Abstract
Background
To investigate whether vascular endothelial growth factor (VEGF) or interleukin-6 (IL-6) contributes to the pathogenesis of macular edema in eyes with branch retinal vein occlusion (BRVO), the correlations between these factors were investigated.
Methods
We studied 25 patients suffering from macular edema with BRVO and 14 patients with nonischemic ocular disease (control group). The degree of retinal ischemia was evaluated in terms of the area of capillary nonperfusion using Scion Images, and the severity of macular edema was examined using optical coherence tomography. Vitreous fluid samples were obtained at the time of vitreoretinal surgery, and VEGF and IL-6 levels in the vitreous fluid and plasma were determined by means of enzyme-linked immunosorbent assays.
Results
Vitreous fluid levels of VEGF and IL-6 were significantly elevated in patients with BRVO compared with control patients (P=0.0011 and P<0.0001, respectively). Also, the vitreous level of VEGF was significantly correlated with that of IL-6 (P=0.0012), and vitreous levels of VEGF and IL-6 were correlated with the size of the BRVO nonperfusion area (P<0.0001 and P=0.0033, respectively). Furthermore, vitreous levels of VEGF and IL-6 were correlated with the severity of macular edema (P=0.0008 and P=0.0191, respectively) and the severity of macular edema of BRVO was significantly correlated with the size of the BRVO nonperfusion area (P=0.0044).
Conclusions
The levels of VEGF and IL-6 are increased in patients with macular edema with BRVO and are significantly correlated with the size of the nonperfusion area and the severity of macular edema. Therefore, they may play a role in macular edema with BRVO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Branch retinal vein occlusion (BRVO) is a common retinal vascular disease sometimes associated with macular edema and results in the loss of visual acuity [13, 17]. BRVO causes blood–retinal barrier (BRB) breakdown by damaging capillary endothelial cells [26], while macular edema is caused by the breakdown of the BRB, leakage of intraretinal fluid from abnormal retinal capillaries, and secretion of vasopermeability factors produced in the retina into the vitreous [1]. A vasopermeability factor produced by tumors was discovered as a substance inducing an increase in vascular permeability [24]. This was confirmed to be the same substance as vascular endothelial growth factor (VEGF), the expression of which was promoted by hypoxia, and attracted attention [1, 25]. Recent studies showed that VEGF causes conformational changes in the tight junctions of retinal vascular endothelial cells and increases vascular permeability [12, 28]. VEGF is localized in various types of retinal cells including retinal pigment epithelial cells, pericytes, endothelial cells, Muller cells, and astrocytes [2, 15], and VEGF is elevated in the vitreous and aqueous humor of patients with central retinal vein occlusion (CRVO) [1, 21]. Many of the effects of VEGF are mediated by other factors, and the events that induce VEGF production can initiate a cascade of factors. However, it is unclear how this occurs. Interleukin-6 (IL-6) is a multifunctional cytokine that may indirectly cause an increase of vascular permeability by inducing the expression of VEGF [8], or else may directly increase endothelial permeability [16].
We previously reported that the aqueous and vitreous levels of VEGF and IL-6 are involved in the pathogenesis of diabetic macular edema (DME) [10, 11], although the relationship between the intraocular expression of VEGF and IL-6 and macular edema with BRVO (as a similar ischemic retinal disease) remains unclear. Fluorescein angiography (FA) demonstrates staining vessel walls and leakage from retinal vessels adjacent to areas of ischemic retinal tissue, suggesting that diffusible agent release from the ischemic retina may occur. To investigate the relationship between these cytokines and macular edema with BRVO, we measured the concentrations of VEGF and IL-6 in the vitreous fluid of patients with BRVO, and also investigated the relationship between the levels of these factors, the size of the nonperfusion area, and the severity of macular edema.
