Summary
Paget’s disease of bone is a disorder of bone remodelling, leading to changes in the architecture and overall appearance of the bone. The disorder may be monostotic or polyostotic and affect any bone in the body, although most commonly it involves the spine, pelvis, skull and femur. This article explores the different imaging modalities used in the assessment of Paget’s disease of bone in its different phases. The relative merits of each imaging modality is discussed with illustrative examples, in particular with respect to radiographs, nuclear medicine bone scan, computed tomography (CT) and magnetic resonance imaging (MRI).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paget’s disease of bone was named after Sir James Paget who first reported the condition as osteitis deformans in 1877 [1]. It is a disease of excessive and abnormal bone remodelling, leading to changes in the architecture and appearance of the bone [2]. The incidence of Paget’s disease is changing, namely reducing, the cause of which is not known [3]. Similarly, the aetiology of Paget’s disease is not fully understood, with theories ranging from genetic to viral causative agents, which is discussed more comprehensively elsewhere in this journal edition. This article discusses the different imaging modalities and their utility in relation to Paget’s disease of bone.
Distribution
Paget’s disease may be monostotic or polyostotic, and the incidence of monostotic disease is increasing. The disease commonly involves the spine and pelvis (30–75 %) sacrum (30–60 %), skull (25–65 %) and femur (25–35 %) [4]. The lower extremities tend to be involved more frequently than the upper extremities, particularly the humerus and scapula; however, the disease can involve any bone in the body [5]. It may be asymptomatic and identified incidentally on imaging performed for unrelated indications. Sometimes the presenting complaint is pain and imaging is thus performed for further investigation [6].
The role of imaging is to detect the disease and its activity, and to determine whether it is the cause of a patient’s particular symptoms.
Phase of disease
Paget’s disease is initially characterised by bone resorption followed by abnormal, disorganised bone deposition, resulting in pathological bone remodelling. Characteristically, the process follows different phases of activity followed by a quiescent phase [7]. The initial phase is termed the active one, during which osteoclastic activity is predominant, thus giving rise to a lytic appearance of the bones. This is followed by a mixed phase, in which there is both osteoclastic and osteoblastic activity, with the osteoblastic activity being predominant. This is then followed by an inactive phase during which the osteoblastic activity declines. A further inactive, sclerotic phase has also been described, during which there is normal or decreased bone activity with persistence of the sclerotic architecture [8]. The different phases of activity have different radiological appearances; however, the hallmark is disorganisation of the bone texture.
Radiographs are a first-line modality for investigating suspected cases of Paget’s disease. In the active phase, the bones typically appear lytic, with excessive bone resorption. In the long bones, the disease typically advances along the diaphysis from a subarticular location. This has been given the term “blade of grass” appearance ([9]; Fig. 1). In the skull the osteolysis may be seen as a zone of lucency, termed osteoporosis circumscripta (Fig. 2). In the tibia, the lytic phase may begin in the diaphysis, rather than in a subarticular location.
In the mixed phase the bones may show features of both the initial lytic and late osteoblastic phase, with the bones displaying osteolysis, coarsening and thickening of the trabeculae; cortical thickening and widening. In the pelvis, this may be seen as thickening of the iliopectineal and ischiopubic lines, a very classic manifestation of Paget’s disease (Fig. 3). Acetabular protrusion is also a common finding. In the spine the vertebral bodies may be expanded, often also involving the posterior elements [10]. In the skull, there may be a “cotton wool appearance” [11, 12].
In the late quiescent phase of Paget’s disease, the previous osteoblastic activity is manifest as osteosclerosis. The coarsened trabecula pattern persists, as does the bony expansion. In the skull, the enlargement of the diploic space is termed a “Tam-O-Shanter” cap, named after the Scottish cap; whereas in the spine, it is labelled as a “picture frame vertebra”.
