Abstract
Purpose
To investigate the correlation between hyperreflective foci (HF) on spectral-domain optical coherence tomography at baseline and visual outcomes after intravitreal bevacizumab injection (IVB) in branch retinal vein occlusion.
Methods
We retrospectively studied 97 eyes of 97 patients with macular edema secondary to BRVO, who were treated with IVB. The eyes were divided into three groups according to the location of HF on SD-OCT: HF in outer retinal layers, HF in inner retinal layers, and no HF. The baseline and final best-corrected visual acuity (BCVA), foveal thickness (FT), external limiting membrane (ELM) status, junction between photoreceptor inner and outer segments (IS/OS) status, and the number of HF were evaluated and compared among three groups.
Results
Baseline BCVA was correlated with baseline FT (R = 0.366, p < 0.001), but final BCVA was not correlated with final FT (R = −0.008, p = 0.942). Baseline BCVA was significantly better in eyes with intact ELM at baseline (p = 0.006), and final BCVA was significantly better in eyes with intact ELM and IS/OS at final visit (p < 0.001, p = 0.003 respectively). At the final visit, 15 of 37 eyes (40.5 %) with HF in outer retinal layers had a disrupted ELM (p = 0.001), while 28 of 37 eyes (75.7 %) with HF in outer retinal layers had a disrupted IS/OS (p < 0.001). Final BCVA was poorer in eyes with HF in outer retinal layers groups than those in the other two groups (p < 0.001), although baseline BCVA was not different between them.
Conclusions
HF on SD-OCT at baseline might predict the photoreceptor status and final VA after IVB in BRVO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Branch retinal vein occlusion (BRVO) is the second most common retinal vascular disease after diabetic retinopathy [1–5], and the development of macular edema (ME) is the most common cause of visual deterioration [6]. Current treatment options for ME include grid laser treatment, intravitreal injection of steroids, surgical procedures, and intravitreal anti-vascular endothelial growth factor (VEGF) agents [7–10].
The major cause for the formation of ME and neovascularization in patients with BRVO seems to be the production of VEGF, a hypoxia-induced endothelial-cell-specific angiogenic and vasopermeable factor [6]. Recently, intravitreal anti-VEGF treatment with bevacizumab has been used to efficiently reduce ME and improve visual acuity (VA). After complete resolution of ME, however, some patients had poor visual outcomes [10], and there may be another factor to predict the final visual outcomes.
Spectral-domain optical coherence tomography (SD-OCT) is widely used to identify individual retinal layer, objectively quantify retinal thickness, and monitor the efficacy of therapy such as the resolution of ME. Also, SD-OCT can provide detailed view of retinal microstructure, including the external limiting membrane (ELM) and the photoreceptor inner and outer segment junction (IS/OS).
Several studies have shown that the integrity of the ELM and IS/OS is associated with visual outcomes in retinal diseases such as BRVO, central retinal vein occlusion, diabetic macular edema, and age-related macular degeneration [11–16]. Those studies demonstrated that disrupted ELM or IS/OS may be a predictor of poor VA after treatment.
The latest SD-OCT can reveal hyperreflective foci (HF) within the outer and inner neurosensory retinal structures in patients with diabetic ME, retinal vein occlusion, exudative age-related macular degeneration, and dry age-related macular degeneration [17–20], which is not detected by ophthalmoscopy. The origin of these foci is not clear, but may represent the initial stage in the development of intraretinal hard exudates or subclinical features of lipoprotein extravasation after the breakdown of the inner blood–retinal barrier [17]. Reduction in HF on SD-OCT has been described after anti-VEGF injection in patients with diabetic ME and exudative age-related macular degeneration [21–23], and has been related to the final VA. The location of HF may be a predictor of final ELM and IS/OS status, and VA in DME [24].
The purpose of this study was to evaluate the correlation between HF on SD-OCT at baseline and foveal photoreceptor status and VA after intravitreal bevacizumab injection (IVB) in BRVO.