Materials and methods
Patients
Undiluted vitreous fluid samples were harvested at the onset of vitrectomy after informed consent was obtained from each subject following an explanation of the purpose and potential adverse effects of the procedure. This study was performed in accordance with the Helsinki Declaration of 1975 (the 1983 revision), and the institutional review board of the Hiroshima University School of Medicine, Hiroshima Prefectural Hospital and Chuden Hospital, also approved the protocol for the collection of vitreous fluid and blood samples. Vitreous fluid samples were obtained from 25 BRVO patients and 14 patients with nonischemic ocular disease (control group) (Table 1). The patients without BRVO included 11 with a macular hole and 3 with an epiretinal membrane, but none had associated proliferative vitreoretinopathy. The BRVO patients included 10 men and 15 women with a mean±SD age of 64.3±10.4 years, and the patients in the control group included 7 men and 7 women aged 64.4±6.7 years (Table 1). The mean duration of BRVO was 3.8±3.0 months (range 1–10 months) (Table 1). Before surgery, retinal photocoagulation was performed on 13 eyes (mean 395 shots, range 64–932 shots). The inclusion criteria for this study were (1) clinically detectable diffuse macular edema or cystoid macular edema of more than 1-month duration before vitrectomy, (2) a best-corrected visual acuity worse than 20/40 before vitrectomy, and (3) prolonged macular edema even after retinal photocoagulation. Significant macular edema was defined as retinal thickening of one optic disc area or greater in size, involving the fovea [5]. Exclusion criteria for this study included (1) previous intraocular surgery, (2) diabetes with or without retinopathy, (3) iris rubeosis, and (4) a history of ocular inflammation and vitreoretinal disease. Vitrectomy was performed at the Hiroshima University School of Medicine, Hiroshima Prefectural Hospital and Chuden Hospital.
Fundus findings
All patients with BRVO who presented at the Hiroshima University School of Medicine, Hiroshima Prefectural Hospital and Chuden Hospital, were evaluated by biomicroscopic examination using a fundus contact lens with a slit-lamp, and those who had macular edema with BRVO were enrolled in the study. The fundus findings were preoperatively confirmed by standardized fundus color photography and FA, which was performed with a Topcon TRC-50EX fundus camera, image-net system (Tokyo Optical Co Ltd., Japan), and a preset lens with a slit-lamp.
Preoperative and operative fundus findings were recorded for each subject. One grader independently assessed the ischemic occlusion of BRVO from photographs taken with a Topcon 50° digital fundus camera. Panoramic images were made using Photoshop software (Adobe Systems Inc.) and saved in BMP format. The images were then analyzed using the public domain Scion Image program (developed by the Scion Corporation and available on the Internet at http://www.scioncorp.com/) [4]. Scion Image for Windows is the Windows version of Scion Image, which in turn is a version of the Macintosh program, NIH Image, which was written by the National Institutes of Health. For digital fundus photography, the disc area was circumscribed using a cursor and then measured, and the same procedure was repeated for the nonperfusion area. The area of retinal photocoagulation was excluded in calculating the nonperfusion area. The nonperfusion area divided by the disc area was defined as the degree of retinal ischemia.
Optical coherence tomography (OCT) (Zeiss-Humphrey Ophthalmic Systems, Dublin, CA, USA) was performed on each subject within 1 week before vitrectomy. Each fundus was scanned with a measurement beam focused on the horizontal and vertical planes crossing the central fovea, which was determined from the photograph of the fundus. All eyes were examined at scan lengths of 2.8 mm and 5.0 mm. We defined the foveal thickness at the central fovea as the distance between the inner retinal surface and the retinal pigment epithelium, which included serous retinal detachment, and this measurement was automatically determined by computer. In addition to this, the thickness of the neurosensory retina was defined as the distance between the inner and outer neurosensory retinal surfaces [19], and the OCT-measured foveal thickness was defined as the severity of macular edema. The average preoperative foveal thickness was 518±189 μm (range 275–912 μm).
Sample collection
Samples of undiluted vitreous fluid (300–500 μl) obtained at the time of vitrectomy were collected into sterile tubes and rapidly frozen at −80°C.
Blood samples with EDTA-2Na were simultaneously collected and centrifuged at 3000×g for 5 min at 4°C to obtain the plasma and then aliquotted and stored at −80°C until they were assayed.
Measurement of VEGF and IL-6 levels
The concentrations of VEGF and IL-6 were measured by means of enzyme-linked immunosorbent assay (ELISA) using human VEGF and IL-6 immunoassays (R&D Systems, Minneapolis, MN, USA) [10]. The VEGF kit used can detect two of the four VEGF isoforms (VEGF denoted with a 121 subscript and VEGF indicated by a 165 subscript), and the assays were performed according to the manufacturer’s instructions. The levels of these factors in vitreous fluid and plasma were within the detection range of the assays, with the minimum detectable concentration being 15.6 pg/ml for VEGF with a coefficient of variation (CV) for the intra-assay of 5.4% and CV interassay of 6.7% and 0.156 pg/ml for IL-6 with a CV for the intra-assay of 5.5% and CV interassay of 6.8%.
