Abstract
Purpose
To evaluate the association of normal-tension glaucoma and peripapillary choroidal thickness.
Participants
Sixty-one patients with normal-tension glaucoma in one eye.
Methods
Spectral domain optical coherence tomography (SD-OCT) scans were obtained to estimate peripapillary choroidal thickness in a group of unilateral normal-tension glaucoma patients. The average peripapillary choroidal thicknesses of the glaucomatous eye and the nonglaucomatous eye of each patient were compared, and the choroidal thickness underlying the retinal nerve fiber layer defect in the glaucomatous eye was compared with the choroidal thickness of a compatible position in the contralateral normal eye. The associations of peripapillary choroidal thickness with independent parameters including the presence of glaucoma, age, sex, refractive error, axial length, central corneal thickness, intraocular pressure, visual field mean deviation, visual field pattern standard deviation, and systemic disease were assessed with mixed model univariate and multivariate analyses.
Results
The average peripapillary choroidal thickness was not statistically significantly different in the glaucomatous and nonglaucomatous eyes of the patients (P = 0.52). There was no definite difference between the choroidal thickness underlying the retinal nerve fiber layer defect in the glaucomatous eye and the choroidal thickness of a compatible position in the contralateral normal eye, indicating that there was no correlation of the retinal nerve fiber layer with choroidal thickness. Age (P = 0.004) and axial length (P ≤ 0.0001) were negatively associated with peripapillary choroidal thickness.
Conclusions
In unilateral normal tension glaucoma, there was no significant intereye difference in choroidal thickness measured with SD-OCT. The structural features of the choroid may not be associated with normal-tension glaucoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although glaucoma is one of the leading causes of blindness worldwide, its pathophysiology is still being studied. In addition to a mechanical theory, a vascular theory has been suggested for glaucoma, especially normal-tension glaucoma. In that respect, the relationship of choroidal structure and function to glaucoma has been reported [1, 2]. As the subjects and the methods used to measure choroidal thickness vary, there are disagreements between reports. Histological studies in secondary angle closure glaucoma show diminished choroidal thickness, especially in the parapapillary region, and other angiographic techniques show thinner choroids in glaucoma than in normal eyes [3, 4]. However, the thickness of the choroid in glaucomatous eyes showed an increase of 20 % compared with normal eyes when measured with A- and B-scan echography [5].
To evaluate the relationship between choroidal thickness and glaucoma, we designed a study in patients with unilateral normal-tension glaucoma (NTG). The background for this study is as follows. (1) NTG is suggested to be related to vascular dysregulation. (2) NTG is highly prevalent in South Korea [6, 7]. (3) Although primary glaucoma is believed to be a bilateral disease, there is a gap in the presentation time of glaucoma between the two eyes. We often encounter patients with a normal eye that did not show glaucomatous changes until a long time after the other eye was diagnosed with NTG. (4) To exclude confounding systemic factors, patients with unilateral NTG were selected [8]. Although it is common to compare choroidal thicknesses of normal subjects and glaucoma subjects, investigators cannot examine all systemic and ocular factors that may differ between the two groups, and therefore cannot compensate for confounding factors in the results. Age, axial length, central corneal thickness, and diastolic ocular perfusion pressure are significantly associated with choroidal thickness in glaucoma patients, and others have reported contrary results in normal populations [9, 10]. Unknown factors may still exist that influence choroidal thickness, and these may become a limitation on evaluation [5]. Good reproducibility is reported with Spectralis OCT, and this method provides better visualization of inner and outer retinal segments [11].
We further determined the effects of age, sex, refractive error, axial length, central corneal thickness, intraocular pressure, glaucoma severity, and systemic diseases on peripapillary choroidal thickness.
Methods
The study was approved by the Samsung Medical Center Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. This was a prospective cross-sectional study. After excluding poor images, 61 patients with normal-tension glaucoma in only one eye were enrolled at a glaucoma clinic. All patients underwent full ophthalmic examinations, including visual acuity, refraction, axial length, slit-lamp biomicroscopy, intraocular pressure with Goldmann applanation tonometer, gonioscopy, binocular optic disc evaluation, fundus photography and visual field test (perimetry using a Humphrey visual field analyzer, Carl Zeiss Inc., Dublin, CA, USA, and Swedish Interactive Threshold Algorithm Standard 30–2). The diagnostic criteria for normal-tension glaucoma were as follows: repeatable untreated intraocular pressure <21 mmHg with open angle, glaucomatous optic disc damage and compatible abnormal visual field test results consisting of pattern standard deviation <5 %, glaucoma hemifield test results outside normal limits, or both, in at least two consecutive and reliable examinations. Three glaucoma specialists (WS, HK, C, and CK) independently evaluated the stereo color fundus photographs and automated visual field to differentiate glaucomatous from normal eyes. The contralateral eye of each patient was ophthalmologically normal, no glaucomatous optic disc change with no visual field deficit.
