Abstract
Purpose
To evaluate automatic peripapillary choroidal thickness (PPCT) measurements in a wide area around the optic disc and various established zones in primary open-angle glaucoma (POAG) patients and age- and sex-matched healthy controls using a new swept-source optical coherence tomography (SS-OCT) device.
Study design
Single center cross-sectional observational study.
Methods
A total of 135 POAG patients and 86 healthy subjects were consecutively enrolled. An optic disc 6.0 × 6.0 mm three-dimensional scan OD was obtained using the SS-OCT Triton. A 26 × 26 cube-grid centered in the optic disc was generated to automatically measure choroidal thickness. Seven choroidal zones were established (superior temporal, central, and nasal; inferior temporal, central, and nasal, and the optic nerve head) and compared between healthy controls and POAG patients.
Results
PPCT was significantly thinner in the central superior, nasal superior, and nasal inferior zones of the POAG subjects. Choroidal thickness in the central superior zone was 124.61 ± 54.95 µm in POAG group vs 156.17 ± 80.89 µm in healthy controls (p = 0.029); in the nasal superior zone, 133.84 ± 58.89 µm in the POAG group vs 168.34 ± 73.45 µm in healthy controls (p = 0.012); and in the nasal inferior zone, 113.45 ± 49.93 µm in the POAG group vs 137.47 ± 65.96 µm in healthy controls (p = 0.049).
Conclusion
Compared with healthy subjects, glaucoma patients present with peripapillary choroidal thinning, especially in the central superior, nasal superior, and nasal inferior zones. The new SS-OCT could be a useful tool to evaluate choroidal thinning, and it could be an additional support to facilitate glaucoma diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main theories regarding glaucoma pathogenesis stress the importance of optic nerve head (ONH) vascularization. The peripapillary choroid provides the main blood supply to the prelaminar region of the ONH [1]. As perfusion anomalies contribute to glaucoma [2], several studies suggest that peripapillary choroidal thickness (PPCT) could potentially serve as a clinical indicator for diagnosing glaucoma [3,4,5].
In many cases, however, ONH analysis is difficult due to features such as a tilted position, a complex distribution of ONH margins, or the presence of drusen or myopic crescents. In these and other situations, supplementary tests such as PPCT analysis could greatly benefit the clinician [6].
The choroid is a dynamic structure, and a thinner choroid is associated with older age [7, 8], higher intraocular pressure (IOP) [9], higher myopia and longer axial length [10, 11]. Efforts to introduce PPCT into the routine clinical arsenal of glaucoma diagnosis, however, have been impeded by measurement difficulties and a relative lack of knowledge about the normal distribution of PPCT [12].
Automated choroidal segmentation over a relatively wide section of PPTC allows for a quantitative and accurate approximation of the actual topography of this area. A better understanding of the normal PPCT distribution will help to elucidate the role of PPCT in glaucoma pathogenesis and facilitate diagnosis [13, 14].
Currently, the differences between normal and glaucomatous eyes are measured from a radial scan surrounding the ONH with a fixed diameter of 3.6 mm at 12 discrete locations (30° apart), rather than on a grid created from many points on the peripapillary choroid [15, 16]. The latest swept-source optical coherence tomography (SS-OCT) technology, however, allows for the measurement of a 26 × 26 cube-grid area of the peripapillary choroid and could provide valuable information about the actual disposition and distribution of a wide area of choroidal tissue surrounding the ONH.
The main aim of this study was to improve our knowledge regarding the thickness distribution of different zones around the ONH by comparing a population of control healthy eyes with a population of glaucomatous eyes, and to define the choroidal thickness distribution in both groups. We then compared the average thicknesses of different topographic zones between glaucomatous and normal eyes to explore the differences and to discuss the potential role of PPTC measurements in routine clinical practice.
