Summary
Aim
Reasonable application of laboratory parameters in prevention, diagnosis, treatment and therapy monitoring of osteoporosis.
Target groups
Physicians from different specialist disciplines (general medicine, geriatrics, gynaecology, urology, internal medicine—especially endocrinology and metabolism, nephrology, laboratory medicine, rheumatology, nuclear medicine, orthopaedics, paediatrics, rehabilitation and physical medicine, radiology, social medicine, transplantation medicine, accident surgery), moreover social insurances, hospitals and self-help groups.
Background
Evaluation of aetiology of bone disorders, widening of the therapeutic spectrum for diseases of bone and knowledge on biochemical markers of bone turnover. Improvements in judging the success of therapy and in monitoring the compliance of patients. Research perspectives.
Bases
Scientific literature and guidelines, consensus meetings.
Résumé
Basic and specialized laboratory investigations are important in differentiation between primary and secondary osteoporosis for an adequate therapy. Biochemical markers of bone turnover are an additional aid in evaluation of individual fracture risk. These markers identify responders to bone therapy faster than surveillance of bone mineral density, which helps to improve patient’s compliance too. Characteristics, preanalytic precautions and applications are presented for selected markers of bone resorption and formation and for parameters regulating bone metabolism.
Zusammenfassung
Ziel
Sinnvoller Einsatz der Labordiagnostik zur Prävention, Diagnose, Therapie und Therapieüberwachung der Osteoporose.
Zielgruppe
Ärztinnen und Ärzte für Allgemeinmedizin, Geriatrie, Gynäkologie, Urologie, Innere Medizin (besonders Endokrinologie und Stoffwechsel), Nephrologie, Med. und Chem. Labordiagnostik, Onkologie, Rheumatologie, Nuklearmedizin, Orthopädie, Pädiatrie, Rehabilitation und Physikalische Medizin, Radiologie, Sozialmedizin, Transplantationsmedizin, Unfallchirurgie, sowie Sozialversicherungsanstalten, Krankenanstalten, Selbsthilfegruppen.
Hintergrund
Abklärung der Ätiologie von Knochenerkrankungen. Wachsendes Spektrum der Therapiemöglichkeiten von Knochenerkrankungen und der biochemischen Marker des Knochenstoffwechsels. Verbesserungen in der Beurteilung des Therapieerfolgs und bei der Überwachung der Compliance von Patienten. Forschungsperspektiven.
Grundlagen
Wissenschaftliche Literatur, Leitlinien und Konsens-Gespräche.
Fazit
Routine- und Spezial-Laboruntersuchungen sind für die Unterscheidung zwischen primärer und sekundärer Osteoporose und für die Wahl einer angemessenen Therapie wichtig. Biochemische Marker des Knochenumbaus sind ein zusätzliches Hilfsmittel bei der Abschätzung des individuellen Frakturrisikos. Mit diesen Markern kann ein Ansprechen auf eine knochenspezifische Therapie rascher erfasst werden als mit der Überwachung der Knochenmineraldichte, dies hilft auch die Compliance der Patienten zu verbessern. Eigenschaften, Präanalytik und Anwendung von ausgewählten Markern für Knochen- Resorption und Anbau und von Parametern, die den Knochenstoffwechsel regulieren, werden präsentiert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Once clinically relevant alterations of bone structures are detected by imaging techniques, laboratory investigations serve for further exploration. Discovery of the causes of osteoporosis is an important challenge for the medical laboratory. If reports are positive, targeted treatment may result from basal or expanded laboratory investigations.
Analyses of biochemical bone-markers serve for another purpose, namely for an estimation of the phenomena of the dynamic process of bone turnover. During life, bone metabolism differs in velocity and balance between resorption and formation. Understandably enough, a predominant resorption process together with an elevated rate of turnover will soon become clinically relevant. Thus, the most important prognostics are the estimations of the turnover rate and the net balance. Proving changes in bone turnover by specific therapeutic interventions is another assignment of biochemical bone markers [1, 2].
Osteoporosis
Definition, clinical aspects, epidemiology
Osteoporosis is a systemic skeletal disease, which was characterized by WHO by a decreased bone mineral density (BMD) and microarchitectural deterioration of bone tissue, resulting in an increased risk of fractures [3, 4]. Typical predilection sites for osteoporotic fractures are the distal forearm, vertebral bodies of thoracic and lumbar spine as well as the hip region.