The different phases may coexist in one bone at any one time and display an array of radiographic features. Identification of Paget’s disease may depend on the activity of the disease, for example, if a patient is asymptomatic during a quiescent phase of disease, this particular area of the body may not be imaged and, therefore, the status of disease in this bone will not be identified. This is relevant in determining whether a patient has monostotic or polyostotic disease. A whole-body radiographic survey in conjunction with a bone scan may be used to determine the activity and distribution of disease [13].
Imaging modalities—strengths and weaknesses
Nuclear medicine scintigraphy (“bone scan”) is an important radiological modality for assessment of Paget’s disease, providing functional imaging as opposed to anatomical imaging. The ability to image the whole skeleton enables assessment of the extent and distribution of active disease. To undertake the study, the patient is injected intravenously with radioactive technetium-99 m which is labelled to hydroxymethylene diphosphonate (Tc99m-HDP). The radioisotope, being a diphosphonate, targets osteoblasts and is a marker of osteoblastic activity. This therefore allows excellent assessment of the phases of Paget’s disease where there is osteoblastic activity. Thus, the initial lytic phase, where osteoclastic activity predominates, can be underestimated in extent on a bone scan [14]. Bone scans are sensitive in Paget’s disease but not specific, as any process causing increased osteoblastic activity will be identified with increased radioisotope uptake. Conversely, although sometimes the pathology is very apparent on a bone scan owing to the intense osteoblastic activity, it is difficult to appreciate on other modalities, as there may not yet be any major structural changes (Fig. 4).
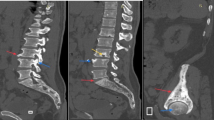
Computed tomography (CT) is complementary to radiographs in assessing Paget’s disease. It provides anatomical detail and gives a three-dimensional assessment of the bone structure. This can be particularly important in assessing the spine, where bony detail can be obscured by overlapping abdominal or thoracic structures (Fig. 5). CT gives excellent detail of the cortical and trabecular bone and is particularly suited to showing the trabecular and cortical thickening, osseous expansion and osteolysis (Fig. 6). In the spine, Paget’s disease may manifest as vertebral sclerosis, which may be termed an “ivory vertebra.” Differentiating Paget’s disease involving the vertebra from malignant conditions may depend on identifying expansion of the posterior elements and trabecular thickening [15]. CT may also be very helpful in assessing the base of skull and facial bones, where radiographs provide limited detail owing to the complex bony anatomy.
T8 vertebral Paget’s disease. Sagittal T1 and T2 MRI images (a) show subtle vertebral expansion and heterogeneity in the marrow signal (arrows) compared with the normal adjacent levels. On CT (b) the vertebral expansion, sclerosis and change in trabecular pattern is more readily appreciated (arrows)
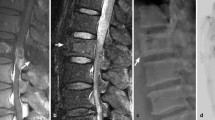
Magnetic resonance imaging (MRI) may also be used in assessment of Paget’s disease; however, the pathology may be subtle and vary depending on the phase of disease. MRI gives a good depiction of bone marrow and soft tissues; however, it is less useful for imaging trabecular bone. Commonly in Paget’s disease, the normal fatty marrow persists and the main clue to the diagnosis on MRI is alteration of the expected contour of the bone (Fig. 7). The spine is commonly imaged with MRI for back pain and changes of Paget’s disease may be overlooked if correlation is not performed with other imaging, such as radiographs [10]. During active remodelling of the long bones, there may be regions of high short tau inversion recovery (STIR) signal within the cortex. In a more quiescent phase, the cortex typically shows low signal on all sequences [16]. The medullary cavity generally shows a seemingly normal fatty marrow signal, like in unaffected bones, spuriously suggesting it is not involved. There may be regions of heterogeneous T1 and T2 signal, corresponding to hypervascular marrow. There may also be regions showing low signal on both T1 and T2 images, representing fibrous tissue or mineralised areas. Following contrast, there may be greater enhancement compared with uninvolved bone, suggesting hyperaemia in the Pagetic bone [17, 18]. Sometimes the manifestation of Paget’s disease can be subtle and overlap with other conditions, namely sclerotic metastatic disease. In cases where definitive diagnosis of Paget’s disease is not possible on imaging grounds alone, biopsy may be required.
