Abstract
Purpose
The purpose of this study is to compare choroidal thickness in juvenile open angle glaucoma (JOAG) and healthy controls using spectral domain optical coherence tomography (SD-OCT) and study its correlations.
Methods
In this case–control study, 56 eyes of 28 JOAG patients and an equal number of controls were recruited. SD-OCT was used to measure the choroidal thickness (ChT), in the macular region at 5 locations: subfoveal, 1500 µm and 3000 µm nasal and temporal to the foveal center, and in the peripapillary region at 6 locations: up to 1500 µm, nasal and temporal to the disc, respectively. The ChT and its correlations with age, intraocular pressure, cup-to-disc ratio, central corneal thickness, mean deviation, and axial length were studied.
Results
The average macular ChT in JOAG was 306.30 ± 56.49 µm vs. 277.12 ± 64.68 µm in controls. The average peripapillary ChT in JOAG was 197.79 ± 44.05 µm vs. 187.24 ± 38.89 µm in controls. The average total ChT (p = 0.042), the average macular ChT (p = 0.022), the subfoveal ChT (p = 0.022), the ChT 1500 µm (p < 0.001), and 3000 µm temporal to the fovea (p = 0.002) were significantly thicker in the JOAG group. In the JOAG group, the average macular ChT had a significant negative correlation with age, whereas axial length was positively correlated with the average peripapillary ChT.
Conclusions
In this South Asian cohort of JOAG, the average total ChT, average macular ChT, subfoveal ChT, and ChT at 1500 µm, and 3000 µm temporal to the fovea were significantly thicker when compared to healthy controls.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Juvenile open angle glaucoma (JOAG) is a rare disorder diagnosed between the ages of 3 and 40 years [1,2,3]. It is believed to occur as a result of the immaturity of outflow pathways (angle dysgenesis) and is usually inherited as an autosomal dominant trait with mutations in the myocilin protein [4,5,6]. Intraocular pressure (IOP) is usually high, with medical therapy frequently insufficient and requiring surgery[7,8,9].
The maximum blood flow per mass of tissue in the human body is found in the choroid [10,11,12]. The vulnerability of the peripapillary choroidal circulation to IOP rise has been studied with conflicting findings; however, fluorescein angiography studies in normal tension, ocular hypertension, and open angle glaucoma has revealed perfusion abnormalities of the optic nerve head, choroid, and retina, impaired ocular blood flow, filling defects, and delays in peripapillary choroidal filling, which may contribute to glaucomatous optic neuropathy [10,11,12,13,14,15]. Ocular blood flow is an elaborate and complicated network involving the retinal, choroidal, and retrobulbar vasculature [10]. There is evidence that choroidal thickness (ChT) and circulation are involved in glaucoma pathogenesis and may be compromised in chronic open angle glaucomas [11, 12]. In angle closure subjects, the choroid is thicker, and in primary open angle glaucomas, it is not significantly thinner or thicker when compared with healthy controls [11, 12] It is questionable whether the process of open angle glaucoma largely affects or involves blood perfusion in the choriocapillaris and if ChT is a reliable additional diagnostic biomarker in glaucoma pathogenesis. Optical coherence tomography (OCT) allows for a quantitative assessment of the choroid, which may be combined with a qualitative and functional assessment to provide assistance in glaucoma management. Choroidal thickness has not been studied in JOAG. Since there are conflicting results and the relationship is unclear, further studies on glaucoma pathomechanisms are warranted.
The rationale of this study is to compare ChT between JOAG and age-, gender-, central corneal thickness (CCT)-, and axial-length (AL)-matched controls and to observe any differences and correlations with other parameters.
Methods
Study design: A cross-sectional case–control study.