Regarding recent epidemiologic and demographic data from the Western world, it can be assumed that about 46 % of women and 22 % of men will incur an osteoporotic fracture beyond 50 years of age (lifetime fracture risk) [5]. By 2050 the number of global hip fractures will increase to about 6 million per year [6], despite the fact that in some countries, including Austria, a clear levelling-off or even decrease in hip fracture incidence has been demonstrated [7, 8]. Osteoporotic fractures raise huge debits for the health budget as well as substantial bio-psycho-social burden for all persons concerned. Especially fractures of hip and vertebral bodies result in dramatic restraints in quality of life and are associated with an increased rate of mortality [9]. The dramatic increase in frequency of osteoporotic fractures raised the need for adequate methods to determine the individual risk of fractures in these context biochemical markers of bone turnover gained relevance.
Diagnosis of osteoporosis
In suspect bone loss, the five main pillars of the diagnostic exploration include detailed anamnesis and risk evaluation, clinical investigations, conventional X-ray of thoracic and lumbar spine, measurement of BMD and laboratory investigations.
Anamnesis and risk evaluation
An exact anamnesis is important for the diagnosis as well as for the estimation of the fracture risk, which is essential to derive accurate therapeutic measures. The main risk factors are summarized in Table 1 [10].
WHO issued a risk-score to assess 10 years of fracture risk. The FRAX® (fracture risk assessment) tool is based on individual patient models that integrate the risks associated with clinical risk factors with (or without) BMD at the femoral neck [11]. The FRAX® models have been developed from studying population-based cohorts from Europe, North America, Asia and Australia. In their most sophisticated form, the FRAX® tool is computer-driven (questionnaire at http://www.shef.ac.uk/FRAX/). Simplified paper versions, based on the number of risk factors are also available. The FRAX® output is a 10-year probability of hip fractures and a 10-year probability of a major osteoporotic fracture (spine, forearm or shoulder fractures). The FRAX® tool is an important step towards individual case finding and a turn away from the T-score pragmatism (Table 2), which rated BMD results as a simple decision for diagnosis and therapy.
During diagnosis it is important to differentiate between primary osteoporosis and secondary generalized osteoporosis (Tables 3 and 4; Fig. 1), because this has a strong impact on therapeutic measures. Secondary osteoporosis may emerge from various disorders of the endocrine system or of the gastro-intestinal tract, from kidney insufficiency and other disorders [12–15]. Furthermore, loss of bone mineral content may be due to medications e.g. continuous cortisone therapy [16–23]. The differential diagnosis of metabolic osteoporosis needs a stepwise approach, corresponding to the severity of disease and including the diagnostic spectrum of various medical disciplines (Table 4).
Clinical investigations
In manifest osteoporosis the painful degenerative skeletal changes emerge predominantly from the axial skeleton, because of altered geometry of the spine. Characteristics are increased kyphosis of the thoracic spine, protruded abdomen, narrowing or diminished crista-costa space and in most cases loss in body height.
Conventional X-ray
X-ray investigations of thoracic and lumbar spine are essential, because about 25–30 % of all osteoporotic fractures, including vertebrate body fractures, occur in cases of non-osteoporotic lowered BMD [3].
BMD
Gold-standard is the dual-energy X-ray absorptiometry (DXA). BMD is expressed in g/cm2, thus it is not a density in the common physical dimension, but in fact it considers the bone diameter, which has a determining influence on bone fragility [24, 25]. BMD measurements are important to estimate the individual fracture risk (see above and references [26–28]) and to differentiate between minor and severe forms of bone loss (Table 2).
Laboratory
Investigations of routine parameters (“basal laboratory”) are useful when differentiating between primary and secondary metabolic osteoporosis. Underlying diseases can be detected by additional laboratory analyses which may also serve to secure a diagnosis and to provide pretherapeutic starting values for therapy monitoring (Table 3).