Patients under 18 years of age or with a best corrected visual acuity of <20/40, inflammatory eye disease, exfoliation or pigment dispersion syndrome, ocular trauma, disease involving the retina and choroid, nonglaucomatous optic neuropathy, or visual field loss were excluded.
Peripapillary choroidal thickness was measured with the OCT (Spectralis software, version 4.0; Heidelberg Engineering, Heidelberg, Germany) by the same experienced operator.
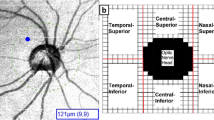
After acquiring the images, two readers (blinded to the diagnostic and other ocular parameters of the subjects) measured the choroidal thickness as described in a previous study [12]. One segmentation line was placed on the RPE/Bruch’s membrane interface to represent the inner, and another line was placed on the sclerochoroidal interface to represent the outer boundaries of the choroid (Fig. 1). We excluded patients with a poorly visualized interface. The retinal thickness algorithm function was then used to automatically generate the thickness of the choroid. A 360° 3.4-mm diameter peripapillary circle scan was performed using a standard protocol for RNFL assessment, centered on the optic disc, and represented as a linear strip and choroidal thickness for every 30° of the circle. To assess reproducibility, two independent readers individually measured peripapillary choroidal thicknesses at every 30° interval of the circle, and one reader measured choroidal thickness three times on different days.
Measurement of peripapillary choroidal thickness. The boundaries of the choroid were measured by manual segmentation. Upper red line indicates the RPE/Bruch’s membrane interface and the lower red line indicates the sclerochoroidal interface. The green line, which the operator can move, shows the choroidal thickness calculated automatically using the retinal thickness algorithm function of SD-OCT
We compared the choroidal thickness of the glaucomatous eye and the nonglaucomatous eye by two methods. The primary analysis compared the average peripapillary choroidal thicknesses of the two eyes. Then we calculated the proportion of patients who showed statistically significant differences in the peripapillary choroidal thicknesses of the two eyes. We further compared the choroidal thickness underlying the retinal nerve fiber layer defect in the glaucomatous eye and the choroidal thickness of a compatible position in the contralateral normal eye (Fig. 2).
Comparison of peripapillary choroidal thickness between the glaucomatous eye and the nonglaucomatous contralateral eye. a Normal eye with no retinal nerve fiber defect or glaucomatous optic nerve changes (arrow). b Glaucomatous eye; the arrow indicates the retinal nerve fiber defect (at 5–6 o’clock). The choroidal thickness underlying the retinal nerve fiber defect was measured. The choroidal thickness under the intact retinal nerve fiber layer at a compatible location in the contralateral nonglaucomatous eye (at 6–7 o’clock) was also measured
Statistical analysis was conducted using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). Intraobserver and interobserver agreement was evaluated with the intraclass correlation coefficient (ICC). 95 % confidence intervals (CI) for ICC were corrected by Bonferroni’s method due to multiple testing. The peripapillary choroidal thicknesses of the two eyes were compared using the two statistical methods mentioned previously. The first method was the paired t test and the second was the chi-square test for one-way frequency, after calculating the percentage of patients who showed inter-eye differences in choroidal thickness among the total subjects. The same statistical method was applied when comparing the choroidal thickness underlying the retinal nerve fiber layer defect lesion in the glaucomatous eye and the choroidal thickness of a compatible position in the contralateral normal eye. The associations between peripapillary choroidal thickness and various factors were analyzed using mixed model univariable and multivariable analyses, in which the independent variables were the presence of glaucoma, age, sex, refractive error, axial length, central corneal thickness, intraocular pressure, visual field mean deviation, visual field pattern standard deviation, and systemic disease.
