Abstract
Anti-vascular endothelial growth factor agents reduce macular edema and improve vision in eyes with macular edema associated with retinal vein occlusion (RVO), including branch RVO (BRVO) and central RVO. However, not all eyes with resolved macular edema show satisfactory best corrected visual acuity. Photoreceptor impairment can mostly explain the vision loss in these cases. Photoreceptor damage can be caused by subretinal hemorrhage in the central fovea and hard exudates or their precursor derived from concentrated lipoproteins originating from leaky retinal vessel extravasation. The contribution of neuron impairment in the inner retina, including the impairment of bipolar and ganglion cells by ischemia, indicated by the presence of a non-perfusion area (NPA), to vision loss in eyes with BRVO is insignificant. This is because the papillomacular bundle area is usually spared from NPAs in BRVO cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common cause of vision loss in eyes with retinal vein occlusion (RVO), including branch RVO (BRVO) and central RVO (CRVO), is macular edema, which in many cases can be successfully treated or managed with intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents [1,2,3]. However, regardless of treatment eyes with RVO and macular edema do not always recover satisfactory vision after the macular edema has been resolved. This is also true for eyes with the absence of late complications such as vitreous hemorrhage, neovascular glaucoma, and traction retinal detachment due to the neovascularization of the disc or retina during the chronic stages of the disease.
This review discusses the mechanisms of vision loss beyond macular edema in eyes with RVO and macular edema, and speculates on the histopathological process for macular edema increasing vision loss in eyes with RVO.
Three mechanisms causing vision loss in eyes with macular edema associated with RVO
Decreased vision due to macular edema is usually reversible in acute cases of RVO. Central foveal thickness (CFT) representing the degree of macular edema parallels visual deterioration and recovery in the clinical course of treatment with the intravitreal injection of anti-VEGF agents for eyes with RVO and macular edema (Fig 1).
Line graph of central foveal thickness (CFT) and LogMAR best corrected visual acuity (BCVA) in a left eye with inferior temporal branch retinal vein occlusion (BRVO) with macular edema treated with intravitreal bevacizumab (IVB) in an 84-year-old man. CFT is rapidly diminished after IVB and subsequently experiences a gradual increase. The changes in CFT are roughly synchronized with the increase and subsequent decrease of BCVA. The color fundus photograph at baseline is inserted
Whenever the macular edema is resolved after either a single or repetitive treatments of intravitreal anti-VEGF agents, visual recovery is satisfactory in many eyes with RVO and macular edema. However, some eyes with RVO and resolved macular edema show incomplete visual recovery.
Eyes in the “BRAVO” study received monthly intravitreal ranibizumab (IVR) for six months, followed by IVR pro re nata (PRN) for six months. Out of the 131 cases in the group treated with 0.5 mg of IVR, as many as 86% attained a 250 µm CFT or thinner at month 12 of the study period, while only 66% attained a “good” BCVA score of 20/40 or better [4]. This means that 20% or more of cases had “poor” BCVA scores (lower than 20/40) despite having a dry macula with a CFT of 250 µm or thinner.
In the “CRUISE” study, the counterpart of the “BRAVO” study, IVR was administered monthly followed by PRN in eyes with macular edema secondary to CRVO. Out of the 130 cases treated with 0.5 mg IVR, 78% attained a dry macula with a CFT of 250 µm or thinner at month 12 of the study period, yet only 43% showed a BCVA score of 20/40 or better [5].
In the “VIBRANT” study, monthly or bimonthly intravitreal aflibercept injections (IAI) were administered to 91 eyes with macular edema secondary to BRVO. 95% of the 91 cases treated with monthly IAI for 24 weeks followed by bimonthly IAI for 48 weeks attained a dry macula at week 52 of the study period, and 85% of them attained a BCVA score of 20/40 or better [6].
These results show that BCVA scores are not necessarily high enough in eyes without residual macular edema after treatment with intravitreal anti-VEGF agents.
Photic signals are delivered through the normally transparent neural retina, and are received by photoreceptors located in the outer retina. Then, the visual signal is transmitted from photoreceptors to bipolar cells and ganglion cells via unmyelinated axons. Therefore, to cause reduced vision in eyes with resolved macular edema irreversible factors must be present either in the photoreceptors at the fovea or in the inner retinal neurons connected to the foveal cones.