Methods
Patients
We retrospectively reviewed the medical records of the patients who underwent IVB for ME in BRVO at the Department of Ophthalmology in Konkuk University Medical Center from January 2008 to January 2013. We excluded patients with high myopia (>8 diopter), glaucoma, vitreous hemorrhage, past history of uveitis, subfoveal hard exudates, combined retinal diseases such as epiretinal membrane, macular hole, age-related macular degeneration, and other ocular pathologies that affected VA, especially with significant cataract graded at more than NO3 or NC3 according to the Lens Opacity Classification Scheme.
At the initial visit, all patients underwent comprehensive ophthalmic examination including measurement of best-corrected visual acuity (BCVA), intraocular pressure, slit-lamp biomicroscopy, indirect ophthalmoscopy, color fundus photography, fluorescein angiography, and SD-OCT (Spectralis HRA + OCT®; Heidelberg Engineering, Heidelberg, Germany).
The study followed the tenets of the Declaration of Helsinki, and was approved by the local institutional review board. All patients were treated with an injection of intravitreal bevacizumab (1.25 mg/0.05 ml), and BCVA, color fundus photography, and SD-OCT were evaluated at every visit. Basically, most of patients received three IVB at 4–6 week intervals, and ME was resolved on SD-OCT in all of the patients who were treated with three IVB. However, additional treatment was not performed when ME was completely resolved on SD-OCT after one or two IVB. The final visit was defined as the last visit after the intravitreal injections.
SD-OCT measurement
A 9 mm × 6 mm area of the macular region centered on the fovea was examined using the SD-OCT with display mode; volume scans of 49 sections were centered on the fovea, and nine B-scan images at each section were averaged. Thirty-degree radial scans centered on the fovea were also obtained in a clockwise manner in all eyes. The eye-tracking system of the device was used to assure that the correct position was maintained during the scanning process. The morphologic features of ME, HF, hard exudates, ELM, IS/OS, and foveal thickness (FT) were analyzed with SD-OCT by two experienced masked graders (Kim, HC & Chung, H).
The status of the ELM and IS/OS was classified into intact or disrupted status. Intact ELM and IS/OS was defined as a continuous hyperreflective line. Disrupted ELM and IS/OS was defined as loss or irregularity of the hyperreflective line corresponding to the ELM and IS/OS. For disrupted ELM and IS/OS, the percentage of disruption of the ELM and IS/OS was evaluated manually by measuring the length of disruption on horizontal and vertical SD-OCT images within 500 μm in either direction of the fovea [25–27]. The percentage of disruption along the ELM and IS/OS layer was averaged to generate a number between 0 (no ELM or IS/OS disruption) and 100 % (total disruption of ELM or IS/OS) [26, 27].
FT was automatically calculated as an average retinal thickness within a circle having a 500 μm radius centered on the fovea (software version 5.1.2.0).
The presence of HF was defined as having been observed in at least one scan but not all scans. Therefore, if any single HF was detected in either raster scans or radial scans, it was considered as the presence of HF. If no HF was detected in both scans, it was considered as the absence of HF.
We categorized the study eyes into three groups according to the location of HF on SD-OCT: HF in the outer retinal layers (from the ELM to the retinal pigment epithelium within the 1 mm area centered on the fovea, Fig. 1), HF in the inner retinal layers (from the nerve fiber layer to the outer nuclear layer, Fig. 2), and no HF (no HF throughout all retinal layers, Fig. 3). The eyes with HF both in the inner retinal layers and in the outer retinal layers were classified as HF in the outer retinal layers; we divided the eyes with HF according to the presence of the HF in the outer retinal layers.