Statistical analysis
All analyses were performed using SAS System 6.12 software (SAS Institute Inc., Cary, NC, USA). The data are presented as the frequencies or means (±SD), and the results are presented as means (±SD) or geometric means (±SD) for data shown on a logarithmic scale. The Mann–Whitney U test and Welch’s t test were used to compare IL-6 and VEGF concentrations between BRVO patients and control subjects. To examine correlations and the relationship among angiogenic factors, the size of the nonperfusion area, and the severity of macular edema, Spearman’s rank-order correlation coefficients were calculated, and the correlations were graphically represented by means of linear regression analysis. A two-tailed P value of less than 0.05 was considered to be statistically significant.
Results
Vitreous levels of VEGF and IL-6
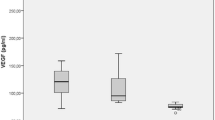
Vitreous fluid levels of VEGF were significantly elevated in patients with BRVO [1263 pg/ml (31.2–4,710)] compared with those of the control subjects [59.0 pg/ml (31.2–109) (P=0.0011; Fig. 1a). Vitreous fluid levels of IL-6 were significantly elevated in patients with BRVO [42.1 pg/ml (0.945–192)] compared with control subjects [1.29 pg/ml (0.3–3.68)] (P<0.0001; Fig. 1b).
a Vascular endothelial growth factor (VEGF) concentrations in the vitreous fluid of nonischemic control and branch retinal vein occlusion (BRVO) patients with macular edema (*P=0.0011). b Interleukin-6 (IL-6) concentrations in the vitreous fluid of nonischemic control and BRVO patients with macular edema (*P<0.0001)
Linearity of the regression model was evaluated by means of residual plot analysis in the regression diagnosis. Based on this result, a linear regression model was better than a nonlinear polynomial regression model. There was also a significant relationship between the vitreous concentration of VEGF and that of IL-6 in the BRVO patients (ρ=0.6086, P=0.0012). A higher level of VEGF was associated with a higher level of IL-6 (y=0.02x+17.89) (Fig. 2).
The vitreous levels of VEGF were significantly correlated with the size of the nonperfusion area of BRVO (ρ=0.8632, P<0.0001). Vitreous levels of IL-6 were also significantly correlated with the size of the nonperfusion area of BRVO (ρ=0.5642, P=0.0033). A greater size of the nonperfusion area was associated with higher increases in the levels of VEGF and IL-6 (y=50.44x+75.44 and y=1.01x+17.66, respectively) (Fig. 3a,b).
a Correlation between the degree of retinal ischemia and the vitreous levels of vascular endothelial growth factor (VEGF). The degree of retinal ischemia was evaluated in terms of the capillary nonperfusion area using Scion Images. VEGF levels in the vitreous fluid were significantly correlated with the size of the nonperfusion area of branch retinal vein occlusion (BRVO) (ρ=0.8632, P<0.0001). b Correlation between the degree of retinal ischemia and the vitreous levels of IL-6. IL-6 levels in vitreous fluid were significantly correlated with the size of the nonperfusion area of BRVO (ρ=0.5642, P=0.0033). A greater size of the nonperfusion area was associated with higher increases in the levels of VEGF and IL-6 (y=50.44x+75.44 and y=1.01x+17.66, respectively)
The vitreous levels of VEGF were significantly correlated with the severity of macular edema of BRVO (ρ=0.6250, P=0.0008). Vitreous levels of IL-6 were also significantly correlated with the severity of macular edema of BRVO (ρ=0.4653, P=0.0191). A higher level of VEGF or IL-6 was associated with a greater increase in the retinal thickness (y=0.08x+412.90 and y=2.03x+434.10, respectively) (Fig. 4a,b). In addition, the severity of macular edema of BRVO was significantly correlated with the size of the nonperfusion area of BRVO (ρ=0.5494, P=0.0044). A greater size of the nonperfusion area was associated with a greater increase in the retinal thickness (y=4.28x+417.38) (Fig. 5).
a Correlation between the severity of macular edema and vitreous levels of vascular endothelial growth factor (VEGF). The severity of macular edema was evaluated using optical coherence tomography (OCT). VEGF levels in vitreous fluid were significantly correlated with the severity of macular edema (ρ=0.6250, P=0.0008). b Correlation between the severity of macular edema and the vitreous levels of interleukin-6 (IL-6). IL-6 levels in vitreous fluid were significantly correlated with the severity of macular edema (ρ=0.4653, P=0.0191). A higher level of VEGF or IL-6 was associated with a greater increase in the retinal thickness (y=0.08x+412.90 and y=2.03x+434.10, respectively)
There was no significant difference in the level of VEGF or IL-6 between the group with retinal photocoagulation (13 eyes) and the group without retinal photocoagulation (12 eyes) (data not shown, P=0.7852 and P=0.2766, respectively).
