained by the Cirrus HD-OCT (Fig. 1a) onto the images of early-phase FA (Fig. 1b) and late-phase ICGA (Fig. 1e) obtained by HRA2 using Adobe Photoshop (cs2; Adobe Systems Incorporated) (Fig. 1f, g). Due to color contrast of the topographic map, it was hard to identify the areas where the retinal thickness was more than 300 µm or 350 µm. Therefore, we defined the area where the retinal thickness was more than 400 µm (orange-red to white in the Cirrus HD-OCT color chart) as the area of the edema. The measured area of the retinal edema (mm2) and the number of MAs were determined by using the Image J software (developed by Wayne Rasband, National Institutes of Health; available at http://rsb.info.nih.gov/ij/index.html) as previously described [17, 19]. Moreover, we also separated the density of MAs inside of the retinal edema from the density of MAs outside of the retinal edema.
OCT and FA/ICGA for calculating density of MAs (Case 4). Cirrus HD-OCT image (6 × 6 mm) (a), early-phase of FA (b) and ICGA (c), and late-phase of FA (d) and ICGA (e). Retinal topographic map of the HD-OCT was overlaid onto the early-phase of FA (f) and the late-phase of ICGA (g). Areas of the retina where the thickness was more than 400 μm are depicted as orange-red to white in the retinal topographic map (encircled by the yellow line)
ICGA-guided navigated focal laser photocoagulation
ICGA-guided navigated focal laser photocoagulation was performed with the Navilas® Laser System. Its principal operation is described elsewhere [20]. In brief, it combines imaging, laser application planning, and treatment using a computer-based device.
The navigated laser photocoagulation performed in this study was digitally planned based on late-phase ICGA images acquired by HRA2 at an angle of 30 degrees and the placement of single spots on the focal spots of leakage. The location of hyperfluorescent spots detected by late-phase ICGA was compared with OCT thickness map, and hyperfluorescent spots inside the edema were picked out for focal laser photocoagulation.
Laser photocoagulation parameters were as follows: spot size 50–100 μm; pulse duration 20–100 msec; and laser power 50–120 mW at a wavelength of 532 nm. To avoid atrophic creep, we used a short duration (20 msec) with small spot size (50 μm) setting, especially inside the parafoveal area (<1500 μm). Out of the parafoveal area (>1500 μm), we sometimes used a spot size (100 μm) with 100 msec duration for complete closure of MAs. Color snap images taken just after each laser shot were used to individually manipulate the laser power to values around 80 mW in order to achieve a pale grayish laser burn (Fig. 2). All laser photocoagulation operations were performed by the same surgeon (M.N.).
The images of Case 4 for ICGA-guided NAVILAS laser photocoagulation. The same eye as shown in Fig. 1. The late-phase ICGA images (a, b) and laser spots were planned (yellow circle) onto the image taken by Navilas® (c, d) before treatment. The fundus image taken just after laser photocoagulation showed pale grayish laser burns on each planned spots (e, f). Yellow dashed square (a, c, e) indicate the cropped and magnified area shown as below (b, d, f). Green arrows show the laser planned (b) and coagulated microaneurysms (f)
If the edema persisted and ICGA showed remnants or newly developed focal hyperfluorescence points at 3 months after the initial laser treatment, an additional laser treatment was administered. Distances from the center of fovea to the closest MAs were measured on the pre-planned Navilas® images, with the calculations based on the central retinal vein diameter (125 μm).
Endpoints
The primary endpoint was defined as the change in CRT at 6 months after laser photocoagulation. Secondary endpoints were defined as changes in the BCVA and MV at 6 months after the laser treatment, and complications of the laser treatment.
Statistical analysis
The results are expressed as the mean ± standard deviation. A paired t-test was used to compare the baseline BCVA, CRT, and MV values before and at 6 months after treatment. The density of MAs (/mm2) on FA and ICGA was compared by use of ANOVA with Tukey test. P values less than 0.05 were considered significant for all analyses. All statistical analyses were performed using the StatMate IV Statistical Analysis System for Microsoft Excel Version 4.01 (Atoms).