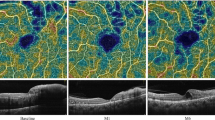
Representative case of BRVO with hyperreflective foci in the outer retinal layers. Fundus findings and SD-OCT images of a 38-year-old man with macular edema secondary to BRVO. The initial BCVA was 20/100. a Pretreatment SD-OCT image shows macular edema with serous retinal detachment accompanied by hyperreflective foci in the outer retinal layers (black arrows). b After three intravitreal bevacizumab injections, macular edema resolved, but IS/OS disruption was detected (black arrow). The hyperreflective foci were not detected. The final BCVA was 20/25
Representative case of BRVO with hyperreflective foci in the inner retinal layers. Fundus findings and SD-OCT images of a 73-year-old woman with macular edema secondary to BRVO. The initial BCVA was 20/40. a Pretreatment SD-OCT image shows macular edema with cystoid spaces accompanied by hyperreflective foci in the inner retinal layers (black arrows). b After two intravitreal bevacizumab injections, macular edema completely resolved and the photoreceptor was intact. The hyperreflective foci were not detected. The final BCVA was 20/25
Representative case of BRVO with no hyperreflective foci. Fundus findings and SD-OCT images of a 57-year-old woman with macular edema secondary to BRVO. The initial BCVA was 20/63. a Pretreatment SD-OCT image shows macular edema with cystoid spaces without hyperreflective foci. b After three intravitreal bevacizumab injections, macular edema completely resolved, and photoreceptor was intact. The final BCVA was 20/20
The number of HF within parafoveal area was subjectively determined in all patients by classifying them into three categories at baseline and final visit (A = few, representing approximately 2–10 HF; B = moderate, representing 11–20 HF; C = many, representing 21 or more HF) as described in previous studies [22, 23]. Classification was performed by two experienced masked graders (Kim, HC & Chung, H).
Statistical analyses
BCVA was converted to the logarithm of the minimal angle of resolution (logMAR) equivalents before statistical analyses. All values are presented as a mean ± standard deviation (SD). For patient characteristics at baseline, ANOVA test was used to compare the three groups (HF in the outer retinal layers, HF in the inner retinal layers, and no HF group) for age, BCVA (logMAR), FT, follow-up period, and number of injections, followed by pairwise multiple comparison using Duncan’s method. The Chi-square test was used to analyze the initial patient characteristics such as sex, systemic diseases (diabetes mellitus, hypertension), number of eyes with hard exudates, and number of eyes with serous retinal detachment. Also, Student's t-test was used to compare the number of HF at baseline between HF in the outer retinal layers group and HF in the inner retinal layers group. The relationship between the logMAR BCVA and the FT was analyzed using the Pearson correlation coefficient. Student's t-test was used to compare two groups (intact and disrupted ELM group at baseline and final visit, intact and disrupted IS/OS group at final visit respectively) with regard to BCVA (logMAR) at baseline and final visit. ANOVA test was used to compare the three groups for BCVA (logMAR), FT at final visit. Chi-square test was used to analyze the relationship between three groups for ELM and IS/OS status. Then, multiple linear regression analysis with backward elimination was employed to analysis the association of final BCVA (logMAR) with baseline prognostic parameters including baseline BCVA (logMAR), FT, ELM status, the location and number of HF, age, sex, and systemic diseases. A p-value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS v. 17.0 for Windows software (SPSS, Chicago, IL, USA).
Results
The baseline characteristics of the patients are shown in Table 1. Ninety-seven eyes of 97 patients (46 males and 51 females) with ME secondary to BRVO were examined. The mean age was 57.59 ± 11.35 years (range, 38–84 years), and the mean follow-up period was 5.91 ± 3.37 months (range, 3–21 months). Mean BCVA (logMAR) at baseline was 0.49 ± 0.32, and mean FT at baseline was 547.68 ± 183.83 μm. The number of eyes with HF in the outer retinal layers was 37, those with HF in the inner retinal layers was 43, and those with no HF was 17. There were no significant differences among the three groups with regard to age, sex, systemic diseases, follow-up period, and mean number of injections, but there was a significant difference among the three groups with regard to the number of patients with hard exudates and serous retinal detachment.