Recruitment
Inclusion criteria were patients younger than 40 years of age diagnosed with JOAG [56 eyes of 28 patients] who were recruited from 1st August, 2021 to 31st July, 2022, with convenience sampling after a comprehensive eye exam including visual acuity, refraction, Goldmann tonometry, gonioscopy, slit lamp exam, fundus evaluation, pachymetry, optical coherence tomography of the optic disc, nerve fiber layer assessment and ganglion cell complex thickness evaluation and SITA-standard central 24–2 perimetry (Humphrey visual field analyser Model 720, Carl Zeiss® Meditex Dublin, CA, USA). JOAG was diagnosed on the basis of an open angle on gonioscopy, glaucomatous optic disc changes, raised intraocular pressure or visual field defects on Humphrey perimetry central 24–2 with reliable indices. Other causes of possible glaucoma like secondary glaucoma, previous glaucomatous damage, steroid usage, uveitis, trauma, angle closure was excluded out on the basis of history and examination. These JOAG subjects were included at first presentation to the clinic at the stage of diagnosis, before topical medication was started; which was done after perimetry and OCT imaging. Inclusion criteria for healthy controls (56 eyes of 28 subjects) (age, gender, CCT, and axial length-matched (p > 0.05)) was best-corrected Snellen visual acuity of 6/6 (LogMAR 0.0), normal anterior segments, intraocular pressure (IOP) ≤ 21 mm Hg, healthy, perfused optic discs and no retinal pathology. Controls were recruited from the OPD after a thorough eye exam, and then pachymetry, axial length measurement, and OCT scans were performed. Systemic diseases were excluded as well on the basis of history. Informed consent was taken from the participants as well as controls. The study was approved by the Ethical Review Committee of Fauji Foundation Hospital, Rawalpindi, Pakistan [492/RC/FFH/RWP], which is according to the Declaration of Helsinki.
Ophthalmic examination
The examination protocol followed for every case included slit lamp biomicroscopy (Takagi Slit lamp microscope SM-10N®) with fundus assessment (Volk Superfield® lens), Goldmann applanation tonometry (Haag-Streit®), and Posner 4-mirror gonioscopy (Ocular®). Quantel Medical® Axis- II PR France A-scan was used to calculate axial length (AL) via the contact method [11 MHz], optical pachymetry, and then optical coherence tomography of the optic nerve head and macula were done with SOCT Copernicus REVO80® SD-OCT (Optopol Technology, Software Version 10.0.1, Poland) (840 nm superluminescent diode source, transverse resolution of 12 µm, axial resolution of 5 µm, and 80 000 A-scan/sec scanning speed).
Choroidal thickness measurement
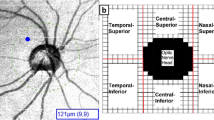
The subjects were explained the OCT examination procedure that it was a chin and forehead mounted, non-contact device and that they need to look into the lens and focus on the internal fixation target and blink freely. They were asked to hold still while the images were captured manually, taking care to prevent motion, blink, or other errors in examination. The chorioretinal setting of the OCT was used for clear visualization of the choroid. For peripapillary choroid assessment, a horizontal 7 X 7 mm 3D macular scan was taken centred at the fovea with a scan angle set to 0°, and a horizontal 6 X 6 mm 3D optic disc scan was taken using an internal fixation target. For the macular choroid evaluation, a 7 × 7 mm macular 3D scan was taken separately. All images were manually captured ensuring a quality index (QI) ≥ 7 without any errors or artefacts. To reduce diurnal variability, all measurements were made between 9:00 am and 12:00 noon. A single skilled observer performed the ChT analysis twice in the macular region. For macular choroidal thickness, the vertical distance between the hyper-reflective lines corresponding to the retinal pigment epithelium and the sclera-choroidal junction were measured manually with a ‘line measuring tool’ to determine the ChT. Each eye underwent a total of five measurements at the foveal centre, nasally and temporally, 1500 µm, and 3000 µm from the foveal center, also measured manually with the line measuring tool. (Fig. 1) The peripapillary choroidal thickness was measured twice with the line measurement tool, nasally & temporally, 500 µm, 1000 µm, and 1500 µm from the Bruch’s membrane opening (BMO), identified manually in every case. (Fig. 2) The choroidal thickness measurement has been detailed in a previous study [16].
Statistical analysis
The data was analysed using IBM SPSS statistics version 20. For the variables, frequencies, means, and standard deviations were computed for the descriptive data. Both eyes were included in the data which was entered as within subjects’ fashion. The average macular ChT, the average peripapillary ChT, the average total ChT, retinal nerve fiber layer (RNFL) thickness in four quadrants and on average, the average and sectoral ganglion cell complex (GCC) thickness were computed via SPSS. The paired t-tests were used to compare means between the JOAG group and controls. The means of the variables were compared across genders and between the two eyes using the independent t-tests. The correlation between the variables was evaluated using Pearson's correlation coefficient and linear regression analysis. A p-value of 0.05 or lower was regarded as significant. The missing data analysis was done for the JOAG group. Regression based imputation was used for the missing data.