Three mechanisms of vision loss may be considered in eyes with macular edema associated with RVO (Table 1): (1) the impairment of the photoreceptors in the fovea; (2) the impairment of the neurons in the inner retina; and (3) the direct effects of macular edema itself causing neuro-retinal swelling. Vision loss from the former two mechanisms may probably be irreversible, and may contribute to a sustained loss of vision after the resolution of macular edema.
Vision loss due to impairment of photoreceptors in the fovea
Impaired photoreceptors in eyes with resolved macular edema
Photoreceptor impairment in eyes with resolved macular edema is demonstrated morphologically in the outer nuclear layer (ONL), the external limiting membrane (ELM), the ellipsoid zone (EZ), and the interdigitation zone (IZ). The nomenclature and its abbreviation are based on the recent report on consensus nomenclature for the classification of retinal and choroidal layers and bands visible on spectral domain-optical coherent tomography (SD-OCT) images [7].
Several reports have investigated the association of the qualitative changes and quantitative parameters in OCT images indicating photoreceptor integrity with final BCVA in eyes with RVO and macular edema. Ota et al. [8] found that final BCVA depends on the third high reflectance band (HRB), which corresponds to the IZ, in a fovea observed in resolved macular edema following BRVO. Subsequent reports demonstrate a significant correlation between final BCVA and the continuity of the inner and outer segments of the photoreceptors (the IS/OS line) corresponding to the EZ and ELM in eyes with resolved or persistent macular edema following RVO [9,10,11,12,13,14]. It is also reported that a preserved photoreceptor layer in the fovea, determined by the integrity of the ELM or the EZ in baseline OCT images taken prior to anti-VEGF treatment, can predict final BCVA [15,16,17].
In addition to the EZ continuity, a bulge at the central fovea called the “foveal bulge” is a good marker of foveal photoreceptor soundness, and its presence is generally associated with a BCVA of 1.0 or better [18]. These reports indicate that photoreceptor impairment may significantly contribute to the recovery of BCVA in eyes with macular edema associated with RVO.
Mechanisms of impaired photoreceptors through macular edema
Blood to photoreceptors is supplied from choroidal circulation through the retinal pigment epithelium (RPE) and not from retinal circulation, so it is difficult to speculate whether retinal circulatory disturbances caused by RVO impair the photoreceptors in the outer retina. Subretinal blood and exudates containing lipoproteins, which may derive from the leaking of retinal vessels through the ELM, are possible causes of photoreceptor impairment.
Subretinal blood containing red blood cells is sometimes observed in eyes with macular edema associated with RVO [19, 20]. Blood in the subretinal space may be harmful to photoreceptors, especially in eyes with age-related macular degeneration (AMD) or retinal arterial macroaneurism [21]. The baseline subretinal hemorrhage (SRH) in eyes with BRVO is associated with poorer final BCVA [20, 22], probably due to foveal photoreceptor damage inflicted by the hemorrhage [23].
There are three mechanisms for subretinal blood impairing photoreceptors: (1) toxic effects of released iron ion from hemoglobin in the SRH, (2) traction of photoreceptor outer segments during clot retraction, and (3) formation of a diffusion barrier between photoreceptors and the RPE [21]. In vivo studies show that blood in the subretinal space induced the apoptosis of photoreceptors [24, 25].
In addition to subretinal blood, photoreceptors may also be impaired by subretinal fluid containing high concentrations of lipoproteins, which may be the precursors of hard exudates. OCT has revealed hyperreflective foci (HRF) representing extravasated lipoproteins [26, 27], which may appear in the subretinal space penetrating the discontinuous ELM in eyes with macular edema associated with diabetic retinopathy [28, 29] and BRVO [30]. It is demonstrated that final BCVA was poorer in eyes with macular edema associated with BRVO containing HRF in outer retinal layers [31] and in those with submacular serous retinal detachment. [32]
Persistent macular edema and impairment of photoreceptors
Although vision loss due to retinal swelling caused by macular edema in the acute stage of RVO appears to be clinically reversible, delays in treatment with intravitreal anti-VEGF or corticosteroids to reduce retinal swelling may sometimes limit the ultimate recovery of vision. In a study of a group receiving monthly sham treatments for six months followed by a PRN regimen of IVR for six months, BCVA at 12 months was inferior to that of the group receiving monthly IVR for six months followed by a PRN regimen of IVR for 6 months [4]. Similar clinical results were also observed in eyes with CRVO and macular edema in the “GENEVA” study using dexamethasone intravitreal implants [33]. It is also reported that delayed treatment with anti-VEGF agents reduced BCVA recovery in eyes with BRVO and macular edema [34]. In addition to vascular changes in eyes with persisting macular edema caused by RVO, including microaneurysms and leaky capillaries [35], irreversible changes may develop in photoreceptors due to subretinal blood or exudates.