Materials and methods
Study subjects
One hundred thirty-five right eyes of 135 glaucoma patients between 60 and 80 years of age who visited our department during the recruitment period were consecutively enrolled in this cross-sectional observational study. Given the influence of age on choroidal thickness [7, 8], and to homogenize study sample, we established an age range for recruitment. All patients were enrolled prospectively in the Glaucoma Diagnostic protocol at Miguel Servet University Hospital, Zaragoza, Spain. Eighty-six age-and sex-matched healthy subjects who visited our department during the recruitment period were selected and enrolled as the control group. Written informed consent was obtained from all participants after explanation of the nature and potential consequences of the research. The study protocol followed the tenets of the Declaration of Helsinki, and ethics approval was obtained from the Clinical Regional Ethics Committee of Aragón (CEICA).
Inclusion criteria consisted of a clinical diagnosis of POAG at a previous visit at least 1 year earlier. The diagnosis of POAG was based on characteristic optic nerve damage on slit-lamp examination (defined as a definite notch in the neuroretinal rim or absence of the neuroretinal rim not due to another known cause) with corresponding visual field (VF) defects, an open-appearing anterior chamber angle, and increased IOP (> 21 mmHg). A glaucomatous VF defect was defined as the presence of three or more significant (p < 0.05) nonedge continuous points with at least 1 at the p < 0.01 level on the same side of the horizontal meridian in the pattern deviation plot, and classified as “outside normal limits” on the Glaucoma Hemifield Test, confirmed on two consecutive VF examinations.
Subjects were excluded if they had vision loss secondary to another eye condition, any laser procedure in the previous 2 months, previous filtration surgery or previous cataract surgery. Other exclusion criteria included extreme refractive errors, such as high myopia (− 6.0 or higher), hyperopia (+ 6.0 or higher), or astigmatism (± 3.0 or higher), acute angle closure glaucoma, and evidence of macular pathologies, vascular or inflammatory diseases, or optic nerve neuropathies other than glaucoma. As we used refractive error as a comparison variable, pseudophakic eyes were also excluded. Participants with clinically significant lenticular opacity using the LOCS III classification were also excluded [17]. The exclusion criteria for lenticular opacity were nuclear color/opalescence greater than NC2 and NO2, respectively, cortical cataract greater than C2, and posterior subcapsular cataract greater than or equal to P1.
Ophthalmological examination
All subjects underwent a complete ophthalmologic examination, including measurements of best-corrected visual acuity (BCVA) (using a Snellen chart at 4 m) and IOP (using a calibrated Goldmann applanation tonometer), slit-lamp examination of the anterior segment, and fundus evaluation. The Humphrey 24-2 Swedish Interactive Threshold Algorithm Standard perimeter (Zeiss Meditec, Dublin, CA, USA) was used to evaluate the VF. Only reliable VFs, defined as those with < 20% fixation errors and < 33% false positives or false negatives were used.VF and OCT examinations were performed within a 2-month period.
Optical coherence tomography measurement
All participants included in the current study had SS-OCT measurements obtained using Deep Range Imaging (DRI) OCT Triton (Topcon Corporation, Tokyo, Japan), software IMAGEnet 6 version 1.19, through dilated pupils. An optic disc of 6.0 × 6.0 mm three-dimensional scan was obtained. This scan combines automatically morphometric optic disc parameters (such as disc area and vertical cup-to-disc ratio) and various peripapillary parameters, including retinal nerve fiber layer and choroidal thicknesses. The subjects were seated and properly positioned. All DRI-OCT images were obtained by a single well-trained technician. The DRI-OCT Triton includes the new SMARTTrack™ tool, which enhances tracking, corrects for motion, and guides the operator to reduce potential errors when acquiring images. Only eyes with good-quality scans were included in the analysis. Good-quality SS-OCT images were defined as those with signal strength ≥ 40 (maximum = 100), and without motion artifacts, involuntary saccades, or overt misalignment of de-centration.
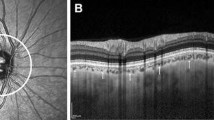
Following data acquisition, a 26 × 26 cube-grid centered in the optic disc was generated to automatically measure choroidal thickness. This grid included 676 cubes of 200 µm around the ONH, including the 88 central cubes corresponding to the ONH area that were not analyzed; thus the DRI-OCT Triton displays choroidal thickness for a total of 588 peripapillary cubes (Fig. 1a). The Bruch membrane and choroidal–scleral interface were delineated with the segmentation algorithm implemented by Topcon.