Vitreous and plasma levels of VEGF and IL-6
Vitreous levels of VEGF were significantly higher than the plasma levels [79.0 pg/ml (15.6–244), P<0.0001; Table 2], and there was no correlation between the VEGF vitreous and plasma levels of this cytokine (ρ=−0.1409, P=0.5018). Also, vitreous levels of IL-6 were significantly higher than the plasma levels [6.46 pg/ml (0.15–139), P<0.0001; Table 2], and no correlation was observed between the vitreous and plasma concentrations of this cytokine (ρ=−0.1606, P=0.4432).
Vitreous and plasma levels of VEGF and IL-6 versus clinical factors
Neither the size of the nonperfusion area nor the severity of macular edema was significantly correlated with the presence of hypertension (data not shown, P=0.5374 and P=0.2895, respectively), and there was no significant relationship between the vitreous fluid levels of VEGF and age or hypertension (data not shown, P=0.9844, P=0.6069, respectively). There was also no significant relationship between the vitreous fluid levels of IL-6 and these factors (data not shown, P=0.1637 and P=0.5056, respectively).
Discussion
The present study showed that the vitreous fluid levels of VEGF and IL-6 were significantly elevated in patients with BRVO compared with control subjects. Since VEGF is involved in the pathogenesis of not only vasopermeability increase but also vascular occlusion through the increased expression of adhesion molecules, the elevation of VEGF and IL-6 may cause BRVO [14, 18]. However, the levels of VEGF and IL-6 were low in the control group without BRVO including hypertensive patients, suggesting that VEGF and IL-6 elevation is unlikely to precede the onset of BRVO, and that their expression is likely elevated after its onset.
The vitreous level of VEGF was significantly correlated with that of IL-6 in the BRVO patients. Although a correlation between VEGF and IL-6 in DME has been reported [10], there have been no reports of a correlation in BRVO-associated macular edema. IL-6 indirectly induces VEGF [8], but it is not clear how IL-6 and VEGF interact in BRVO-associated macular edema.
We speculated that the expression of VEGF and IL-6 is also increased by ischemia in BRVO and evaluated the degree of retinal ischemia in terms of the area of capillary nonperfusion. The vitreous levels of VEGF and IL-6 were significantly correlated with the size of the nonperfusion area of BRVO, and our results suggest that a higher degree of ischemia is associated with the elevated expression of VEGF and IL-6. These findings are supported by previous reports that found that VEGF mRNA expression in retinal pericytes is increased in concordance with the extent of hypoxia [2], and that IL-6 mRNA expression in cultured endothelial cells under hypoxic conditions is elevated in a time-dependent manner [3, 20, 29].
Quantitative analysis of the retinal thickness by OCT has been reported to be clinically useful as a method of evaluating the therapeutic effects on macular edema [6, 23]. By measuring the foveal thickness using OCT as the severity of macular edema with BRVO, we found that the vitreous levels of VEGF and IL-6 were correlated with the severity of macular edema. Furthermore, the severity of macular edema of BRVO was significantly correlated with the size of the nonperfusion area of BRVO. Patients with BRVO show significant breakdown of the BRB [7, 26], and this may contribute to vasogenic macular edema with BRVO. Intravitreous injection of VEGF produces retinal edema, dilated and tortuous vessels, and capillary closure in adult primates [27]. In addition, VEGF causes the rearrangement of actin filaments and increases endothelial permeability by promoting the phosphorylation of the tight junction proteins ZO-1 and occludin [12, 28]. Similar to VEGF, IL-6 induces increased endothelial permeability in vitro through the rearrangement actin filaments, thus changing the shape of endothelial cells in a dose- and time-dependent manner [16]. In addition, increased IL-6 expression affects the integrity of protein ZO-1, leading to endothelial barrier dysfunction [9]. The results of this study and past reports suggest that vascular occlusion is accompanied by increased expression of VEGF and IL-6 in patients with BRVO, resulting in BRB breakdown and increased vascular permeability and leading to the development and progression of macular edema in BRVO. Three patients with extensive vascular occlusion involving one or more quadrants of the fundus had marked macular edema (increases in retinal thickness). In addition, the findings frequently showed the fundus to be associated with increased vascular permeability such as hemorrhages and hard exudate (data not shown). We previously reported that aqueous and vitreous levels of VEGF and IL-6 are correlated with the severity of DME and increased vascular permeability in patients with DME [10, 11]. The results of this study suggest that increased intraocular expression of VEGF and IL-6 may play a role in macular edema in patients with BRVO as in patients with DME, which is a similar ischemic retinal disease.