Results
Patient characteristics
Table 1 presents the characteristics of the 6 patients enrolled in this study. The mean age of the patients was 66.8 ± 4.8 years (range 58–72 years).
The structural types’ patterns included cystoid macular edema (CME) in 6 eyes and sponge-like retinal swelling in 2 eyes. There was no SRD type in this study. Prior to the start of the study, previous DME treatment had included vitrectomy (2 eyes), sub-Tenon injection of triamcinolone acetonide (TA) (Kenacort; Bristol-Myers Squibb,) (4 eyes), intravitreous injection of TA (MaQaid; Wakamoto Pharmaceutical Co., Ltd.) (3 eyes), ICGA-guided focal laser photocoagulation (1 eye) and multiple injections of anti-VEGF therapy (ranibizumab) 4 years prior to the study (1 eye) (Table 2). In 1 patient who had previously received an intravitreous injection of TA, steroid glaucoma developed and her IOP could not be controlled with topical eye drops alone. She, therefore, underwent selective laser trabeculoplasty 1 year prior to this study. At enrolling to this study her IOP was well under control. As seen in Table 2, 3 eyes were phakic and 5 eyes were pseudophakic.
FA/ICGA and OCT findings
Findings in all eyes included, (1) increased retinal thickness with center involvement on the OCT macular thickness map; (2) symmetrically increased retinal thickness on B-scan OCT; (3) fluorescein leakage starting from early phase and continuously increasing to late phase in FA were noted and classified as diffuse macular edema. However, ICGA revealed hyperfluorescence spots detected in both the early- and late-phase. The hyperfluorescence spots revealed by late-phase ICGA were located in the thickened retina that was detected by the macular cube OCT map in all eyes (Fig. 1f, g).
The density of MAs in FA and ICGA
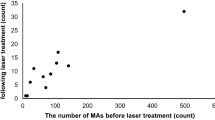
The density of the MAs (/mm2) inside the edema in early-phase FA was 4.54 ± 0.25 and outside the edema it was 1.96 ± 0.71. The density of the MAs (/mm2) inside the edema in late-phase ICGA was 1.26 ± 0.63 and outside it was 0.22 ± 0.09 (Fig. 5). Late-phase ICGA showed significantly smaller density of MAs than early-phase FA (p<0.01 for inside edema, p<0.05 for outside edema) (Fig. 3).
Laser Settings
The mean number of spots was 22 (range 5–81), with a spot size of 50–100 μm, duration time of 20–100 msec, and a power of 50–120 mW. The mean distance from the center of fovea to the closest MAs was 624.8 ± 377.7 μm (range 336.0–1438.9 μm).
CRT, BCVA and MV
The mean CRT was 524.13 ± 154.83 μm at baseline, and 381.50 ± 125.71 μm at 6 months after laser treatment. There was a significant difference between the two values (p<0.05) (Fig. 4).
The mean LogMAR BCVA, which was 0.49 ± 0.33 before laser treatment, improved significantly at 6 months after treatment to 0.39 ± 0.33 (p<0.05) (Fig. 5). There was also a significant reduction in the MV from 12.9 ± 1.9 μm3 to 11.6 ± 1.2 μm3 (p<0.05) (Fig. 6).
At month 3, 5 out of the 8 eyes (63%) underwent additional ICGA-guided navigated focal laser photocoagulation due to confirmation of remnants or newly developed MAs by ICGA (Table 2). Overall, mean closure rate of MAs was 60 %. In 3 of the 5 eyes (60%) that required additional ICGA-guided navigated focal laser photocoagulation at month 3, a plano contact lens (Navilas® Zero Power Lens, Ocular Instruments Inc.) for focal laser was applied due to poor fixation and heavy blinking. During the 6 months after the ICGA-guided navigated focal laser photocoagulation, no recurrence of edema was observed. In addition, there were no complications during laser treatments in any of the patients.
Representative case (Case 1)
Two years prior to being enrolled in the study, a 56-year-old woman underwent vitrectomy combined with phacoemulsification and intraocular lens implantation due to DME. The fellow eye, which had received a previous intravitreous injection of TA, developed steroid-induced glaucoma. Her IOP could not be controlled by topical medication. As a result, she was treated by selective laser trabeculoplasty in order to lower the IOP.