Results
Mean age of the participants in the JOAG group was 20.79 ± 8.68 years [Range 8–38 years]. The majority were female in 20 cases (71.4%) and the rest were males; 8 (28.6%). The mean subfoveal ChT in the JOAG group was 380.09 ± 71.64 µm vs. 344.71 ± 82.72 µm in the control group (p = 0.022). The average macular ChT in the JOAG group was 306.30 ± 56.49 µm vs. 277.12 ± 64.68 µm in the control group (p = 0.022). The average peripapillary ChT in the JOAG group was 197.79 ± 44.05 µm vs. 187.24 ± 38.89 µm in the control group (p = 0.232). The average total ChT in the JOAG group was 247.11 ± 40.02 µm vs. 228.09 ± 45.55 µm in the control group (p = 0.042). The choroid was observed to be thicker in the JOAG group in all locations except 3000 µm nasal to the fovea. ChT was significantly more in the JOAG group at the subfoveal location, 1500 µm & 3000 µm temporal to the fovea, at an average at the macula, and at an average total (p < 0.05). Figure 3 displays total choroidal thickness, average macular choroidal thickness, and peripapillary choroidal thickness between JOAG and healthy controls.
Table 1 depicts the baseline characteristics, macular and peripapillary ChT and paired differences between the two groups.
The average and inferior RNFL thickness were significantly less in the JOAG group (p < 0.05). The sectoral and average RNFL thickness in the two groups are displayed in Table 2.
The sectoral GCC was compared between the two groups in 6 locations; superior, superonasal, superotemporal, inferior, inferonasal, inferotemporal, and on average and was found to be significantly less in the JOAG group in all locations (p < 0.001), shown in Table 3. Figure 4 displays the average RNFL and GCC thicknesses of the two groups.
All the variables were analysed for correlation with the Pearson’s correlation coefficient and linear regression analysis. The subfoveal ChT (r = -0.367 p = 0.005) and average macular ChT were significantly negatively correlated with age (r = -0.364 p = 0.006). The average peripapillary ChT was positively correlated with axial length (r = 0.268 p = 0.046).
Discussion
Glaucoma is a multifaceted optic neuropathy, characterized by neurodegeneration of retinal ganglion cells, retinal nerve fiber layer thinning, optic nerve head changes, and a controversial mechanism of damage. Its genesis, progression, and visual field loss have also been linked to the choroid, in addition to other known factors like IOP, age, family history, genetics, myopia, vascular dysregulation, blood pressure, and CSF pressure, according to research conducted previously. [12, 17,18,19,20,21]
Role of choroid in glaucoma pathogenesis
Amongst all the vascular beds in the human body, the choroid has the highest perfusion rate with a blood flow rate of 1400 mL/min per 100 g tissue [17,18,19,20,21]. The choroidal vasculature is responsible for about 70–80% of the ocular blood flow (OBF) [17,18,19,20,21]. Neurohumoral and local processes are used to autoregulate the choroidal blood flow (ChBF). Although, ChBF remains stable over a high range of ocular perfusion pressure (OPP), it is thought to have impaired autoregulation in glaucomatous eyes. Vascular factors may be responsible for glaucoma pathogenesis. Hence, the choroid merits being thought of as a prospective participant. The peripapillary region appears to be impacted by the choroidal vasculature, and the choroid may affect IOP modulation in open angle glaucoma [14, 22,23,24]. The uveoscleral outflow occurs into the supraciliary and suprachoroidal space and may also be affected by the choroid. Aqueous has direct access from the anterior chamber to the ciliary muscle's interstitial spaces, and from there to the supraciliary and suprachoroidal regions, as there is no continuous cellular layer on the anterior iris face. There is ongoing debate on the method by which fluid from the supraciliary and suprachoroidal areas leaves the eye: either by diffusion or by osmotic absorption by the choroid and then into the vortex veins [14, 22,23,24]. Being a dynamic tissue, the ChBF can be directly impacted by a number of variables, including OPP, blood pressure, IOP, and even emotional and stress levels. While measuring choroidal characteristics, the circadian cycle and other relevant physiologic elements should be taken into account. It is crucial to understand how the macular and peripapillary choroidal circulation and glaucomatous optic neuropathy (GON) are related [25,26,27,28,29].