At baseline in four eyes, the number of HF was graded to be few (grade A), in 25 eyes to be moderate (grade B), and in 51 eyes to be many (grade C). Grading of three categories matched in 145 of 160 cases (90.6 %) before and after bevacizumab injection in HF in the outer and inner retinal layers group. For the unmatched 15 cases, the evaluation was performed to make an agreement by the two graders together. The number of HF at baseline was not significantly different between HF in the outer retinal layers group and HF in the inner retinal layers group (Table 1). Also, we analyzed the number of HF after bevacizumab injection. The number of HF became fewer in 63 eyes (78.75 %), was stable in six eyes (7.50 %), and became increased in 11 eyes (13.75 %). The mean number of HF at baseline reduced significantly from 25.71 ± 10.03 to 15.44 ± 9.01 at final visit in all patients (p < 0.001). The change in the number of HF was correlated positively with the change in logMAR BCVA (R = 0.202, p = 0.047) and FT (R = 0.239, p = 0.018).
Baseline BCVA (logMAR) was significantly better in eyes with intact ELM at baseline (p = 0.006), and final BCVA (logMAR) was significantly better in eyes with intact ELM and IS/OS at final visit (p < 0.001, p = 0.003, respectively) (Table 2).
Baseline FT was significantly different among the three groups (p = 0.006), but final FT was not significantly different among the three groups (p = 0.331) after IVB. FT change was significantly different among the three groups (p = 0.002) (Table 3).
Baseline BCVA (logMAR) was correlated positively with baseline FT (R = 0.366, p < 0.001), but final BCVA (logMAR) was not correlated with final FT (R = −0.008, p = 0.942).
ELM status at baseline was not significantly different among three groups (p = 0.253) (Table 4). At the final visit, 15 of 37 eyes (40.54 %) with HF in the outer retinal layers had a disrupted ELM (p = 0.001), while 28 of 37 eyes (75.68 %) with HF in the outer retinal layers had a disrupted IS/OS (p < 0.001). For eyes with HF in the inner retinal layers, three of 43 eyes (6.98 %) had a disrupted ELM, and 10 of 43 eyes (23.26 %) had a disrupted IS/OS at final visit. For eyes with no HF, two of 17 eyes (11.76 %) had a disrupted ELM, and three of 17 eyes (17.65 %) had a disrupted IS/OS at final visit.
The mean percentage of disruption of ELM at baseline was 81.90 ± 23.84 % (range, 16.80∼100 %), and at final visit was 50.39 ± 28.51 % (range, 13.70 ∼100 %). The mean percentage of disruption of ELM at baseline reduced significantly at final visit (p < 0.001). The mean percentage of disruption of IS/OS at final visit was 48.47 ± 27.95 % (range, 11.70∼100 %). The IS/OS disruption length at final visit was correlated positively with the number of HF at final visit (R = 0.359, p = 0.027), and also correlated positively with the final logMAR BCVA (R = 0.504, p = 0.001).
Baseline BCVA (logMAR) was worse in eyes with HF in the outer retinal layers group compared with those in the other two groups, although the statistical significance was only marginal (p = 0.054) (Table 3). When we compared the visual outcomes after IVB, final BCVA (logMAR) was poorer in eyes with HF in the outer retinal layers group than those in the other two groups (p < 0.001).
To evaluate the association of final BCVA (logMAR) with baseline prognostic factors, multivariate analysis was performed. We found that old age (p < 0.001), poor baseline BCVA (logMAR) (p < 0.001), and the HF in the outer retinal layers (p < 0.001) were associated with poor visual prognosis independently (Table 5).
Discussion
This study was performed to analyze HF using SD-OCT in patients who were treated with IVB for ME secondary to BRVO. In our study, only 17 eyes (17.5 %) at baseline had no HF detected on SD-OCT, and these eyes had less disrupted ELM and IS/OS at final visit with better final BCVA. The percentage of eyes with no HF was quite similar to that of a previous study by Ogino et al. [18], who reported the absence of HF in 22.4 % of eyes with BRVO (17.5 % vs 22.4 %, p = 0.456). However, that study [18] just analyzed the characteristics of HF in eyes with RVO. To our knowledge, the current study is the first report showing that optical coherence tomographic HF at baseline could be predictive of visual outcomes after IVB for the treatment of macular edema in patients with BRVO.