Possible recovery of vision in eyes with impaired photoreceptors
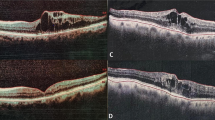
Rhegmatogenous retinal detachment is a major retinal disorder impairing photoreceptors. It is demonstrated that the impaired photoreceptors in the central fovea regenerate after successful macula-off rhegmatogenous retinal detachment surgery [36, 37]. Although the recovery of impaired photoreceptors in eyes with RVO has not been reported, a long-term follow-up could reveal the recovery of the EZ accompanied by vision gain in eyes with RVO showing resolved macular edema and a disrupted EZ at the central fovea (Fig 2).
An example of EZ recovery accompanied by visual gain after four years. Top, color photograph and vertical optical coherent tomography (OCT) of the right eye with macular edema associated with branch retinal vein occlusion (BRVO) in a 70-year-old woman, whose best corrected visual acuity (BCVA) was 0.04 at 42 days after the onset of symptoms. Middle, color photograph and OCT 30 days after the first intravitreal bevacizumab (IVB) shows resolved macular edema and a disrupted ellipsoid zone (EZ) at the central fovea; BCVA was 0.04. Bottom, color photograph and OCT four years later, the woman’s BCVA was 0.9, with an EZ restored to normal at the central fovea
Vision loss due to Impairment of the neurons in the inner retina
Neuronal loss in the inner retina caused by ischemia
It is logical that in eyes with ischemic retinal disorders visual acuity is greatly decreased by the loss of inner retinal neurons such as bipolar and ganglion cells in the papillomacular bundle area; which transport visual signals received by the photoreceptors in the central fovea and are closely associated with central visual acuity. Visual prognosis in eyes with central retinal artery occlusion (CRAO) is generally far worse than in those with branch retinal artery occlusion (BRAO) [38]. This is probably because, in the former case inner retinal neurons in the papillomacular bundle area are damaged, while in the latter they are likely to be spared. At the same time, visual prognosis is very poor in eyes with cilioretinal artery occlusion, a special variant of BRAO in which inner retinal neurons in the papillomacular bundle area are damaged [39]. If, in eyes with CRAO a cilioretinal artery that perfuses a large area in the papillomacular bundle area is spared, BCVA is not greatly reduced because neurons in the papillomacular bundle area are not damaged [40].
Ischemia in eyes with RVO is evidenced by the vascular dropout of a network known as the non-perfusion area (NPA), shown by fluorescein angiography (FA). If ischemia is present in the papillomacular bundle area in eyes with RVO as shown in Fig 3(left), it may result in severe vision loss irrespective of macular edema. Although NPA is implicated in reduced light sensitivity in visual field testing [41], outside of the papillomacular bundle area it may not cause visual acuity deterioration.
Fluorescein angiograms (FA) of ischemic central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO). Left: The left eye of 75-year-old woman with severe ischemic CRVO. Widespread non-perfusion area involves the papillomacular bundle area. The best corrected visual acuity in her left eye was 0.02. Right: The left eye of 60-year-old woman with ischemic BRVO. The papillomacular bundle area is not included in the non-perfusion area. The best corrected visual acuity in her left eye was 1.0
Because in many eyes with RVO the papillomacular bundle area tends to be spared, especially in those with ischemic BRVO (Fig 3, right), reduction of BCVA due to ischemia in the inner retina may be less likely.
Broken foveal capillary networks and vision loss in eyes with RVO
In the late 20th century, when OCT was yet unavailable in everyday clinical practice, macular edema in eyes with RVO was evaluated mainly by FA images. It is reported that eyes with BRVO having intact or complete perifoveal capillary arcades had a better visual prognosis than those having incomplete arcades due to the presence of a parafoveal NPA [42,43,44,45]. These assumptions resulted from possible considerations that ischemia in the inner retina around the central fovea causes neuronal loss, including the loss of bipolar and ganglion cells, which are connected to the foveal cones responsible for normal visual acuity.