Peripapillary choroid grid. a Image of the 26 × 26 cube-grid centered in the optic disc of a right eye obtained with deep range imaging (DRI) optical coherence tomography (OCT) Triton (Topcon Corporation, Tokyo, Japan). This grid includes 676 cubes of 200 µm around the optic disc: the 88 central cubes corresponding to the optic disc area are not measured, and the DRI-OCT Triton automatically displays choroidal thickness in each of the 588 peripapillary cubes. b Schematic representation of the peripapillary choroidal thickness zones defined in this study (superior temporal, superior central, superior nasal, inferior temporal, inferior central, and inferior nasal) bounded by a red line
After two experts carefully examined all images, three eyes were excluded due to improperly defined choroidal boundaries, two eyes were excluded due to poor DRI-OCT image quality, and one eye was excluded during the examination due to poor fixation.
Statiscal analysis
For the statistical analysis, the PPCT OCT grid was divided into the following seven zones: superior temporal, superior central, superior nasal, inferior temporal, inferior central, inferior nasal, and the ONH (Fig. 1b). Study zones were set according to their position around the ONH; the central superior zone included 86 cubes directly positioned above the ONH, and the central inferior zone included the 86 points below the ONH. The four squares beyond the ONH were labeled as temporal superior, temporal inferior (104 cubes each), nasal superior, and nasal inferior (104 cubes each).
Once established, the mean PPCT value for each study zone was obtained for the healthy control and glaucoma groups. The ONH was not examined and therefore not included in the statistical analysis. Only right eyes were selected for the statistical analysis.
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 20.0, SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to assess the sample distribution of the variables. BCVA decimal values were converted to the LogMAR equivalent using the following formula [18]: LogMAR = −Log (Decimal Acuity). For quantitative data following a parametric distribution, differences between groups were compared using Student’s t test. For qualitative data, a Chi square test was used for comparison. A logistic regression analysis was performed to know the diagnostic power of the mean choroidal thickness in each of the areas studied. The Pearson correlation coefficients between PPCT in the different areas analyzed with age, IOP and spherical equivalent were calculated. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 221 right eyes were analyzed for statistical purposes: 135 from glaucoma patients and 86 from healthy controls. Mean age of the POAG patients was 70.35 ± 6.13 years (range 60 to 80 years) and that of the healthy controls was 68.88 ± 4.11 years (range 60 to 80 years). Seventy-three (54%) of the 135 subjects in POAG group and 45 (52%) of the 86 subjects in control group were men. Mean spherical equivalent was − 0.08 ± 1.64 D in the POAG group and 0.07 ± 2.76 D in the control group, and there was no significant difference. There were no statistically significant differences in age, sex, and spherical equivalent between groups (p = 0.118; 0.627, and 0.088, respectively). The study population characteristics are summarized in Table 1.
The peripapillary choroid was thicker in the superior sector in absolute terms; statistical comparison between inferior and superior zones, in each of the temporal, central, or nasal position zones revealed significant differences between the superior and inferior areas in control healthy eyes as well as in glaucomatous eyes (Table 2).
Comparison between control and POAG eyes showed a thinner choroidal layer in every zone in the glaucoma group. The central superior, nasal superior, and nasal inferior zones were significantly thinner in the POAG eyes (Table 3). The choroidal thickness in the central superior zone was 124.61 ± 54.95 µm in the POAG group and 156.17 ± 80.89 µm in the healthy controls (p = 0.029); in the nasal superior zone, 133.84 ± 58.89 µm in the POAG group vs 168.34 ± 73.45 µm in the healthy controls (p = 0.012); and in the nasal inferior zone, 113.45 ± 49.93 µm in the POAG group vs 137.47 ± 65.96 µm in the healthy controls (p = 0.049). None of the zones analyzed showed a statiscally significant value in the logistic regression equation. Slight significant correlations were found between each of the six PPCT areas with age (values ranged from −0.204 to −0.266). We did not find any significant correlation between PPCT and IOP or spherical equivalent.