After vitrectomy, macular edema did not resolve and ICGA-guided conventional focal laser photocoagulation and sub-Tenon’s injection of TA was performed OD. However, since the DME was refractory to these treatments (Fig. 7a), FA and ICGA were then performed (Figs. 7c, d, f, g). From the findings of FA and OCT, the patient was diagnosed as having diffuse edema. However, the mid- to late-phase ICGA images showed the responsible MAs at locations compatible with the thickest part of the retina (Figs. 7a, g), as confirmed by the ICGA-guided navigated focal laser. After obtaining informed consent, ICGA-guided navigated focal laser photocoagulation was performed (Fig. 8). As the ICGA evaluation at month 3 did not find any hyperfluorescence spots at the macula (Fig. 7e, h), it was not necessary to perform any additional laser photocoagulation. OCT showed that there was a decrease in the ME, and the decimal BCVA increased from 0.2 to 0.3 at month 6 without additional treatments (Fig. 7b). No recurrence has been observed for 3.5 years.
Representative case (Case 1). Two years prior to being enrolled in the study, a 56-year-old woman underwent vitrectomy combined with phacoemulsification and intraocular lens implantation due to DME, but macular edema persisted. Although she was treated by sub-Tenon’s capsule injection of triamcinolone acetonide and indocyanine-green angiography (ICGA)-guided focal laser, ME did not resolve and her visual acuity was 0.2 (a). FA/ICGA was carried out, and based on the late-phase ICGA image, we performed navigated focal laser photocoagulation. Although early-phase FA showed the presence of MAs inside the arcade vessels (c), late-phase FA was not able to determine the focal spots of leakage (d). In contrast, late-phase ICGA was able to identify hyperfluorescence spots at the macula (g). As the patient was previously treated by ICGA-guided focal laser, the potential laser scars noted in the hypofluorescence area are likely related to this previous procedure (g). The late-phase ICGA taken 3 months after the initial navigated laser photocoagulation exhibited no remnants of the hyperfluorescence spots at the macula (h). The patient’s vision improved to 0.3 at 6 months, with the CRT and MV also improving (b)
The images of Case 1 for ICGA-guided NAVILAS laser photocoagulation. The same eye as shown in Fig. 7. The late-phase ICGA image was overlaid onto the color fundus image taken by Navilas® before laser (a). Yellow circle (a) indicates the laser planned microaneurysms which are also seen in the planned image with green small circles (b). The fundus photo was take right after laser session (c), and the coagulated microaneurysms were shown with yellow arrows (d). (The planned image was taken one week before laser)
Discussion
This study demonstrates that ICGA-guided navigated focal laser photocoagulation is effective for the treatment of DME. All eyes in this study had been previously treated by other modalities including anti-VEGF, steroids, focal laser photocoagulation or vitrectomy, and subsequently developed chronic DME. As this study was performed between 2013 and early 2014, there were no approved anti-VEGF agents that could be used for the treatment of DME in Japan. Furthermore, the IRB at the Nagoya City University Graduate School of Medical Science did not allow the use of bevacizumab for treatment of ocular diseases during that time period. Based on this background, the only common available treatment modalities for DME in our hospital were lasers and steroids. However, even during the anti-VEGF era where a randomized control study showed the superiority of anti-VEGF treatment to laser or steroids [21], data indicate that patients during this time received fewer injections than patients in major clinical trials, with 57.7% of the patients receiving additional laser or intravitreous injections of TA [22]. Furthermore, the cost of the anti-VEGF agents is a major burden in DME treatments, and thus, has become a worldwide socioeconomic issue [23]. Therefore, it is important for physicians to find a better way to treat DME with either less injections of anti-VEGF drugs or without using these at all.