Conflicting reports on choroid status in open angle glaucoma
There have been conflicting reports on the status of the choroid in primary open angle glaucoma (POAG) with laser doppler flowmetry suggesting reduced choroidal perfusion [30]. In the Leuven Eye Study, which is the largest clinical trial on blood flow in glaucoma utilizing retinal oximetry, dynamic contour tonometry, enhanced-depth OCT, and colour Doppler imaging of the retrobulbar vessels, [31]; comparison of POAG, normal-tension glaucoma (NTG), ocular hypertension (OHT), glaucoma suspects and healthy controls, it was observed that the glaucoma groups had a greater mean ocular perfusion pressure than the control group, with lower retrobulbar velocities, greater retinal venous saturation, and asymmetry in choroidal thickness seen in glaucoma groups. Laser interferometry too has revealed reduced optic disc perfusion in OAG [32]. Color Doppler imaging has shown ocular blood flow (OBF) decrease and choroidal thinning in a recent study on OHT [33]. An OCT angiography study on juvenile OHT subjects has revealed that nasal-inferior and temporal peripapillary vessel density (VD) were slightly lower and the peripapillary temporal VD showed a weakly negative correlation with IOP, while VD of five locations of the radial peripapillary capillaries showed a strong positive correlation with high CDR. There was a slight positive correlation of certain macular regions with high IOP [34].
Histological evidence of loss of choroidal vasculature, choriocapillaris thinning and loss of larger choroidal blood vessels in GON, and choroidal thinning have also been reported [24,25,26,27,28,29]. The depth of the choroid tissue is primarily regarded as a biomarker of sound functional tissue margins, similar to the RNFL thickness. With the rise of OCT investigations, it is strongly advised to synthesize the data available to address ongoing issues with obtaining reliable, repeatable results. [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]
Choroid characteristics in JOAG
Studies depicting a relationship between JOAG and the choroid are scarce, with only Abou Shousha and Nabil [35] reporting their results mixed with POAG; suggesting a thinner choroid in both juvenile and POAG, compared to healthy controls. This is a novel study reporting the results of JOAG and ChT in a South Asian cohort with spectral-domain OCT. In this study, with the exception of the macular area 3000 µm nasal to the fovea, the choroid was found to be thicker in the JOAG group in every location. In the subfoveal region, 1500 µm and 3000 µm temporal to the fovea, at an average at the macula, and at an average total, the ChT was substantially higher in the JOAG group (p < 0.05) when compared with healthy controls. These findings suggest that choroidal vasculature may not be significantly affected in JOAG eyes or that choroidal thinning may not be a feature of this glaucoma sub-type.
Contrasting studies on choroidal thickness and POAG
Numerous researchers have presented conflicting reports on the relationship between either peripapillary or macular or total choroidal thickness and open angle glaucoma, with most reports refuting an association and reporting no significant differences [12, 29, 36,37,38,39,40,41,42,43]. Some have reported a thinner ChT [44, 45] in POAG, while other studies reported a thickened choroid in POAG with increased vessel diameter vertically and luminal area [46]. Sclerotic glaucomatous optic neuropathy [14, 47] has been associated with a thinner ChT and since juvenile patients are less likely to have atherosclerosis, it is possible that decreased ChT may not be a found in JOAG. The relationship between POAG and choroidal thickness is still debatable despite significant attempts at understanding it. Table 4 summarizes the various studies on ChT in various types of open angle glaucomas done previously.
Correlations and associations of choroidal thickness
Significant negative correlations of the subfoveal ChT (r = -0.367 p = 0.005) and average macular ChT (r = -0.364 p = 0.006) were observed with age in our study. The average peripapillary ChT was positively correlated with axial length (r = 0.268 p = 0.046). IOP, CCT, CDR, and MD were not significantly correlated with ChT. Nakakura et al. [48] in their study of ChT and variables in POAG found significant negative correlations with age and axial length and comparisons, but no correlation with CCT or MD. Maul et al.[38] found significant correlations of a thin choroid with older age, greater AL and thicker CCT. Sacconi et al. [43] found a correlation of ChT with age and axial length, but not with IOP. Lin et al. [49] also reported no association of peripapillary ChT with white-on-white MD, but a significant correlation with blue-on-yellow MD in open angle glaucoma. Kutluksaman et al. [42] failed to find correlation of ChT with CDR in their research. Li et al. [50] reported no correlation with MD. So, currently there are conflicting results and more good quality studies are needed to establish a definitive correlation.