Discussion
Using automated choroidal segmentation software in a wide area around the ONH, we found the thinnest PPCT in the inferior sectors of the optic discs in both glaucoma and control eyes; however, in general the POAG group showed thinner choroidal layer pattern compared with the control group, especially in the nasal and central superior sectors. Until OCT technology provided the possibility of accurately measuring choroidal thickness, histology was the only valid approach to determine the boundaries of the choroidal layer. Different microscopic studies have provided controversial results: some reporting a decrease in the choroidal thickness of glaucomatous eyes [19], while others reporting the opposite, probably due to methodological differences [20].
The first OCT analysis evaluating choroidal thicknesses in eyes with and without glaucoma was focused within the macular area, and again discrepancies over choroidal thinning in glaucomatous eyes are reported [21,22,23,24]. It seems that studying the foveal, temporal, nasal macular, or average macular choroidal thicknesses is not the best approach for evaluating glaucomatous changes [15, 16].
PPCT examination seems to be the most useful approach to diagnosing glaucoma [3,4,5]. But relationship between PPCT and blood supply is still controversial in the available literature. In the Beijing Eye Study [2], the findings do not indicate a significant association between PPCT and blood pressure (BP). Gupta et al. [12], using novel automated choroidal segmentation software in an Asian population did not find any association between BP and PPCT either. However, in a recent review performed by Goharian and Sehi [25], the conclusion was that alterations in BP and ocular perfusion pressure could affect choroidal thickness. Tan et al. [26] report that arterial BP had an effect on subfoveal choroidal thickness. New studies are necessary to elucidate the relationship between BP, choroidal thickness and IOP. These studies need to conduct different measurements during the same day of these factors while keeping strict control of vascular factors. With regard to the profile of the PPCT, our finding of the thinnest PPCT in the inferior region is consistent with other studies. Thus, we believe this area with a thinnest choroid may represent consequently an area of lower blood supply (as the peripapillary choroid is the main source of ONH nourishment), which may predispose the inferior region of the optic nerve to glaucomatous ischaemic damage [27, 28].
The development of enhanced depth imaging spectral-domain optical coherence tomography (EDI-OCT) enables a more accurate measurement of choroidal thickness, but provides conflicting results [29] as well. A recent meta-analysis conducted by Lin et al. [30], demonstrates average PPCT in open-angle glaucoma (OAG) as significantly reduced compared with healthy subjects, especially in the superior and nasal sectors, the same as our results. They included different studies performed with various OCT instruments (spectral domain and SS-OCT).Contrary to this, previous meta-analysis conducted by Wang and Zhang [31] and Zhang et al. [32] show no correlation between PPCT and OAG, but both only included studies using EDI-OCT technology, without SS-OCT. A recent publication performed by Song et al. [33] with SS-OCT, found that the global and all 12 clock-hour PPCT, with the exception of the 10 o’clock sector, were thinner in OAG (even after adjusting for age, axial length and disc area). However, macular choroidal thickness did not remain thinner in glaucoma group after adjusting for age, axial length and spherical equivalent.
The discrepancies among authors may be related to the dynamic and variable nature of the choroid and some methodological and operative aspects like manual segmentation or the limited amount of locations evaluated. For example, spectral domain-OCT evaluates the 12 central degrees around the optic nerve through a 3.4-mm lineal-circle scan to produce a total of only100 points [34].
New technologies such as SS-OCT provide the opportunity for better delimitation of the retinal layers, as well as a much improved method for measuring a large number of points (588 cubes) in a wider peripapillary area (5.2 × 5.2 mm, 20 central degrees) that provides topographic information of the choroidal shape and distribution. Theoretically, with these tools, we could obtain useful information especially in glaucoma patients with a tilted or aberrant optic disc. Further investigations focused on the association between the type of glaucomatous disc damage and the pattern of PPCT, are required to better understand the role of the choroid in glaucoma.