Recently, Liegl et al. reported that a precise focal laser with a navigation system significantly reduced the burden of anti-VEGF in the treatment of DME [16]. In addition, we reported the efficacy of navigated focal laser photocoagulation (yellow wavelength) in treatment of refractory DME, and found the possibility to reduce the number of anti-VEGF injections [24]. These reports all support the use of focal lasers to treat DME even in the era of anti-VEGF treatments. Nevertheless, one of the concerns of using focal laser treatment is atrophic creep of the retinal pigment epithelium, which can lead to poor visual prognosis due to central scotoma [25]. To avoid atrophic creep in our procedures, we used a short duration (20 msec) setting for the focal laser, especially when inside the parafoveal area (<1500 μm). The use of short durations with a high power setting is reported to result in less lateral expansion of scars compared with the conventional (100 msec) setting in panretinal photocoagulation [26]. Although the follow-up period in our present study was relatively short (6 months), 7 out of 8 eyes that are being periodically followed have shown no deterioration in the visual acuity at more than 3 years after the focal laser treatment. This suggests that a short pulse setting may help to avoid atrophic creep even after focal laser treatments.
Hirano et al. report that eyes with perifoveal leaking MAs required a larger number of anti-VEGF injections, even when combined with focal/grid laser treatment, due to the fact that the focal/grid laser can only be applied to the outside of the fovea (> 500 μm) [27]. In the present study, however, the mean distance from the center of fovea to the closest MAs was 624.8 ± 377.7 μm, with the MAs in 5 out of the 8 eyes (63%) located at the perifovea (< 500 μm). In addition, use of an eye-tracking system and pre-registered laser planning increased the MAs hit rate by 30% compared to that found with the conventional slit-lamp delivery system [20]. The results of our study show that the utilization of a precise focal laser with a navigation system would be useful for treating perifoveal MAs. That said, however, the re-treatment rates in our study were relatively high (63%) compared to a previous report (18%) [28]. Although we initially performed laser photocoagulation without a contact lens in order to increase patient comfort at the beginning of our study, heavy blinking or poor fixation sometimes made it difficult to perform focal laser photocoagulation even with a navigation system. As a result, a contact lens was required during the re-treatment. Moreover, in contrast to the slit-lamp delivery laser photocoagulation, it is not possible to directly observe the retina during laser photocoagulation with a navigated laser system. In addition, in order to adjust the power setting, it is necessary to check the color snap images taken just after each laser shot on the monitor. Due to these complexities, there might be a high learning curve to adapt to this system.
Clinical practice patterns for the management of DME in Japan indicate that 70% of specialists will choose a focal (direct) laser on MAs as first-line therapy for focal macular edema, whereas more than 70% of specialists choose anti-VEGF agents as first-line therapy for diffuse macular edema [29]. In our study, while all eyes were diagnosed as diffuse edema based on the FA and OCT findings, we were able to easily identify the MAs responsible for the edema using ICGA. Moreover, the density of MAs (/mm2) inside edema was significantly lower in late-phase ICGA than that in early-phase FA. These results indicate that fewer MAs could be used in ICGA-guided laser photocoagulation than in FA-guided laser photocoagulation, which would be beneficial to avoid atrophy.
Indocyanine green (ICG) dye mostly binds to serum proteins such as albumin and lipoproteins [30]. Therefore, the dye barely leaks through blood vessels, with the ICGA-positive MAs persisting after the fading of the plasmatic fluorescence on the late-phase ICGA images. We also speculated that ICG dye might possibly accumulate in the hyaline and collagenous materials around the highly permeable MAs, and thus, become trapped in the enveloping fibrin. These mechanisms may account for the preferential detectability of the leakage points responsible for the macular edema and the enlargement of the spots in the late-phase of ICGA. Although we initially reported on the efficacy of ICGA-guided laser photocoagulation for use in idiopathic macular telangiectasia type 1 [31], we have also used this technique for the treatment of DME [17] and ME associated with retinal vein occlusion. Other reports also confirm the usefulness of ICGA-guided focal laser photocoagulation [32, 33]. Furthermore, it is also suggested that evaluations of the OCT pattern of ME may help in understanding the pathogenesis of edema [34, 35]. As it has been demonstrated that the CME type exhibits the MAs that are responsible for edema [19, 34], our study included the CME type (75%) in our evaluations and confirmed that the use of a focal laser for MAs might be a good treatment modality for the CME type.