Strengths and limitations of the study
Strengths of this study are that it is the first cross-sectional study to report choroidal thickness in exclusively JOAG patients, both at the macula and peripapillary area.
Limitations of this study include small sample size, a single-center study, and manual ChT measurements with SD-OCT. Also, a South Asian ethnicity may be a limitation towards the generalization of findings.
It is absolutely essential to try to understand the exact relationship between ChT, choroidal circulation and GON. The advent of OCT has provided us a direct view of this vascular structure with great importance. Substantial prospective and perhaps longitudinal studies are the need of the hour to delineate the precise connection between glaucoma and the choroid.
Conclusions and future perspectives
When compared to healthy controls, this South Asian JOAG cohort showed significant differences in the average total ChT, average macular ChT, subfoveal ChT, and at 1500 µm and 3000 µm temporal to the fovea; the choroid also on average being thicker in the macula and peripapillary regions. Further OCT studies are warranted to reach conclusive evidence.
Data availability
Data sharing statement: The data for this study is available from Open Science framework with the DOI: https://doi.org/10.17605/OSF.IO/Z564W and link https://osf.io/z564w/?view_only=5d4d064cf9a844acbd38e641cdb0e85d
References
Selvan H, Gupta S, Wiggs JL et al (2022) Juvenile-onset open-angle glaucoma - A clinical and genetic update. Surv Ophthalmol 67(4):1099–1117. https://doi.org/10.1016/j.survophthal.2021.09.001
Yanoff M, Duker JS (2009) Ophthalmology. Third Edition. Mosby: St. Louis.p 12
Ciociola EC, Klifto MR (2022) Juvenile open angle glaucoma: current diagnosis and management. Curr Opin Ophthalmol 1;33(2):97–102. doi: https://doi.org/10.1097/ICU.0000000000000813.
Jafer Chardoub AA, Blair K (2022) Juvenile Glaucoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Gupta V, Srivastava RM, Rao A et al (2013) Clinical correlates to the goniodysgensis among juvenile-onset primary open-angle glaucoma patients. Graefes Arch Clin Exp Ophthalmol 251(6):1571–1576. https://doi.org/10.1007/s00417-013-2262-2
Melamed S, Blum Meirovitch S, Leshno A (2022) Juvenile Open-Angle Glaucoma. In: AlbertDM, Miller JW, Azar DT et al(eds) Albert and Jakobiec's Principles and Practice of Ophthalmology. Springer, Cham https://doi.org/10.1007/978-3-030-42634-7_187
Pathania D, Senthil S, Rao HL et al (2014) Outcomes of trabeculectomy in juvenile open angle glaucoma. Indian J Ophthalmol 62(2):224–228. https://doi.org/10.4103/0301-4738.101074
Qiao Y, Tan C, Chen X, et al (2021) Gonioscopy-assisted transluminal trabeculotomy versus goniotomy with Kahook dual blade in patients with uncontrolled juvenile open-angle glaucoma: a retrospective study. BMC Ophthalmol 16;21(1):395. doi: https://doi.org/10.1186/s12886-021-02159-z
Yeung HH, Walton DS (2010) Goniotomy for juvenile open-angle glaucoma. J Glaucoma 19(1):1–4. https://doi.org/10.1097/IJG.0b013e3181a2fa31
Allingham RR, Damji KF, Freedman S, et al (2011) Shields Textbook of Glaucoma. Sixth Edition. Wolters Kluwer Health/Lippincott Williams & Wilkins; New DehliP 59–720
Jonas JB (2014) Glaucoma and choroidal thickness. J Ophthalmic Vis Res 9(2):151–153
Wang W, Zhang X (2014) Choroidal thickness and primary open-angle glaucoma: a cross-sectional study and meta-analysis. Invest Ophthalmol Vis Sci 28;55(9):6007–14. doi: https://doi.org/10.1167/iovs.14-14996
Plange N, Kaup M, Weber A et al (2004) Fluorescein Filling Defects and Quantitative Morphologic Analysis ofthe Optic Nerve Head in Glaucoma. Arch Ophthalmol 122(2):195–201. https://doi.org/10.1001/archopht.122.2.195