Our study has several limitations. First, we did not collect data on axial length; however, we demonstrated that there was no significant difference in the refractive error among the groups. The axial length and refractive error variables are related, but not interchangeable, as the axial length tends not to change after the second decade of life, except in cases with some pathology. Instead, the refractive error may fluctuate or change due to different factors and, for this reason; we excluded pseudophakic eyes [35]. The results from a recent study by Ikuno and Tano [36] using OCT, however, suggest that refractive error and axial length have a similar relationship with OCT measurements. Second, the choroid can be influenced by circadian changes [26], and we did not perform OCT examinations at the same time in all participants; however, all the OCT scans were acquired in the morning (between 09:00 and 12:00). Third, we only measured choroidal thickness in the right eyes of each participant, and inter-eye differences were not assessed. Fourth, the POAG patients were not divided according to severity, and it would be interesting to investigate whether there is an association between PPCT and glaucoma severity or normal tension glaucoma; or between PPCT and peripapillary retinal nerve fiber layer thickness.
Finally, we have not confirmed the reliability of the automated PPCT measurements obtained with the new SS-OCT (there is only one study that analyzed repeatability of retinal and choroidal thickness with SS-OCT in neovascular age-related macular degeneration) [37]; and it is necessary to investigate the accuracy of this software in other studies. Intradevice repeatability of peripapillary retinal nerve fiber layer thickness and different retinal measurements using the same SS-OCT device (DRI-OCT Topcon) are good, with intraclass correlation coeficients (ICCs) ≥ 0.9 for all sectors in control subjects [38, 39]. Ikuno et al. [40] studied the reliability and reproducibility of manual normal choroidal thickness measurements on EDI-OCT; and interobserver correlation was found to be 0.970 and intervisit correlation was 0.893. So, it's expected that reliabiliby of automated choroidal thickness measurements with SS-OCT will be good.
The present study demonstrates that peripapillary choroidal tissue is thickest superiorly and thinnest inferiorly, both in controls and glaucoma patients. Significant associations were detected between choroidal thickness along different zones of the retinal tissue around the optic disc, and the presence of glaucomatous changes; although these associations were statistically significant in three zones (i.e., central superior, nasal superior, and nasal inferior), the remaining zones were also thinner in glaucomatous eyes. Our findings suggest that glaucoma degeneration affects the choroidal tissue, especially in the superior and nasal areas around the optic nerve.
More studies based on new technology that explores peripapillary thickness in different areas of the choroid, with a careful recording of vascular variables, would clarify the actual role of this evaluation of PPCT in clinical practice.
References
Ho J, Branchini L, Regatieri C, Krishnan C, Fujimoto JG, Duker JS. Analysis of normal peripapillary choroidal thickness via spectral domain opticalcoherence tomography. Ophthalmology. 2011;118:2001–7.
Jiang R, Wang YX, Wei WB, Xu L, Jonas JB. Peripapillary choroidal thickness in adult Chinese: the Beijing Eye Study. Invest Ophthalmol Vis Sci. 2015;56:4045–52.
Roberts KF, Artes PH, O’Leary N, Reis AS, Sharpe GP, Hutchison DM, et al. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol. 2012;130:980–6.
Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012;12:29.
Usui S, Ikuno Y, Miki A, Matsushita K, Yasuno Y, Nishida K. Evaluation of the choroidal thickness using high-penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma. Am J Ophthalmol. 2012;153:10–6.
Sullivan-Mee M, Patel NB, Pensyl D, Qualls C. Relationship between juxtapapillary choroidal volume and beta-zone parapapillary atrophy in eyes with and without primary open-angle glaucoma. Am J Ophthalmol. 2015;160:637–47.
Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011;92(3):189–94.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5.
Arora KS, Jefferys JL, Maul EA, Quigley HA. The choroid is thicker in angle closure than in open angle and control eyes. Invest Ophthalmol Vis Sci. 2012;53:7813–8.
Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–9.
Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50.
Gupta P, Jing T, Marziliano P, Baskaran M, Cheung GC, Lamoureux EL, et al. Peripapillary choroidal thickness assessed using automated choroidal segmentation software in an Asian population. Br J Ophthalmol. 2015;99:920–6.
Duijm HF, van den Berg TJ, Greve EL. Choroidal haemodynamics in glaucoma. Br J Ophthalmol. 1997;81:735–42.
Fuchsjager-Mayrl G, Wally B, Georgopoulos M, Rainer G, Kircher K, Buehl W, et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45:834–9.
Banitt M. The choroid in glaucoma. Curr Opin Ophthalmol. 2013;24:125–9.