Results
Four patients were excluded due to a poorly visualized interface. A total of 61 eyes (31 right and 30 left and glaucomatous eyes) were enrolled in the study. The mean age of the subjects was 52.63 years, and the male to female ratio was 0.97:1. All participants were South Korean. The results of an inter-eye comparison of basic ocular parameters are shown in Table 1. There was no significant difference in refractive error, axial length, and central corneal thickness (all P > 0.05). There was a statistically significant difference in visual field mean deviation and in pattern standard deviation between the glaucomatous eye and nonglaucomatous eye (all P < 0.0001). Intraclass correlation coefficients (95 % CI) for the measures obtained by both observers and intraobservers in peripapillary scans were 0.936 (0.58–0.99) and 0.943 (0.869–0.979), showing good agreement.
Inter-eye comparison of peripapillary choroidal thickness
The average choroidal thickness in the glaucomatous eye was 142.67 ± 53.90, and that in the nonglaucomatous eye was 140.60 ± 49.04. There was no statistically significant difference in these values between the two eyes (P = 0.52). 52.94 % of all patients showed increased choroidal thickness in the glaucomatous eye, while the other 47.06 % showed a decreased valued in the glaucomatous eye. These proportions were not significantly different (P = 0.73). A comparison of the peripapillary choroidal thickness underlying the retinal nerve fiber layer defect in the glaucomatous eye and that underlying the intact retinal nerve fiber layer in the same location in the contralateral normal eye also showed no statistically significant difference (P = 0.96, Fig. 2). In this comparison, 44.12 % of the patients showed increased choroidal thickness in the glaucomatous eye and the rest showed decreased thickness in the glaucomatous eye. Again, these proportions were not statistically significantly different (P = 0.49).
Associations of peripapillary choroidal thickness with various factors
With a mixed model using subject effects as random effects, univariable analysis showed negative associations of age and axial length with average peripapillary choroidal thickness (estimate −1.3, P = 0.03, estimate −15.04, P = 0.04, respectively). There were no statistically significant correlations with other variables, including sex, refractive error, central corneal thickness, intraocular pressure, visual field mean deviation, visual field pattern standard deviation, and systemic disease (all P > 0.05; data not shown). In terms of associations with the peripapillary choroidal thickness underlying the retinal nerve fiber layer defect, only axial length showed a negative correlation (estimate −18.601, P = 0.0004). In mixed model multivariable analysis, age and axial length were significantly negatively associated with average peripapillary choroidal thickness (Table 2). The presence of glaucoma and the degree of glaucoma severity were not statistically significantly associated with peripapillary choroidal thickness. When the peripapillary choroidal thickness underlying the retinal nerve fiber layer defect was analyzed, similar results were obtained (Table 3).
Discussion
Hemodynamic alterations with both low perfusion pressure and insufficient autoregulation are suggested to be related to the pathogenesis of NTG [13]. The role of the choroid in the pathogenesis of glaucoma is incompletely understood, and studies report varying results regarding the function of the choroid in glaucoma [3, 4]. To understand this influence, we limited the subjects to those with unilateral NTG. Glaucoma is believed to be a bilateral disease, so we cannot exclude the possibility that the contralateral normal eye (no glaucoma present yet) has similar conditions to those in the glaucomatous eye. Although Spectralis OCT provides better visualization of the ocular posterior segment and is highly reproducible compared with previous instruments, it reflects the static structure, not the hemodynamics of choroidal circulation flow. It is therefore possible that we cannot detect changes in choroid hemodynamics with Spectralis OCT, even if such changes exist.