The above imaging modalities are used to assess initial status of disease, but also treatment response. Dynamic contrast-enhanced MRI has been used to assess the response of Pagetic bone to bisphosphonate therapy, with patients showing reduced bone enhancement, and therefore reduced vascularity, following treatment [19]. Bone scans can also be used to assess any changes in osteoblastic activity following treatment.
Ultrasound is of limited use in Paget’s disease as the sound waves in the diagnostic spectrum do not penetrate bone and therefore the modality does not give an adequate assessment. However, in Paget’s sarcoma, there may sometimes also be a soft tissue mass which potentially can be assessed with ultrasound.
Fluorine-18 fluorodeoxyglucose positron-emission computed tomography (F-18 FDG-PET-CT) is a functional imaging technique in which positron emitting fluorine-18 is labelled to glucose and data from this component are fused with anatomical imaging from CT. In uncomplicated cases of Paget’s disease, the uptake of F‑18 FDG-PET is modest, as this radiotracer is a marker of glycolysis rather than osteoblastic activity [20–22]. This is in contrast with a conventional Tc99m-HDP bone scan, which is a marker of osteoblastic activity and shows increased uptake in the active phase of disease. F‑18 FDG-PET can be used as a problem-solving tool if the diagnosis of Paget’s disease remains in question on other imaging modalities, as it would not typically be expected to show intense uptake. It may also have some utility in assessment of metastatic disease in cases of Paget’s sarcoma, as malignancy generally has increased F18 FDG-PET uptake; however, this would be performed in a specialist centre. F‑18 FDG-PET is not currently in general clinical use in imaging Paget’s disease of bone, as it is not the optimal radiotracer to show increased bone turnover, carries a high radiation dose, represents a high cost and has relatively limited availability.
Fluorine-18 sodium fluoride positron-emission computed tomography (18 F-NaF PET-CT) is an emerging nuclear medicine technique, where the F‑18 positron emitter is labelled to sodium fluoride which is taken up by the skeleton. Blood clearance is faster and uptake in bone is greater for 18 F-sodium fluoride compared to Tc99m-HDP, and 18 F-NaF PET-CT has the potential to be more sensitive to skeletal pathology compared to Tc99m-HDP bone scanning. However, currently there is insufficient evidence to recommend the routine use of 18 F-NaF PET-CT in Paget’s disease of bone. 18 F-NaF PET-CT is used in imaging skeletal metastases in specialist centres [23] and may replace Tc99m-HDP bone scans in the future, once it becomes more widely available and at a reduced cost.
Complications
Fracture: Pagetic bone is abnormal bone, classified by the World Health Organisation alongside metabolic bone disease. The remodelling of the bone and its disorganisation makes it structurally weaker and more prone to fracture, and, once fractured, it responds differently compared to unaffected bone. Bowing of the tibia and femur may occur, and also in the spine, giving rise to kyphosis [24]. Insufficiency fractures typically are seen on the convexity of long bones and are colloquially termed “banana fractures.” The femur is a common fracture site, often in a subtrochanteric location. In the spine, the weakened pars interarticularis may fracture, resulting in an olisthesis (Fig. 8). Fractures may also involve the vertebral body owing to the weakened bone. Pathological fracture has also been described as a complication of Pagetic bone treated with bisphosphonates secondary to induced osteomalacia [25]. Radiographs are the most appropriate first-line imaging modality for assessing any suspected fracture, with CT reserved for further clarification with equivocal radiographs.
Arthropathy: Osteoarthritis secondary to the altered bone structure and mechanical loading across a joint is a recognised complication of Paget’s disease. One of the commonest sites for arthropathy is the hip, owing to frequent involvement of the pelvis. Protrusio of the acetabulum may be seen secondary to the softening of the bones. Osteoarthritic knee and facet joints are well-known sites of arthropathy complicating Paget’s disease. Similar to suspected fractures, radiographs are the first-line modality for investigating arthropathy. Owing to its greater cost and more limited availability compared to radiography, MRI is typically reserved for cases where more detailed assessment of cartilage and associated internal structures of the joint is needed.