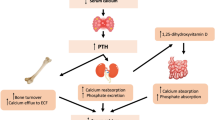
A chronic deficiency in vitamin D results in an impaired bone mineralization. Less severe cases of deficiency or insufficiency cause a hypocalcemic trend by decreased intestinal absorption of calcium, which in turn increase the concentration of the parathyroid hormone (PTH), a stimulant of bone resorption involved in the maintenance of calcium homeostasis. Long-lasting secondary hyperparathyroidism contributes to changes in bone remodelling and osteoporosis in the elderly [29, 30]. An overview on other detrimental consequences of vitamin D deficiency [28, 30] is not a topic of this paper. Vitamin D status is controlled by measurement of 25-hydroxy vitamin D (25OHD); but findings on its association to BMD are controversial [31–34]. However, supplementation with vitamin D and calcium prevented seasonal bone loss during winter [35]. A meta-analysis showed, that sufficient supplementation with vitamin D reduced the risk of hip and nonvertebral fractures [36]. For these reasons we strongly recommend that one include 25OHD analysis in the “basal laboratory”. Similarly, a recent guideline recommends screening for vitamin D deficiency in individuals at risk and advises dosages for oral vitamin D supplementation [37].
Biochemical bone turnover markers are circulating components of bone metabolism, reflecting formation and resorption processes. The biochemical markers are slightly elevated in healthy postmenopausal women due to their deficiency of estrogens and there is an increase in cases of enhanced bone turnover in this group as well. Biochemical markers indicate changes in bone metabolism early, before alterations are reflected by BMD or X-ray of hip and vertebrae [38–40]. Supplementary to BMD measurements they provide information on fracture risk [41]. Therefore, we recommend including at least a bone resorption marker in the “basal laboratory”. Increased levels should be interpreted as a matter of clinical judgement, either as an individual risk factor or as an additional argument to recommend hormone replacement therapy to menopausal women with serious climacteric symptoms, especially with osteopenia. For primary osteoporosis, the bone resorption marker concentration represents the baseline level for individualized therapy according to fracture risk evaluation.
Genetic markers may assess a population based on risk, because of the high genetic disposition of osteoporosis. However, genetic markers are subjected mostly to rare cases (e.g. osteogenesis imperfecta) or scientific questions. The first genetic marker described in 1994 is an association of vitamin D receptor genotypes with low BMD [42]. A genetic assay for a disposition to lactose-intolerance is clinically relevant because of dietary implications [43, 44]. A recent meta-analysis suggested that the collagen type I a1 (COLIA1) Sp1 polymorphism initially described in 1996 may be associated with osteoporotic fracture in postmenopausal women [45].
Résumé (see Fig. 1)
Evidence on individual fracture risk is derived from anamnesis, clinical investigations, conventional X-ray and BMD measurements—however, often without knowledge of a possible underlying disease. Frequently, a “basal laboratory” is not sufficient to explain the aetiology of osteoporosis, because of the abundance of differential diagnoses. Extended analyses of laboratory parameters including hormones may be necessary to confirm suspicion. Biochemical markers of bone metabolism and vitamin D status provide additional information on fracture risk. Treatment depends on the underlying disease and the severity of the bone impairment.
Biochemical markers of bone turnover
Clinical relevance
Prognosis of loss in bone mass
Longitudinal studies have shown two characteristic groups of untreated postmenopausal women: women with high bone turnover lose significantly more BMD than women with normal or low bone turnover [46].
Women with high bone turnover present with elevated concentrations of bone turnover markers in their blood and urine compared with low bone turnover women [47]. In prospective studies on postmenopausal women elevated levels of resorption markers at the beginning were associated with a significant loss of bone mass of the lumbar spine after 1 year compared with low initial marker levels [48]. In a long-term study the decrease of BMD was measured in the fore arm. Women with high bone turnover incurred a 2–6 fold of higher loss in bone mass compared with women with low bone turnover [39]. Thus bone markers are suitable to identify female patients with fast bone loss.
Pre-therapeutic bone turnover markers and fracture risk
Bone turnover markers, especially resorption markers, are an independent predictor for fracture risk, additive to BMD, because elevated turnover markers correlate with elevated fracture risk in osteoporosis. This may be explained by disruptions of trabecula at high turnover rates without detection by densitometry [47, 49]. Three prospective studies, namely EPIDOS [38], Rotterdam [50] and OFELY [40], showed that high resorption markers predict an elevated risk of vertebral fractures, fractures of the femur neck and other peripheral fractures in postmenopausal women. Concentrations exceeding the upper limit of premenopausal women were associated with a two fold elevated fracture risk [51].