One limitation of the present study was the fact that there was only a limited number of patients evaluated along with a short follow-up period (6 months). In addition, we did not compare with conventional FA or ICGA-guided focal laser photocoagulation. In this study, the MAs were located at the perifovea in 63% of eyes, and these patients were especially recommended to be treated by using navigation, rather than conventional laser systems, so our patients’ characteristics might be biased, and it was difficult to compare with this study results with our previous report [17]. One eye had previous treatment history of conventional ICGA-guided focal laser, but the macular edema did not resolve. The location of the closest MA from center of the fovea was 604.7 μm in this case, and navigated ICGA-guide focal laser photocoagulation resolved the edema. This might indicate the advantage of using navigation system for ICGA-guide focal laser.
Furthermore, the original treatments for the refractory DME in this study were performed at a time when anti-VEGF treatment for DME had yet to be approved in Japan. To further clarify the clinical efficacy and benefits of using ICGA-guided navigated focal laser in the anti-VEGF era, a study that examines the combination therapy with ranibizumab or aflibercept will need to be undertaken in the future.
In summary, our study shows that ICGA-guided navigated focal laser photocoagulation was effective for treating DME. Our data additionally suggest that ICGA can detect the MAs responsible for edema, and when using a navigated laser system, even treat perifoveal MAs.
References
Klein R. The epidemiology of diabetic retinopathy: findings from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Int Ophthalmol Clin. 1987;27:230–8.
Stefánsson E, Bek T, Porta M, Larsen N, Kristinsson JK, Agardh E. Screening and prevention of diabetic blindness. Acta Ophthalmol Scand. 2000;78:374–85.
Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–94.
Terasaki H, Ogura Y, Kitano S, Sakamoto T, Murata T, Hirakata A, et al. Management of diabetic macular edema in Japan: a review and expert opinion. Jpn J Ophthalmol. 2018;62:1–23.
Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU. Diabetic Retinopathy Clinical Research Network. Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008;146:649–55.
Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effects to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995;113:1144–55.
Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806.
Takagi H, Otani A, Kiryu J, Ogura Y. New surgical approach for removing massive foveal hard exudates in diabetic macular edema. Ophthalmology. 1999;106:249–57.
Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–7.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL 3rd, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312–8.
Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52.
Thulliez M, Angoulvant D, Le Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132:1317–26.
Dhoot DS, Pieramici DJ, Nasir M, Castellarin AA, Couvillion S, See RF, et al. Residual edema evaluation with ranibizumab 0.5 mg and 2.0 mg formulations for diabetic macular edema (REEF study). Eye (Lond). 2015;29:534–41.
Rayess N, Rahimy E, Ying GS, Bagheri N, Ho AC, Regillo CD, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;159:85–91.
Liegl R, Langer J, Seidensticker F, Reznicek L, Haritoglou C, Ulbig MW, et al. Comparative evaluation of combined navigated laser photocoagulation and intravitreal ranibizumab in the treatment of diabetic macular edema. PLoS One. 2014;9:e113981.
Ogura S, Yasukawa T, Kato A, Kuwayama S, Hamada S, Hirano Y, et al. Indocyanine green angiography-guided focal laser photocoagulation for diabetic macular edema. Ophthalmologica. 2015;234:139–50.
Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–93.
Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Invest Ophthalmol Vis Sci. 2016;57:OCT348–55.
Kozak I, Oster SF, Cortes MA, Dowell D, Hartmann K, Kim JS, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology. 2011;118:1119–24.
Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–14.
Kiss S, Liu Y, Brown J, Holekamp NM, Almony A, Campbell J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;8:1611–21.
Gonder JR, Walker VM, Barbeau M, Zaour N, Zachau BH, Hartje JR, et al. Costs and quality of life in diabetic macular edema: Canadian burden of diabetic macular edema observational study (C-REALITY). J Ophthalmol. 2014;2014:939315.
Kato F, Nozaki M, Kato A, Hasegawa N, Morita H, Yoshida M, et al. Evaluation of navigated laser photocoagulation (Navilas 577+) for the treatment of refractory diabetic macular edema. J Ophthalmol. 2018;2(2018):3978514.