Spaeth G (1975) Fluorescein angiography: its contributions towards understanding themechanisms of visual field loss in glaucoma. Trans Am Ophthalmol Soc 73491- 553
Schwartz B (1994) Circulatory defects of the optic disc and retina in ocular hypertensionand high pressure open-angle glaucoma. Surv Ophthalmol 38supplS23- S34
Nadeem S (2023) Macular choroidal thickness and correlations in a healthy pediatric South Asian cohort: A spectral domain optical coherence tomography study. J Biophotonics 16(7):e202300039. https://doi.org/10.1002/jbio.202300039
Goharian I, Sehi M (2016) Is There Any Role for the Choroid in Glaucoma? J Glaucoma 25(5):452–458. https://doi.org/10.1097/IJG.0000000000000166
Lejoyeux R, Benillouche J, Ong J et al (2022) Choriocapillaris: Fundamentals and advancements. Prog Retin Eye Res 87:100997. https://doi.org/10.1016/j.preteyeres.2021.100997
Forrester JV, Dick AD, Paul G, et al (2016) Chapter 4 - Biochemistry and cell biology. In: The Eye. (Fourth Edition) Saunders, Philadelphia, PA, pp 157–268 https://doi.org/10.1016/B978-0-7020-5554-6.00004-6
Ju WK, Perkins GA, Kim KY et al (2023) Glaucomatous optic neuropathy: Mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog Retin Eye Res 95:101136. https://doi.org/10.1016/j.preteyeres.2022.101136
Schuster AK, Erb C, Hoffmann EM, et al (2020) The Diagnosis and Treatment of Glaucoma. Dtsch Arztebl Int 27;117(13):225–234. doi: https://doi.org/10.3238/arztebl.2020.0225.
Wallman J, Wildsoet C, Xu A et al (1995) Moving the retina: choroidal modulation of refractive state. Vision Res 35(1):37–50. https://doi.org/10.1016/0042-6989(94)e0049-q
Lam K, Lawlor M (2021) Anatomy of the Aqueous Outflow Drainage Pathways. In: Sng CCA, Barton K (eds) Minimally Invasive Glaucoma Surgery. Springer, Singapore, pp 11– 19 https://doi.org/10.1007/978-981-15-5632-6_2
Spraul CW, Lang GE, Lang GK et al (2003) Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res 42:923–932
Yin ZQ, Vaegan MTJ et al (1997) Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma 6(1):23–32
Duijm HF, van den Berg TJ, Greve EL (1997) Choroidal haemodynamics in glaucoma. Br J Ophthalmol 81:735–742
Spraul CW, Lang GE, Lang GK et al (2002) Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res 42(7):923–932. https://doi.org/10.1016/s0042-6989(02)00022-6
Marangoni D, Falsini B, Colotto A et al (2012) Subfoveal choroidal blood flow and central retinal function in early glaucoma. Acta Ophthalmol 90(4):e288–e294. https://doi.org/10.1111/j.1755-3768.2011.02340.x
Jonas JB, Steinmetz P, Forster TM et al (2015) Choroidal Thickness in Open-angle Glaucoma. J Glaucoma 24(8):619–623. https://doi.org/10.1097/IJG.0000000000000063
Samra WA, Pournaras C, Riva C et al (2013) Choroidal hemodynamic in myopic patients with and without primary open-angle glaucoma. Acta Ophthalmol 91(4):371–375. https://doi.org/10.1111/j.1755-3768.2012.02386.x
Abegão Pinto L, Willekens K, Van Keer K et al (2016) Ocular blood flow in glaucoma - the Leuven Eye Study. Acta Ophthalmol 94(6):592–598. https://doi.org/10.1111/aos.12962
Findl O, Rainer G, Dallinger S et al (2000) Assessment of optic disk blood flow in patients with open-angle glaucoma. Am J Ophthalmol 130(5):589–596. https://doi.org/10.1016/s0002-9394(00)00636-x
Bayraktar S, İpek A, Takmaz T et al (2022) Ocular blood flow and choroidal thickness in ocular hypertension. Int Ophthalmol 42(5):1357–1368. https://doi.org/10.1007/s10792-021-02123-2
Chen X, Wang X, Hu X et al (2020) The evaluation of juvenile ocular hypertension by optical coherence tomography angiography. BMC Ophthalmol 20:423. https://doi.org/10.1186/s12886-020-01641-4
Abou Shousha MA, Nabil KM (2017) Comparison between choroidal thickness in normal and glaucomatous eyes using spectral-domain optical coherence tomography. Delta J Ophthalmol 18:104–107