Many etiologies explain the possible nature of HF detected on SD-OCT, but the precise nature and molecular constituents of these foci are unknown. These fine HF were found on SD-OCT, but they could not be found on fundus photographs taken simultaneously with SD-OCT. However, the confluent HF on SD-OCT were detected as hard exudates in the corresponding fundus photograph, and a previous study suggested that these fine foci characterized by the same hyperreflectivity as confluent dots might be small intraretinal protein and/or lipid deposits as precursors of hard exudates [17]. We excluded the patients with subfoveal hard exudates because the purpose of this study was to evaluate the correlation between sparse, fine HF and VA. Coscas et al. [21] also suggested that these hyperreflective dots in exudative AMD were most likely microglia cells activated by inflammation, which subsequently swell and spread in all retinal layers. Another possible explanation for the HF is the result of neurodegenerative processes that have been demonstrated in macular telangiectasia and age-related macular degeneration [28, 29]. Also, Uji et al. [24] documented the close association of these HF with photoreceptor status and VA in DME patients, which prompted us to focus our interest on these HF as a predictor in other retinal vascular diseases such as BRVO in this study.
As observed in previous studies such as DME or AMD [21–23], there was a significant reduction in the number of HF after IVB for BRVO in our study. This result suggests that the retinal tissue integrity was enhanced after anti-VEGF treatment as in DME patients [23].
In the current study, HF in the outer retinal layers group was associated with final ELM and IS/OS disruption (p = 0.001, p < 0.001 respectively), and showed poor visual prognosis compared with other two groups after treatment (p < 0.001). These results suggest that ELM and IS/OS disruption did not recover after IVB in HF in the outer retinal layers group, and this might contribute to final visual outcomes. The percentage of disruption of IS/OS at final visit correlated positively with the number of HF at final visit and final logMAR BCVA. These results indicate that the higher the number of HF to be detected at final visit, the higher the percentage of disruption of IS/OS to be detected after treatment at final visit, and the disruption was responsible for the poor final BCVA. Also, the change in the number of HF was correlated positively with the change in logMAR BCVA and FT. This result suggests that as HF was reduced after treatment, the visual acuity improved and FT decreased.
The FT at baseline was correlated with baseline BCVA. Maculae with HF in the outer retinal layers group were significantly thicker than those in the other groups, suggesting that severe breakdown of the blood–retinal barrier might result in further thickening of the retinal layer. The FT at final visit was not related to the final BCVA. This correlation indicated that thicker FT affected the poor baseline BCVA; however, even after resolution of ME, there was still another factor contributing to the poor final BCVA in eyes with HF in the outer retinal layers group. ELM and IS/OS status at final visit were significantly more disrupted in eyes with HF in the outer retinal layers group, indicating that the photoreceptor status was more likely associated with the final BCVA, rather than FT. Our previous study and other reports showed that the integrity of the ELM and IS/OS status was associated with visual outcomes in several retinal diseases [11–16], which is consistent with the present results (Table 2).
The multiple regression analysis showed that old age, poor baseline BCVA, and HF in the outer retinal layers contributed to poor visual prognosis. Because there was no significant difference among three groups with regard to age (p = 0.327) and baseline BCVA (p = 0.054), these two parameters could be excluded. Therefore, HF in the outer retinal layers might be an independent predictor of final VA.
It was previously reported that the zonulae adherentes between the photoreceptors and Müller cells, forming the ELM, can limit the movement of macromolecules [30–32], and that the normal ELM restricts the migration of extravasated lipoproteins or proteins in the inner retinal layers. A disrupted ELM cannot block the migration of extravasated lipoproteins in the inner retinal layers to the outer retinal layers of the ELM, and makes it possible to pass these extravasated blood constituents through the outer retinal layer. These materials are deposited to the outer retinal layers including the photoreceptors. We hypothesized that these migrated HF might be responsible for the damage of photoreceptor status, so we defined the HF in the outer retinal layers from the ELM to the retinal pigment epithelium. As discussed previously, HF might be a subclinical lipid extravasation as a precursor of hard exudates [17], and these baseline HF in the outer retinal layers could lead to photoreceptor disorganization and discontinuity. For this reason, we could find that the eyes with HF in the outer retinal layers group had significantly more ELM disruption and IS/OS disruption at final visit.