Nerve entrapment: The expansion of the skull which accompanies Paget’s disease may give rise to entrapment of the cranial nerves, with CT being the optimal imaging technique for assessing the skull base. Optic atrophy and papilloedema may arise secondary to skull base enlargement [26]. Deafness may arise secondary to involvement of the middle ear ossicles. Other rare complications include anosmia, trigeminal neuralgia, and facial and bulbar palsy [27]. Expansion of the vertebral bodies may cause spinal stenosis and consequent entrapment of the cauda equina nerve roots, or cord compression. CT and MRI are often used in a complementary manner for investigating this complication, with CT better showing the osseous expansion and MRI detailing the nerve entrapment. Peripheral nerves may also be entrapped, such as the sciatic nerve between the ischium and lesser trochanter, best imaged with MRI.
Malignancy: Pagetic bone may undergo malignant transformation, with cases of osteosarcoma, chondrosarcoma and giant cell tumour reported [5, 24, 28–35]. In Avellino, a town in Italy, four members of one family have been identified as having Paget’s disease of bone complicated by giant cell tumour. This suggests that there may be both a genetic and environmental aetiology to both diseases [36]. Cases of lymphoma arising in Pagetic bone and metastatic disease have also been reported; however, it is not clear whether this is related to the Paget’s disease or coincidental [37, 38].
The greater the disease burden of Paget’s disease, the higher the chance of malignant transformation, with some estimates at between 5–10 % in polyostotic disease [35]. Cases of multifocal sarcomatous degeneration in patients with Paget’s disease have also been reported [39]. The commonest sites of sarcomatous degeneration reflect the typical distribution of disease, with the femur, pelvis (Fig. 9) and humerus comprising the majority of disease locations. Radiographic signs of malignant degeneration are similar to non-Pagetic bone and include osteolysis, cortical destruction, pathological fracture, periosteal reaction and soft tissue mass. On MRI the replacement of normal fatty marrow by tumour can easily be identified [34]. Appearances of sarcomatous degeneration on bone scan may be variable and could be seen as a focal region of relatively decreased radioisotope uptake in the tumour cells against a background of increased isotope uptake in Pagetic bone. This limits the utility of bone scans in identifying malignant degeneration and their use is superseded by MRI. CT may also be used to show any bone destruction associated with the malignancy and any associated soft tissue mass (Fig. 10). Occasionally, ultrasound can be used to assess a soft tissue mass associated with the bone pathology.
Paget’s sarcoma in the left inferior pubic ramus. Frontal radiograph of the pelvis (a) showing destruction of the left inferior pubic ramus. MRI images (b, c) show more clearly the large soft tissue mass. Ultrasound can be useful in the assessment of Paget’s sarcoma, showing a soft tissue mass with abnormal vasculature, as demonstrated on colour Doppler (d)
Paget’s sarcoma right proximal femur presenting as a pathological fracture (a) through a lucent area of bone destruction. The bone destruction was not initially appreciated and the fracture was managed with an internal fixation (b). Subsequent imaging showed further bone destruction with a large soft tissue mass which required resection. Specimen radiograph (c) and gross specimen (d)
Pseudotumour: This is a rare complication of Paget’s disease and is manifest as a large soft tissue mass with extreme bony expansion secondary to the severe bone proliferation and disorganisation, sometimes with haemorrhagic components. This may be mistaken for a malignancy and biopsy is often warranted for histological confirmation [32, 40]. A case of subperiosteal ganglion has also been associated with Paget’s disease of the bone, occurring in the acetabulum [41].