However, several recent studies using OCT angiography (OCTA) demonstrate that defects in capillary networks around the fovea induce photoreceptor damage, which can explain reduced BCVA irrespective of inner retinal neuron impairment. Among the reports studying the correlation between capillary networks around the fovea and visual prognosis in a small number of eyes with BRVO [46,47,48], Wakabayashi et al. [48] studied OCTA images in a 3 × 3 mm area around the fovea in 85 eyes with resolved macular edema caused by BRVO. They demonstrate that vascular perfusion area in the deep capillary plexus (DCP) was correlated with final BCVA, and with the integrity of the photoreceptors evaluated with ELM, EZ, and IZ. This indicates that poor visual prognosis in eyes with reduced vascular perfusion in the DCP around the fovea may be associated with photoreceptor damage through impaired blood supply. The authors speculate that reduced blood flow in the DCP damaged the synaptic region between the inner nuclear layer and the outer plexiform layer, which resulted in photoreceptor damage.
Vision loss not associated with photoreceptor damage but primarily due to ischemic impairment of the neurons in the inner retina may be uncommon, especially in eyes with BRVO and macular edema. To investigate whether vision loss is due to impaired neurons in the inner retina, NPAs at the nasal side of the foveal capillary networks rather than those around the central fovea need to be studied, as these correspond to the neurons connecting the foveal cones and the papillomacular bundle in the nerve fiber layer.
Favorable visual prognosis in eyes with partial ischemia in the macular area
It is reported that partial ischemia in the macular area may favor visual prognosis rather than hinder it. Contrary to earlier reports emphasizing the negative effects of ischemia on visual prognosis, Finkelstein [49] demonstrates that eyes with macular edema following BRVO and incomplete macular perfusion showing NPA in the macular area which remain untreated for three years following diagnosis, have better visual prognosis than those with complete macular perfusion without NPA in the macular area. One of the explanations for this paradoxical finding may be that eyes with NPA in the macular area have fewer vessels leaking intravascular fluids than completely perfused eyes, leading to less severe macular edema causing irreversible photoreceptor damage (Fig 4).
Early and late fluorescein angiography (FA) images of eyes with branch retinal vein occlusion (BRVO) showing incomplete or complete macular perfusion. Top left, an eye with BRVO showing incomplete macular perfusion with a non-perfusion area (NPA) in the macular area. Top right, an eye with complete macular perfusion. Bottom left, a late FA image of the eye shown in the top left with less leakage from the affected vessels. Bottom right, a late FA image of the eye shown in the top right with marked leakage from the affected vessels
Macular edema is induced by VEGF, and the intravitreal concentration of VEGF is higher in eyes with RVO and NPA than in those without NPA [50]. However, the dropout of retinal venules and capillaries, the effector vessels responding to increased VEGF, results in reduced leakage of intravascular fluid into the intrastromal space of the retina leading to less marked macular edema and less impaired vision.
In the “BRITER” study, eyes with BRVO and macular edema were treated with an individualized stabilization criteria-driven PRN regimen of ranibizumab. The eyes with macular ischemia at baseline appeared to experience better visual gain than those without ischemia, although the statistical assessment of these data was not significant [51].
Vision loss due to neuro-retinal swelling caused by macular edema
Mechanisms of vision loss by macular edema itself
The severity of macular edema is evaluated by CFT. Although changes in CFT and BCVA are closely correlated in the acute stage of BRVO with macular edema (Fig 1), the mechanism of vision impairment in eyes with increased CFT has never been clearly explained. Retinal swelling due to macular edema may result both in reduced light intensity transmitted to the photoreceptors and in impairment of transduction of visual signals from the photoreceptors to the bipolar cells.
Reduced transparency in the neural retina due to macular edema
It is possible that increased light scattering due to macular edema in the neural retina causes a reduction of transparency in the neural retina along the visual axis and leads to decreased visual acuity. One textbook states that macular edema creates multiple interfaces between separated retinal cells, which results in light scattering and decreases the neural retina’s transparency [52].
Images of eyes with persistent macular edema secondary to RVO obtained from high-quality SD-OCTs rarely show the honeycomb structure that causes light scattering. Rather, such eyes often show a large cyst in front of the foveal cones, which does not appear to interfere with light transmission (Fig 5).