There is disagreeement among reports about the relationship of the choroid and glaucoma, due to the different study designs used and subjects included. Tanabe et al. [14] report that the thinner choroid inferior to the optic disc may be a natural anatomical architecture of normal eyes, and this area may be more susceptible to hypoxia or elevated intraocular pressures, indicating a possible role of the choroid in glaucoma. In histological sections of eyes with secondary angle closure, decreased choroidal thickness was observed [3]. Spaide also mentions that patients with age-related choroidal atrophy may be at higher risk for glaucoma [15]. However, Margolis and Spaide [16] report that choroidal thickness seems to vary topographically within the posterior pole. It is hard to simply compare the results of previous studies because of differences in the measurement methods used and study subjects included [3, 4, 16]. Erlich and coauthors report no significant difference in peripapillary choroidal thickness between patients with NTG and those with primary open angle glaucoma (P > 0.05 in all sectors, P = 0.92 in global choroidal thickness) [17]. In another cross-sectional study, peripapillary choroidal thickness was significantly thinner in the normal and normal-tension glaucoma patients in inferior, inferonasal, and inferotemporal locations [18]. However, a significant correlation of RNFL with choroidal thickness were observed in the superior sector, but they included only 50 NTG patients in their study [18]. Our results did not show a significant difference in average peripapillary choroidal thickness due to the presence of normal-tension glaucoma. Glaucoma is often a disease with focal damage, especially to the inferotemporal or superotemporal retinal nerve fiber layers. Thus, we further compared the peripapillary choroidal thickness specific to a retinal nerve fiber layer defect with the peripapillary choroidal thickness of an area with intact retinal nerve fiber in the contralateral normal eye. In this approach, there was no statistically significant difference between the two eyes. The peripapillary choroidal thickness in the glaucomatous eye in our study was 142.67 ± 53.90 and that in the nonglaucomatous eye was 140.60 ± 49.04, lower than the average normal peripapillary choroidal thickness reported: 203.83 ± 68.36 [9]. In studies suggesting that the choroid contributes to glaucoma, the authors supposed that the blood supply to the prelaminar portion of the optic nerve is derived in part from the choroid [1]. However, the retinal nerve fiber layer—the site of the glaucomatous defect—is dependent on the central retinal artery, and there are extensive anastomoses between the capillary bed of the anterior optic nerve head and the prelaminar and laminar regions. If insufficient choroid does cause glaucomatous optic nerve injury, an accompanying retinal nerve fiber layer defect should be observed. When we analyzed the structural features of the choroid with Spectralis OCT, we found definite differences in the presence of glaucoma, but the retinal nerve fiber defect was not associated with choroidal thickness.
Among the ocular and systemic parameters, peripapillary choroidal thickness was negatively associated with age and axial length. In particular, the dependence of choroidal thickness on axial length was stronger than the influence of age (estimated value −29.06 for axial length vs. −1.99 for age). Changes in eye length associated with defocus are modulated by changes in both scleral growth and choroidal thickness, the net effects of which result in an anterior or a posterior movement of the retina toward the image plane [19]. In this context, we wondered whether a defocus, i.e., visual impairment, in advanced glaucoma could change the choroid. According to these results, the degree of visual field defect was not associated with choroidal thickness. Also, similar to the results obtained in other studies, the retinal nerve fiber layer was not found to be associated with choroidal thickness [12]. Another study reports that central corneal thickness is also associated with choroidal thickness, but our study showed no association. These differences could result from differences in the subjects included in the two studies [8]. In future studies using Spectralis OCT, these influencing factors, especially age and axial length, should be considered.
In conclusion, this study suggests that there is no significant inter-eye difference in choroidal thickness in patients with unilateral normal-tension glaucoma. Therefore, the structural features of the choroid may not be associated with normal-tension glaucoma. In addition, age and axial length should be considered when investigators measure choroidal thickness with Spectralis OCT.
References
Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–48.
Hayreh SS, Revie IH, Edwards J. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54:461–72.
Kubota T, Jonas JB, Naumann GO. Decreased choroidal thickness in eyes with secondary angle closure glaucoma. An aetiological factor for deep retinal changes in glaucoma? Br J Ophthalmol. 1993;77:430–2.
Yin ZQ, Vaegan, Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6:23–32.
Cristini G, Cennamo G, Daponte P. Choroidal thickness in primary glaucoma. Ophthalmologica. 1991;202:81–5.
Galassi F, Giambene B, Varriale R. Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2011;52:4467–71.
Kim CS, Seong GJ, Lee NH, Song KC. Prevalence of primary open-angle glaucoma in central South Korea: the Namil Study. Ophthalmology. 2011;118:1024–30.
Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118:1571–9.
Ho J, Branchini L, Regatieri C, Krishnan C, Fujimoto JG, Duker JS. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011;118:2001–7.
Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–6.
Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119:119–23.
Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–5.
Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93.
Tanabe H, Ito Y, Terasaki H. Choroid is thinner in inferior region of optic disks of normal eyes. Retina. 2012;32:134–9.
Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–10.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5.
Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011;92:189–94.
Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012;28(12):29. doi:10.1186/1471-2415-12-29.
Read SA, Collins MJ, Sander BP. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010;51:6262–9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suh, W., Cho, H.K. & Kee, C. Evaluation of peripapillary choroidal thickness in unilateral normal-tension glaucoma. Jpn J Ophthalmol 58, 62–67 (2014). https://doi.org/10.1007/s10384-013-0290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-013-0290-4