Abegão Pinto L, Willekens K, Van Keer K, Shibesh A, Molenberghs G, Vandewalle E, et al. Ocular blood flow in glaucoma—the Leuven Eye Study. Acta Ophthalmol. 2016. doi:10.1111/aos.12962.
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6.
Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–91.
Yin ZQ, Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6:23–32.
Spraul CW, Lang GE, Lang GK, Grossniklaus HE. Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res. 2002;42:923–32.
Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118:1571–9.
Mwanza JC, Hochberg JT, Banitt MR, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–5.
Hirooka K, Fujiwara A, Shiragami C, Baba T, Shiraga F. Relationship between progression of visual field damage and choroidal thickness in eyes with normal-tension glaucoma. Clin Exp Ophthalmol. 2012;40:576–82.
Cennamo G, Finelli M, Iaccarino G, de Crecchio G. Choroidal thickness in open-angle glaucoma measured by spectral-domain scanning laser ophthalmoscopy/optical coherence tomography. Ophthalmologica. 2012;228:47–52.
Goharian I, Sehi M. Is there any role for the choroid in glaucoma? J Glaucoma. 2016;25:452–8.
Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–6.
Hayreh SS. The blood supply of the optic nerve head and the evaluation of it—myth and reality. Prog Retin Eye Res. 2001;20:563–93.
Hayreh SS. The 1994 Von Sallman Lecture. The optic nerve head circulation in health and disease. Exp Eye Res. 1995;61:259–72.
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Lin Z, Huang S, Xie B, Zhong Y. Peripapillary choroidal thickness and open-angle glaucoma: a meta-analysis. J Ophthalmol. 2016. doi:10.1155/2016/5484568.
Wang W, Zhang X. Choroidal thickness and primary open-angle glaucoma: a cross-sectional study and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55:6007–14.
Zhang Z, Yu M, Wang F, Dai Y, Wu Z. Choroidal thickness and open-angle glaucoma: a meta-analysis and systematic review. J Glaucoma. 2016;25:e446–54.
Song YJ, Kim YK, Jeoung JW, Park KH. Assessment of open-angle glaucoma peripapillary and macular choroidal thickness using swept-source optical coherence tomography (SS-OCT). PLoS ONE. 2016;11:e0157333.
Zhang X, Wang W, Aung T, Jonas JB, Wang N. Choroidal physiology and primary angle closure disease. Surv Ophthalmol. 2011;60:547–56.
Chen FK, Yeoh J, Rahaman W, Patel PJ, Tufail A, Da Cruz L. Topographic variation and interocular symmetry of macular choroidal thickness using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:975–85.
Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:3876–80.
Hanumunthadu D, Ilginis T, Restori M, Sagoo MS, Tufail A, Balaggan KS, et al. Repeatability of swept-source optical coherence tomography retinal and choroidal thickness measurements in neovascular age-related macular degeneration. Br J Ophthalmol. 2016. doi:10.1136/bjophthalmol-2016-308999.
Lee SY, Bae HW, Kwon HJ, Seong GJ, Kim CY. Repeatability and agreement of swept source and spectral domain optical coherence tomography evaluations of thickness sectors in normal eyes. J Glaucoma. 2017;26:e46–53.
Min JK, Lee S, Kim JS, Woo JM, Yang HS. Effects of diabetic macular edema on repeatability of retinal nerve fiber layer thickness measurements at the macular and peripapillary area using swept-source optical coherence tomography. Curr Eye Res. 2017;42:307–14.
Ikuno Y, Maruko I, Yasuno Y, Miura M, Sekiryu T, Nishida K, et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5536–40.
Acknowledgements
This manuscript has been edited by SciTechEdit International, LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
L. E. Pablo, None; M. P. Bambo, None; B. Cameo, None; B. Ferrández, None; N. Güerri, None; V. Polo, None; J. M. Larrosa, None; J. M. -Montañés, None; E. G. -Martin, None.
About this article
Cite this article
Pablo, L.E., Bambo, M.P., Cameo, B. et al. The use of zonal analysis of peripapillary choroidal thickness in primary open-angle glaucoma. Jpn J Ophthalmol 62, 41–47 (2018). https://doi.org/10.1007/s10384-017-0538-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-017-0538-5