Color photograph and optical coherent tomography (OCT) image of the right eye of a 70-year-old woman with persistent macular edema associated branch retinal vein occlusion (BRVO). A large foveal cyst without the honeycomb structure, which is located in front of the photoreceptors in the central fovea, does not appear to reduce light transmission from scattering. Best corrected visual acuity (BCVA) was 0.2
Recently, an optical fiber theory of the Müller cells has been proposed in which the long tubular structure of the Müller cells functions as an optic fiber [53, 54]. The morphology of Müller cells at the center of the fovea is different from those in the peripheral retina [55], so it is difficult to attribute vision loss to the disorganization of Müller cells in the fovea caused by macular edema.
Impaired neural transduction in the axons of retinal neurons in eyes with macular edema
Retinal edema may cause changes in the extracellular environment of retinal neurons, including ion concentration, osmotic pressure, and the pH in retinal tissue. Because the axons of various neurons in the neural retina do not have myelin sheaths, compromised extracellular conditions may easily interfere with neural transduction through the unmyelinated axons of photoreceptors and bipolar cells, a possibility that has not yet been fully investigated. Yasuda et al. [56] report that the implicit time of the flicker electroretinogram was prolonged in eyes with macular edema associated with CRVO, and was shortened by the resolution of macular edema after the intravitreal injection of anti-VEGF agents. These results may indicate that vision loss due to macular edema is attributed to impaired neural transduction in the axons of neurons in the retina.
A poor correlation is reported between CFT and BCVA in eyes with RVO and macular edema [57], which supports the mechanism of impaired neural transduction, rather than light scattering, for reduced vision in eyes with macular edema. Differences in BCVA among eyes with macular edema of equivalent CFT could be explained by reduced neuronal transduction caused by different composition of electrolytes in the extracellular space. Although changes in CFT and changes in BCVA were correlated with each other in a longitudinal clinical study, a cross-sectional study revealed a poor association between BCVA and CFT before treatment for macular edema in eyes with RVO [58].
Conclusion
In conclusion, there are three mechanisms of vision reduction in eyes with macular edema associated with RVO. The first involves the swelling of retinal tissue, a result of macular edema itself, which could explain the reversible loss of BCVA. The second involves impaired photoreceptors, evidenced by a disruption or thinning of the ELM, the EZ, and the IZ, which appears to be irreversible after two to three years. The last involves impaired neurons of the inner retina in the papillomacular bundle area connected to the photoreceptors in the central fovea, which may in rare cases contribute to impaired BCVA in eyes with macular edema associated with BRVO.
References
Ehlers JP, Kim SJ, Yeh S, Thorne JE, Mruthyunjaya P, Schoenberger SD, et al. Therapies for macular edema associated with branch retinal vein occlusion: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:1412–23.
Larsen M, Waldstein SM, Boscia F, Gerding H, Mones J, Tadayoni R, et al. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: twelve-month results of the CRYSTAL Study. Ophthalmology. 2016;123:1101–11.
Yeh S, Kim SJ, Ho AC, Schoenberger SD, Bakri SJ, Ehlers JP, et al. Therapies for macular edema associated with central retinal vein occlusion: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:769–78.
Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–602.
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9.
Clark WL, Boyer DS, Heier JS, Brown DM, Haller JA, Vitti R, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT Study. Ophthalmology. 2016;123:330–6.
Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. International Nomenclature for Optical Coherence Tomography P. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014;121:1572–8.
Ota M, Tsujikawa A, Murakami T, Kita M, Miyamoto K, Sakamoto A, et al. Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol. 2007;91:1644–9.
Ota M, Tsujikawa A, Kita M, Miyamoto K, Sakamoto A, Yamaike N, et al. Integrity of foveal photoreceptor layer in central retinal vein occlusion. Retina. 2008;28:1502–8.
Ota M, Tsujikawa A, Murakami T, Yamaike N, Sakamoto A, Kotera Y, et al. Foveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2008;145:273–80.
Shin HJ, Chung H, Kim HC. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011;89:e35–40.
Wolf-Schnurrbusch UE, Ghanem R, Rothenbuehler SP, Enzmann V, Framme C, Wolf S. Predictors of short-term visual outcome after anti-VEGF therapy of macular edema due to central retinal vein occlusion. Invest Ophthalmol Vis Sci. 2011;52:3334–7.