Conclusion
Imaging plays an important role in the diagnosis and follow-up of Paget’s disease. The imaging modalities are complementary, with radiographs remaining vital for disease characterisation and assessing complications. CT provides further detail of bony architecture when needed, particularly in the spine. Functional imaging, namely nuclear medicine bone scanning, is useful for demonstrating the metabolic activity of the disease and providing a broad overview of the whole skeleton. MRI predominantly images the marrow and can therefore underestimate the presence of disease; however, it is invaluable for assessment of complications, such as spinal stenosis and sarcomatous degeneration. With the incidence of Paget’s disease declining, it is useful to review the characteristic imaging features to ensure that the medical community remains aware of its appearance.
References
Paget J. On a form of chronic inflammation of bones (osteitis deformans ). Med Chir Trans. 1877;60:37–64.
Resnick D. Paget disease of bone: Current status and a look back to 1943 and earlier. Am J Roentgenol. 1987;150(2):249–56.
Cundy T. Is Paget’s disease of bone disappearing ? Skeletal Radiol. 2006;35:350–1.
Guyer PB. Paget’s disease of bone : the anatomical distribution. Metab Bone Dis Relat Res. 1981;3(4–5):239–41.
Ansari S, Bonar F, Stalley P, Brown W. Paget’s sarcoma of the patella. Skeletal Radiol. 2015;44:1057–63.
Pratt H, Sampson M. Paget’s disease of bone presenting as chest pain and shoulder immobility. Clin Radiol. 2005;60:407–9.
Al-rashid M, Ramkumar DB, Raskin K, Schwab J, Hornicek F, Santiago A, et al. Paget disease of bone. Orthop Clin North Am. 2015;46(4):577–85.
Wilgram JW. Radiographical and pathological assessment of the activity of Paget’s disease of bone. Clin Orthop Relat Res. 1977;127:43–54.
Schubert F, Siddle KJ, et al. Diaphyseal Paget’s disease : an unusual finding in the tibia. Clin Radiol. 1984;35:71–4.
Dell’Atti C, Cassar-Pullicino VN, Lalam RK, Tins BJ, Tyrrell P. The spine in Paget’s disease. Skeletal Radiol. 2007;36:609–26.
Smith R. Paget’s disease of bone: past and present. Bone. 1999;24(5):1S–2S.
Tehranzadeh J, Fung Y, Donohue M, Anavim A, Pribram HW. Computed tomography of Paget disease of the skull versus fibrous dysplasia. Skeletal Radiol. 1998;27:664–72.
Guanabens N, Rotes D, Holgado S, Gobbo M, Descalzo M, Gorordo J, et al. Implications of a new radiological approach for the assessment of Paget disease. Calcif Tissue Int. 2012;91:409–15.
Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. Radiographics. 2003;23(2):341–58.
Beaudouin C, Dohan A, Nasrallah T, Parlier C, Touraine S, Ea K, et al. Atypical vertebral Paget’s disease. Skeletal Radiol. 2014;43:991–5.
Whitten CR, Saifuddin A. Pictorial review MRI of Paget’s disease of bone. Clin Radiol. 2003;58:763–9.
Boutin RD, Spitz DJ, Newman JS, Lenchik L, Steinbach LS. Complications in Paget disease at MR imaging. Radiology. 1998;209(3):641–51.
Vande Berg BC, Malghem J, Lecouvet FE, Maldague B. Magnetic resonance appearance of uncomplicated Paget’s disease of bone. Semin Musculoskelet Radiol. 2001;5(1):69–78.
Libicher M, Kasperk C, Daniels-Wredenhagen M, Heye T, Kauczoe H, Nawroth P, et al. Dynamic contrast-enhanced MRI for monitoring bisphosphonate therapy in Paget’s disease of bone. Skeletal Radiol. 2013;42:225–30.
Cook G, Maisey M, Fogelman I. Fluorine-18-FDG PET in Paget’ s disease of bone. J Nucl Med. 1997;38:1495–7.
Woo J‑H, Kim S, Choi SJ, Lee YH, Ji JD, Song GG. Diagnosis of Paget’s disease of the pelvis using F‑18 FDG PET/CT. Int J Rheum Dis. 2010;13(4):e51–e54.
Park ET, Kim S. Radiography, Bone scan, and F‑18 FDG PET/CT imaging findings in a patient with Paget’s disease. Nucl Med Mol Imaging. 2010;44(1):87–9.