The levels of both VEGF and IL-6 in the vitreous fluid were higher than their corresponding plasma levels, while there was no correlation between the vitreous and plasma levels of VEGF or IL-6 as shown in Table 2. These results suggest that the elevation of VEGF and IL-6 levels in the vitreous fluid is not related to breakdown of the BRB and/or ocular blood. Although VEGF [2, 15] and IL-6 [22] are produced by cells in the ocular tissue, the sites of production and the expression of their receptors in eyes with macular edema with BRVO have not been clarified. Accordingly, further investigations are required to define the relationship between macular edema with BRVO and these molecules.
In conclusion, the current study showed that vitreous levels of VEGF and IL-6 are significantly higher in the vitreous fluid of patients with macular edema with BRVO than in control subjects, and that vitreous levels of VEGF and IL-6 are significantly correlated with the size of the nonperfusion area and severity of macular edema. In addition, the severity of macular edema of BRVO is significantly correlated with the size of the nonperfusion area of BRVO. Thus, increased intraocular expression of VEGF and IL-6 may play a role in macular edema in patients with BRVO.
References
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA (1995) Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 113:1538–1544
Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL (1999) Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol 277:L1057–L1065
Arnarsson A, Stefansson E (2000) Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Investig Ophthalmol Vis Sci 41:877–879
Battaglia PM, Saviano S, Ravalico G (1999) Grid laser treatment in macular branch retinal vein occlusion. Graefe Arch Clin Exp Ophthalmol 237:1024–1027
Campochiaro PA (2004) Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Investig Ophthalmol Vis Sci 45:922–931
Chahal PS, Fallon TJ, Kohner EM (1986) Measurement of blood–retinal barrier function in central retinal vein occlusion. Arch Ophthalmol 104:554–557
Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ (1996) Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271:736–741
Desai TR, Leeper NJ, Hynes KL, Gewertz BL (2002) Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 104:118–123
Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S (2003) Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 110:1690–1696
Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S (2002) Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 133:70–77
Gardner TW, Antonetti DA, Barber AJ, Lieth E, Tarbell JA (1999) The molecular structure and function of the inner blood–retinal barrier. Penn State Retina Research Group. Doc Ophthalmol 97:229–237
Gutman FA, Zegarra H (1974) The natural course of temporal retinal branch vein occlusion. Trans Am Acad Ophthalmol Otolaryngol 78:178–192
Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP (2002) Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 160:501–509
Lutty GA, McLeod DS, Merges C, Diggs A, Plouet J (1996) Localization of vascular endothelial growth factor in human retina and choroids. Arch Ophthalmol 114:971–977
Maruo N, Morita I, Shirao M, Murota S (1992) IL-6 increases endothelial permeability in vitro. Endocrinology 131:710–714
Michels RG, Gass JD (1974) The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol 78:166–177
Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, Adamis AP (2000) Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 156:1733–1739
Otani T, Kishi S (2000) Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol 129:487–494
Pearlstein DP, Ali MH, Mungai PT, Hynes KL, Gewertz BL, Schumacker PT (2002) Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler Thromb Vasc Biol 22:566–573
Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E (1998) Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 105:412–416
Planck SR, Dang TT, Graves D, Tara D, Ansel JC, Rosenbaum JT (1992) Retinal pigment epithelial cells secrete interleukin-6 in response to interleukin-1. Investig Ophthalmol Vis Sci 33:78–82
Saika S, Tanaka T, Miyamoto T, Ohnishi Y (2001) Surgical posterior vitreous detachment combined with gas/air tamponade for treating macular edema associated with branch retinal vein occlusion: retinal tomography and visual outcome. Graefe Arch Clin Exp Ophthalmol 239:729–732
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Silva RM, Faria de Abreu JR, Cunha-Vaz JG (1995) Blood–retina barrier in acute retinal branch vein occlusion. Graefe Arch Clin Exp Ophthalmol 233:721–726
Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP (1996) Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103:1820–1828
Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA (1999) Cellular mechanisms of blood–retinal barrier dysfunction in macular edema. Doc Ophthalmol 97:217–228
Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D (1995) Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem 270:11463–11471
Acknowledgements
The authors thank Drs. Makiko Yamasaki, Koji Jian, Masaki Imada, Tomoko Yokoyama and Eiichirou Sugimoto for their assistance in collecting the vitreous and plasma samples and in performing the ophthalmological examinations. We also thank Katsunori Shimada of the Department of Biostatistics, STATZ Corporation, Tokyo, for assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noma, H., Minamoto, A., Funatsu, H. et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefe's Arch Clin Exp Ophthalmo 244, 309–315 (2006). https://doi.org/10.1007/s00417-004-1087-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-1087-4