Zhang Z, Yu M, Wang F et al (2016) Choroidal Thickness and Open-Angle Glaucoma: A Meta-Analysis and Systematic Review. J Glaucoma 25(5):e446–e454. https://doi.org/10.1097/IJG.0000000000000275
Verticchio Vercellin A, Harris A, Stoner AM, et al (2022) Choroidal Thickness and Primary Open-Angle Glaucoma-A Narrative Review. J Clin Med 23;11(5):1209. doi: https://doi.org/10.3390/jcm11051209
Maul EA, Friedman DS, Chang DS et al (2011) Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology 118(8):1571–1579. https://doi.org/10.1016/j.ophtha.2011.01.016
Li F, Huo Y, Ma L et al (2021) Clinical Analysis of Macular Choroidal Thickness in Pseudoexfoliative Glaucoma and Primary Open-Angle Glaucoma. J Ophthalmol 16(2021):3897952. https://doi.org/10.1155/2021/3897952
Hosseini H, Nilforushan N, Moghimi S et al (2014) Peripapillary and macular choroidal thickness in glaucoma. J Ophthalmic Vis Res 9(2):154–161
Wang YX, Xu L, Shao L, et al (2014) Subfoveal choroidal thickness and glaucoma. The Beijing Eye Study 2011. PLoS One 11;9(9):e107321. doi: https://doi.org/10.1371/journal.pone.0107321
Ehrlich JR, Peterson J, Parlitsis G et al (2011) Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res 92(3):189–194. https://doi.org/10.1016/j.exer.2011.01.002
Mwanza JC, Hochberg JT, Banitt MR, et al (2011) Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci 18;52(6):3430–5. doi: https://doi.org/10.1167/iovs.10-6600
Kutluksaman B, Yavas GF, Inan S, et al (2019) Choroidal Thickness and its Correlations with Ocular Parameters in Primary Open-angle Glaucoma. Beyoglu Eye J 25;4(3):179–189. doi: https://doi.org/10.14744/bej.2019.86570
Sacconi R, Deotto N, Merz T et al (2017) SD-OCT Choroidal Thickness in Advanced Primary Open-Angle Glaucoma. J Glaucoma 26(6):523–527. https://doi.org/10.1097/IJG.0000000000000661
Cennamo G, Finelli M, Iaccarino G et al (2012) Choroidal thickness in open-angle glaucoma measured by spectral-domain scanning laser ophthalmoscopy/optical coherence tomography. Ophthalmologica 228(1):47–52. https://doi.org/10.1159/000336903
Roberts KF, Artes PH, O’Leary N et al (2012) Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol 130(8):980–986. https://doi.org/10.1001/archophthalmol.2012.371
Nakakura S, Yamamoto M, Terao E, et al (2014) The whole macular choroidal thickness in subjects with primary open angle glaucoma. PLoS One 28;9(10):e110265. doi: https://doi.org/10.1371/journal.pone.0110265.
Lin Z, Huang S, Huang P, et al (2017) The diagnostic use of choroidal thickness analysis and its correlation with visual field indices in glaucoma using spectral domain optical coherence tomography. PLoS One 13;12(12):e0189376. doi: https://doi.org/10.1371/journal.pone.0189376.
Li F, Huo Y, Ma L et al (2021) Correlation Analysis between Macular Choroidal Thickness and Visual Field Mean Defect in Primary Open-Angle Glaucoma. J Ophthalmol 9(2021):5574950. https://doi.org/10.1155/2021/5574950
Funding
No funding was provided for this research.
Author information
Authors and Affiliations
Contributions
Manuscript writing, supervision, data curation, formal analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical Review Committee of Fauji Foundation Hospital, Rawalpindi, Pakistan granted approval for this study which is according to the Declaration of Helsinki [492/RC/FFH/RWP].
Consent for publication
Informed consent was obtained from the patients.
Competing interests
The author has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nadeem, S. Choroidal thickness in juvenile open angle glaucoma: insights from a south asian case–control study. Graefes Arch Clin Exp Ophthalmol (2024). https://doi.org/10.1007/s00417-024-06495-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00417-024-06495-w