Lin FI, Rao JE, Mittra ES, Nallapareddy K, Chengapa A, Dick DW, et al. Prospective comparison of combined 18 F-FDG and 18 F-NaF PET/CT vs. 18 F-FDG PET/CT imaging for detection of malignancy. Eur J Nucl Med Mol Imaging. 2012;39(2):262–70.
Lander PH, Hadjipavlou AG. Paget disease with contraction of long bones. Radiol Radiol Soc N Am. 1986;159(2):471–2.
MacGowan J, Pringle J, Morris VH, Stamp T. Gross vertebral collapse associated with long-term disodium etidronate treatment for pelvic Paget’s disease. Skeletal Radiol. 2000;29:279–82.
Isasi C, Sanz J, Hijos M, Vaquero M, Saucedo G, Andreu L. Successful treatment of optic neuropathy in osteitis deformans. Rheumatology. 2002;41:948–50.
Bone HG. Nonmalignant complications of Paget’s disease. J Bone Miner Res. 2006;21(Suppl 2):P64–P68.
Dizon G, Ritchie A, Myskow M. Case report: benign giant cell tumour associated with Paget’s disease of bone. Clin Radiol. 1995;50:269–71.
Hoch B, Hermann G, Klein MJ, Abdelwahab I, Springfield D. Giant cell tumor complicating Paget disease of long bone. Skeletal Radiol. 2007;36:973–8.
Brandolini F, Bacchini P, Moscato M, Bertoni F. Chondrosarcoma as a complicating factor in Paget’s disease of bone. Skeletal Radiol. 1997;26:497–500.
Theodorou DJ, Theodorou SJ, Kakitsubata Y. Imaging of Paget disease of bone and its musculoskeletal complications: review. Am J Roentgenol. 2011;196:64–75.
Gamage N, Kashima T, Mcnally M, Gibbons C, Smith R, Ostlere S, et al. Giant-cell-rich pseudotumour in Paget’s disease. Skeletal Radiol. 2013;42:595–9.
Mcnairn JDK, Damron TA, Landas SK, Ambrose JL. Benign tumefactive soft tissue extension from Paget’s disease of bone simulating malignancy. Skeletal Radiol. 2001;30:157–60.
Sundaram M, Khanna G, El-khoury GY. T1-weighted MR imaging for distinguishing large osteolysis of Paget’s disease from sarcomatous degeneration. Skeletal Radiol. 2001;30(7):378–83.
Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(Suppl 2):P58–63.
Rendina D, Mossetti G, Soscia E, Sirignano C, Insabato L, Viceconti R, et al. Giant cell tumor and Paget’s disease of bone in one family: geographic clustering. Clin Orthop Relat Res. 2004;421:218–24.
Yu T, Squires F, Mammone J, DiMarcangelo M. Lymphoma arising in Paget’s disease. Skeletal Radiol. 1997;26(12):729–31.
Fenton P, Resnick D. Metastases to bone affected by Paget’s disease. A report of three cases. Int Orthop. 1991;15(4):397–9.
Vuillemin-bodaghi V, Parlier-cuau C, Cywiner-golenzer C. Multifocal osteogenic sarcoma in Paget’s disease. Skeletal Radiol. 2000;29(6):349–53.
Tins BJ, Davies AM, Mangham DC. MR imaging of pseudosarcoma in Paget’s disease of bone: a report of two cases. Skeletal Radiol. 2001;30:161–5.
Mansour R, Nanni M, Muthukumar T, Butt S, Cassar-Pullicino VN. Subperiosteal ganglion associated with Paget s disease of bone. Skeletal Radiol. 2005;34:419–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Winn, R. Lalam, and V. Cassar-Pullicino declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Winn, N., Lalam, R. & Cassar-Pullicino, V. Imaging of Paget’s disease of bone. Wien Med Wochenschr 167, 9–17 (2017). https://doi.org/10.1007/s10354-016-0517-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-016-0517-3