The beneficial effect of IVB for the treatment of various retinal vascular diseases has been shown in other studies [3, 9, 10]. One study dealt with the predictive factors after IVB for BRVO, and reported that the prognostic factors for improvement after IVB were baseline BCVA, patient age, and duration of BRVO [33]. In our study, poor baseline BCVA in eyes with HF in the outer retinal layers group showed poor final BCVA, which is consistent with the previous study. We suggest that the location and the number of HF before the treatment could be another prognostic factor to predict visual outcomes after treatment of BRVO. It is believed to be meaningful that we could use SD-OCT not just for the measurement of ME, but also for the predictor after treatment.
The limitations of this study were its retrospective nature, relatively small sample size, and short follow-up period. In addition, we may have missed HF in eyes that were considered as the absence of HF, because the amount, size, and exact location of HF appeared to be highly variable in our study. These HF were round or oval shapes, and of different size regardless of the location of HF. We mainly analyzed the volume scans as well as six radial cross-sectional scans. We also double-checked the 17 eyes without HF to confirm that there was absence of HF in our study. Nevertheless, we are not sure that they did not miss HF which were not scanned. This is a drawback of our retrospective study. Furthermore, the subjective judgement and classification according to the number of HF is another limitation of our study. Prospective, randomized clinical study with a specific software-based demarcation and calculation of HF will be required to reveal the precise pathogenesis and predictive role of these HF in the patients with BRVO in the near future.
This study suggests the clinical relevance of the HF in BRVO patients, which were not detected by indirect ophthalmoscopy and color fundus photography. Baseline HF in the outer retinal layers on SD-OCT might predict the final photoreceptor status and poor BCVA, although ME improved after treatment in BRVO.
References
Jaulim A, Ahmed B, Khanam T, Chatziralli IP (2013) Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina 33:901–910
Channa R, Smith M, Campochiaro PA (2011) Treatment of macular edema due to retinal vein occlusions. Clin Ophthalmol 5:705–713
Feucht N, Schonbach EM, Lanzl I, Kotliar K, Lohmann CP, Maier M (2013) Changes in the foveal microstructure after intravitreal bevacizumab application in patients with retinal vascular disease. Clin Ophthalmol 7:173–178
Lee JY, Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, Park KH, Jo YJ (2013) Baseline characteristics and risk factors of retinal vein occlusion: a study by the Korean RVO Study Group. J Korean Med Sci 28:136–144
Sakimoto S, Kamei M, Suzuki M, Yano S, Matsumura N, Sakaguchi H, Gomi F, Nishida K (2013) Relationship between grades of macular perfusion and foveal thickness in branch retinal vein occlusion. Clin Ophthalmol 7:39–45
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Patz A (1984) Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol 98:374–375
Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF (2005) Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye (Lond) 19:65–71
Demir M, Oba E, Gulkilik G, Odabasi M, Ozdal E (2011) Intravitreal bevacizumab for macular edema due to branch retinal vein occlusion: 12-month results. Clin Ophthalmol 5:745–749
Hoeh AE, Ach T, Schaal KB, Scheuerle AF, Dithmar S (2009) Long-term follow-up of OCT-guided bevacizumab treatment of macular edema due to retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 247:1635–1641
Ota M, Tsujikawa A, Murakami T, Kita M, Miyamoto K, Sakamoto A, Yamaike N, Yoshimura N (2007) Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol 91:1644–1649
Ota M, Tsujikawa A, Kita M, Miyamoto K, Sakamoto A, Yamaike N, Kotera Y, Yoshimura N (2008) Integrity of foveal photoreceptor layer in central retinal vein occlusion. Retina 28:1502–1508
Shin HJ, Lee SH, Chung H, Kim HC (2012) Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 250:61–70