Domalpally A, Peng Q, Danis R, Blodi B, Scott IU, Ip M, et al. Association of outer retinal layer morphology with visual acuity in patients with retinal vein occlusion: SCORE Study Report 13. Eye (Lond). 2012;26:919–24.
Yunoki T, Miyakoshi A, Nakamura T, Fujita K, Fuchizawa C, Hayashi A. Treatment of macular edema due to branch retinal vein occlusion with single or multiple intravitreal injections of bevacizumab. Jpn J Ophthalmol. 2012;56:159–64.
Sakamoto A, Tsujikawa A, Ota M, Yamaike N, Kotera Y, Miyamoto K, et al. Evaluation of potential visual acuity in eyes with macular oedema secondary to retinal vein occlusion. Clin Exp Ophthalmol. 2009;37:208–16.
Kim M, Yu SY, Kim ES, Bae SH, Park JH, Yu HG, et al. Intravitreal ranibizumab for macular edema secondary to retinal vein occlusion. Ophthalmologica. 2012;227:132–8.
Kang HM, Chung EJ, Kim YM, Koh HJ. Spectral-domain optical coherence tomography (SD-OCT) patterns and response to intravitreal bevacizumab therapy in macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2013;251:501–8.
Hasegawa T, Ueda T, Okamoto M, Ogata N. Presence of foveal bulge in optical coherence tomographic images in eyes with macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2014;157(390–6):e1.
Spaide RF, Lee JK, Klancnik JK Jr, Gross NE. Optical coherence tomography of branch retinal vein occlusion. Retina. 2003;23:343–7.
Muraoka Y, Tsujikawa A, Murakami T, Ogino K, Miyamoto K, Yoshimura N. Branch retinal vein occlusion-associated subretinal hemorrhage. Jpn J Ophthalmol. 2013;57:275–82.
Hochman MA, Seery CM, Zarbin MA. Pathophysiology and management of subretinal hemorrhage. Surv Ophthalmol. 1997;42:195–213.
Zhao L, Li B, Feng K, Han L, Ma Z, Liu Y. Bevacizumab treatment for acute branch retinal vein occlusion accompanied by subretinal hemorrhage. Curr Eye Res. 2015;40:752–6.
Muraoka Y, Tsujikawa A, Takahashi A, Iida Y, Murakami T, Ooto S, et al. Foveal damage due to subfoveal hemorrhage associated with branch retinal vein occlusion. PLoS One. 2015;10:e0144894.
Bhisitkul RB, Winn BJ, Lee OT, Wong J, Pereira Dde S, Porco TC, et al. Neuroprotective effect of intravitreal triamcinolone acetonide against photoreceptor apoptosis in a rabbit model of subretinal hemorrhage. Invest Ophthalmol Vis Sci. 2008;49:4071–7.
Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S, Asato R, et al. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One. 2013;8:e53338.
Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C, et al. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–20.
Deak GG, Bolz M, Kriechbaum K, Prager S, Mylonas G, Scholda C, et al. Effect of retinal photocoagulation on intraretinal lipid exudates in diabetic macular edema documented by optical coherence tomography. Ophthalmology. 2010;117:773–9.
Vujosevic S, Torresin T, Berton M, Bini S, Convento E, Midena E. Diabetic macular edema with and without subfoveal neuroretinal detachment: two different morphologic and functional entities. Am J Ophthalmol. 2017;181:149–55.
Ota M, Nishijima K, Sakamoto A, Murakami T, Takayama K, Horii T, et al. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002.
Tsujikawa A, Sakamoto A, Ota M, Kotera Y, Oh H, Miyamoto K, et al. Serous retinal detachment associated with retinal vein occlusion. Am J Ophthalmol. 2010;149(291–301):e5.
Kang JW, Lee H, Chung H, Kim HC. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitreal bevacizumab for macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2014;252:1413–21.
Gallego-Pinazo R, Dolz-Marco R, Pardo-Lopez D, Martinez-Castillo S, Lleo-Perez A, Arevalo JF, et al. Ranibizumab for serous macular detachment in branch retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol. 2013;251:9–14.
Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–60.
Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, Park KH, et al. Improved visual outcome with early treatment in macular edema secondary to retinal vein occlusions: 6-month results of a Korean RVO study. Jpn J Ophthalmol. 2014;58:146–54.
Iesato Y, Imai A, Hirano T, Toriyama Y, Murata T. Effect of leaking capillaries and microaneurysms in the perifoveal capillary network on resolution of macular edema by anti-vascular endothelial growth factor treatment. Jpn J Ophthalmol. 2016;60:86–94.
Kobayashi M, Iwase T, Yamamoto K, Ra E, Murotani K, Matsui S, et al. Association between photoreceptor regeneration and visual acuity following surgery for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2016;57:889–98.
Ra E, Ito Y, Kawano K, Iwase T, Kaneko H, Ueno S, et al. Regeneration of photoreceptor outer segments after scleral buckling surgery for rhegmatogenous retinal detachment. Am J Ophthalmol. 2017;177:17–26.
Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn J Ophthalmol. 2004;48:490–2.
Kunikata H, Tamai M. Cilioretinal artery occlusions following embolization of an artery to an intracranial meningioma. Graefes Arch Clin Exp Ophthalmol. 2006;244:401–3.
Patel PS, Sadda SR. Retinal artery obstructions. In: Ryan SJ, editor. Retina. 5th ed. Philadelphia: Elsevier inc; 2013. p. 1012–25.
Iijima H. Reduced light sensitivity due to impaired retinal perfusion in branch retinal vein occlusion. Jpn J Ophthalmol. 2018;62:151–7.
Patz A, Yassur Y, Fine SL, Finkelstein D, Orth DH. Branch retinal venous occlusion. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:OP373–8.
Gass JDM. Stereoscopic atlas of macular diseases, diagnosis and treatment. 2nd ed. St. Luis: C.V. Mosby; 1977. p. 282–4.
Michels RG, Gass JD. The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:OP166–77.
Clemett RS, Kohner EM, Hamilton AM. The visual prognosis in retinal branch vein occlusion. Trans Ophthalmol Soc UK. 1973;93:523–35.
Samara WA, Shahlaee A, Sridhar J, Khan MA, Ho AC, Hsu J. Quantitative optical coherence tomography angiography features and visual function in eyes with branch retinal vein occlusion. Am J Ophthalmol. 2016;166:76–83.
Kadomoto S, Muraoka Y, Ooto S, Miwa Y, Iida Y, Suzuma K et al. Evaluation of macular ischemia in eyes with branch retinal vein occlusion: an Optical Coherence Tomography Angiography Study. Retina. 2018;38:272–82.
Wakabayashi T, Sato T, Hara-Ueno C, Fukushima Y, Sayanagi K, Shiraki N, et al. Retinal microvasculature and visual acuity in eyes with branch retinal vein occlusion: imaging analysis by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:2087–94.
Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol. 1992;110:1427–34.
Noma H, Minamoto A, Funatsu H, Tsukamoto H, Nakano K, Yamashita H, et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2006;244:309–15.
Tadayoni R, Waldstein SM, Boscia F, Gerding H, Pearce I, Priglinger S, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology. 2016;123:1332–44.
Ahmed I, Ai E. Cystoid macular edema. In: Yanoff M, Duker JS, editors. Ophthalmology. 1st ed. Amsterdam: Elsevier; 1999. p. 8.34.2.
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40.
Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–78.
Gass JD. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol. 1999;117:821–3.
Yasuda S, Kachi S, Ueno S, Piao CH, Terasaki H. Flicker electroretinograms before and after intravitreal ranibizumab injection in eyes with central retinal vein occlusion. Acta Ophthalmol. 2015;93:e465–8.
Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, Jumper JM, et al. SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116:504–12.
Ou WC, Brown DM, Payne JF, Wykoff CC. Relationship between visual acuity and retinal thickness during anti-vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol. 2017;180:8–17.
Acknowledgements
This study was supported in part by a JSPS KAKENHI Grant Number 16K11263 (Grant-in-aid for scientific Research (C)) from the Japan Society for the Promotion of Science (JSPS), (Hiroyuki Iijima).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Iijima, None.
About this article
Cite this article
Iijima, H. Mechanisms of vision loss in eyes with macular edema associated with retinal vein occlusion. Jpn J Ophthalmol 62, 265–273 (2018). https://doi.org/10.1007/s10384-018-0586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-018-0586-5