Morgan CM, Schatz H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology. 1989;96:96–103.
Jain A, Blumenkranz MS, Paulus Y, Wiltberger MW, Andersen DE, Huie P, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol. 2008;126:78–85.
Hirano T, Toriyama Y, Iesato Y, Imai A, Hirabayashi K, Nagaoka T, et al. Effect of leaking perifoveal microaneurysms on resolution of diabetic macular edema treated by combination therapy using anti-vascular endothelial growth factor and short pulse focal/grid laser photocoagulation. Jpn J Ophthalmol. 2017;61:51–60.
Neubauer AS, Langer J, Liegl R, Haritoglou C, Wolf A, Kozak I, et al. Navigated macular laser decreases retreatment rate for diabetic macular edema: a comparison with conventional macular laser. Clin Ophthalmol. 2013;7:121–8.
Ogura Y, Shiraga F, Terasaki H, Ohji M, Ishida S, Sakamoto T, et al. Clinical practice pattern in management of diabetic macular edema in Japan: survey results of Japanese retinal specialists. Jpn J Ophthalmol. 2017;61:43–50.
Baker KJ. Binding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteins. Proc Soc Exp Biol Med. 1966;122:957–63.
Hirano Y, Yasukawa T, Usui Y, Nozaki M, Ogura Y. Indocyanine green angiography-guided laser photocoagulation combined with sub-Tenon’s capsule injection of triamcinolone acetonide for idiopathic macular telangiectasia. Br J Ophthalmol. 2010;94:600–5.
Ueda T, Gomi F, Suzuki M, Sakaguchi H, Sawa M, Kamei M, et al. Usefulness of indocyanine green angiography to depict the distant retinal vascular anomalies associated with branch retinal vein occlusion causing serous macular detachment. Retina. 2012;32:308–13.
Paques M, Philippakis E, Bonnet C, Falah S, Ayello-Scheer S, Zwillinger S, et al. Indocyanine-green-guided targeted laser photocoagulation of capillary macroaneurysms in macular oedema: a pilot study. Br J Ophthalmol. 2017;101:170–4.
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Foveal cystoid spaces are associated with enlarged foveal avascular zone and microaneurysms in diabetic macular edema. Ophthalmology. 2011;118:359–67.
Murakami T, Uji A, Ogino K, Unoki N, Horii T, Yoshitake S, et al. Association between perifoveal hyperfluorescence and serous retinal detachment in diabetic macular edema. Ophthalmology. 2013;120:2596–603.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Nozaki, Grant (Novartis), Speaker fees (Novartis, Bayer, Santen, Senju, Accura, Topcon, Kowa, Pfizer, Chuo Sangyo, JOIA), Equipment (Ellex); A. Kato, Grant (Novartis), Speaker fees (Novartis, Santen); K. Suzuki, None; M. Yoshida, Grant (Novartis), Speaker fees (Novartis, Santen, Bayer, Sanwa Kagaku, Senju, Chuo Sangyo, Kowa, JOIA); T. Yasukawa, Grant (Novartis), Speaker fees (Novartis, HOYA, Santen, Bayer, Pfizer, Alcon, Sanwa Kagaku, Senju, BAUSCH+LOMB, Ono Yakuhin, Nitten, Chuo Sangyo, Nikken), Equipment (Nidek), Medical supplies (HOYA); Y. Ogura, Grants (Novartis, Wakamoto, HOYA, Santen, Bayer, Alcon, Senju), Speaker fees (Novartis, HOYA, Santen, Bayer, Alcon, Sanwa Kagaku, Senju, BAUSCH+LOMB, Kowa, Topcon, Kissei), Consultant fees (Wakamoto, Alcon, Janssen Pharmaceutical), Equipment (Ellex).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Miho Nozaki
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nozaki, M., Kato, A., Yasukawa, T. et al. Indocyanine green angiography-guided focal navigated laser photocoagulation for diabetic macular edema. Jpn J Ophthalmol 63, 243–254 (2019). https://doi.org/10.1007/s10384-019-00662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-019-00662-x