Hayashi H, Yamashiro K, Tsujikawa A, Ota M, Otani A, Yoshimura N (2009) Association between foveal photoreceptor integrity and visual outcome in neovascular age-related macular degeneration. Am J Ophthalmol 148:83–89
Otani T, Yamaguchi Y, Kishi S (2010) Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina 30:774–780
Oishi A, Hata M, Shimozono M, Mandai M, Nishida A, Kurimoto Y (2010) The significance of external limiting membrane status for visual acuity in age-related macular degeneration. Am J Ophthalmol 150:27–32
Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C (2009) Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 116:914–920
Ogino K, Murakami T, Tsujikawa A, Miyamoto K, Sakamoto A, Ota M, Yoshimura N (2012) Characteristics of optical coherence tomographic hyperreflective foci in retinal vein occlusion. Retina 32:77–85
Folgar FA, Chow JH, Farsiu S, Wong WT, Schuman SG, O'Connell RV, Winter KP, Chew EY, Hwang TS, Srivastava SK, Harrington MW, Clemons TE, Toth CA (2012) Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci 53:4626–4633
Christenbury JG, Folgar FA, O'Connell RV, Chiu SJ, Farsiu S, Toth CA (2013) Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 120:1038–1045
Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot-Thoraval F, Bandello F, Souied E (2013) Hyperreflective dots: a new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica 229:32–37
Framme C, Wolf S, Wolf-Schnurrbusch U (2010) Small dense particles in the retina observable by spectral-domain optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci 51:5965–5969
Framme C, Schweizer P, Imesch M, Wolf S, Wolf-Schnurrbusch U (2012) Behavior of SD-OCT-detected hyperreflective foci in the retina of anti-VEGF-treated patients with diabetic macular edema. Invest Ophthalmol Vis Sci 53:5814–5818
Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, Muraoka Y, Ellabban AA, Yoshimura N (2012) Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol 153:710–717
El-Emam S, Chhablani J, Barteselli G, Wang H, Lee SN, Kozak I, Cheng L, Freeman WR (2013) Correlation of spectral domain optical coherence tomography characteristics with visual acuity in eyes with subfoveal scarring after treatment for wet age-related macular degeneration. Retina 33:1249–1257
Oster SF, Mojana F, Brar M, Yuson RM, Cheng L, Freeman WR (2010) Disruption of the photoreceptor inner segment/outer segment layer on spectral domain-optical coherence tomography is a predictor of poor visual acuity in patients with epiretinal membranes. Retina 30:713–718
Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR (2010) The association between percent disruption of the photoreceptor inner segment–outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 150:63–67
Baumuller S, Charbel Issa P, Scholl HP, Schmitz-Valckenberg S, Holz FG (2010) Outer retinal hyperreflective spots on spectral-domain optical coherence tomography in macular telangiectasia type 2. Ophthalmology 117:2162–2168
Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA (2009) Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology 116:488–496
Scholl S, Kirchhof J, Augustin AJ (2010) Pathophysiology of macular edema. Ophthalmologica 224(Suppl 1):8–15
Bunt-Milam AH, Saari JC, Klock IB, Garwin GG (1985) Zonulae adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci 26:1377–1380
Tsujikawa A, Sakamoto A, Ota M, Kotera Y, Oh H, Miyamoto K, Kita M, Yoshimura N (2010) Serous retinal detachment associated with retinal vein occlusion. Am J Ophthalmol 149:291–301
Jaissle GB, Szurman P, Feltgen N, Spitzer B, Pielen A, Rehak M, Spital G, Heimann H, Meyer CH (2011) Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 249:183–192
Conflict of interest
The authors have no financial interest in any aspect of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, JW., Lee, H., Chung, H. et al. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitreal bevacizumab for macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 252, 1413–1421 (2014). https://doi.org/10.1007/s00417-014-2595-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2595-5