Resorption markers reflect future loss in bone mass and increase the predictive value of BMD in an additive fashion (Fig. 2) [38, 47, 52]. Inconsistent prospective studies on the value of formation markers for prognosis of fractions exist, however a correlation of formation marker levels with fracture risk is still a matter of debate [52].
Combined assessment of BMD and bone resorption rate to predict hip fracture risk in the elderly. Low BMD was defined by a T score £ 2.5. High bone resorption was defined by CTX above the upper limit (> mean + 2SD) of the premenopausal range. Low BMD and elevated bone resorption marker are independent risk factors for hip fracture. Modified from Garnero P et al. [38], with permission.
Planning of therapy considering bone turnover markers
In planning a therapy one must consider, that all medications will have side effects, individual tolerance and possible interferences with other drugs or contraindications because of comorbidity. Fortunately, several bone-specific medications exist with different mechanisms of action.
Antiresorptiva like bisphosphonates are more efficient in osteoporotic patients with fast bone turnover (elevated marker levels) than in cases of low turnover. Patients with high levels of circulating formation markers benefit by this therapy in a reduction of fracture rate [53]. Compared with bisphosphonates, the selective estrogen receptor modulator Raloxifen exhibits less severe side effects, even in long-term treatment [54]. The MORE study demonstrated its efficacy not only in prevention of fractures but also in the therapy of postmenopausal osteoporosis [55], which is characterized by pretherapeutically elevated bone marker levels. A new antiresorptive therapy makes use of an antibody (Denosumab), which inhibits a factor necessary for the development and activity of osteoclasts [56]. In advanced osteoporosis refractive to antiresorptive therapy, because of new fractures or sustained low BMD and high bone markers, another strategy makes use of the anabolic effect of intermittent PTH. A daily application of the recombinant PTH fragment Teriparatide causes improvements of bone microarchitecture, BMD and reduces fracture risk [57, 58]. Antiresorptive as well as anabolic actions on bone are claimed for strontium-ranelate; treatment with this drug leads to an increase of BMD and reduction of fracture risk [59].
Surveillance of therapy with bone turnover markers
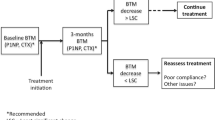
Bone turnover markers are suitable to monitor therapy of osteoporosis. Especially at the beginning of therapy their rating surpassed BMD measurements because of faster change [60]. During antiresorptive therapy resorption markers decline about 30–70 % compared with initial values within 3–6 months, reaching a plateau thereafter [61]. Similarly, the formation markers decrease, but the decrease is less pronounced and it occurs somewhat later. A connection was observed between the extent of the marker decline and the reduction of the fracture risk [62]. As a rule, changes in BMD can be mostly observed after 1 year but the response to antiresorptive therapy by markers can be observed much earlier [60]. With antiresorptive therapy, bone turnover markers should be measured before; at 3–6 and 12 months [63]. Thereafter, yearly controls are adequate. An insufficient drop in marker concentrations indicates failure in therapy or flawed compliance of the patient [1].
A fast increase of formation markers occurs within 1 month of anabolic PTH therapy and a plateau is reached within 3–12 months. This is in line with the initial anabolic action of pulsatile PTH. In contrast, the resorption marker’s increase is delayed for several months indicating subsequent osteoclast activation and start up of bone remodelling [64]. The increase in formation markers 1 month after starting a therapy with the PTH fragment Teriparatide correlated well with an improvement of bone structures as confirmed by biopsies [65]. With anabolic therapy bone turnover markers should be measured before starting and at 1–3 and 12 months.
Hopes, that a combination therapy with antiresorptive and anabolic agents has benefits, were forced to be abandoned for the first time. Indeed, the positive effect of PTH on BMD was blunted by the bisphosphonate alendronate [66]. This was reflected by biochemical markers of bone turnover (Fig. 3). Alendronate quickly reduced bone resorption and bone formation soon after. Overall, the rate of turnover was reduced within 1 year. Monotherapy with PTH resulted in an enhanced turnover rate by raising bone formation and bone resorption later on as well. Combined therapy led to a slight reduction of the turnover rate compared with the baseline.
Median percent changes in the serum concentrations of biochemical markers of bone formation (panel a: N-terminal propeptide, of type 1 collagen) and bone resorption (panel b: C-terminal telopeptide of type 1 collagen) during antiresorptive therapy with bisphosphonate (Alendronate), anabolic therapy with intermittent parathyroid hormone and combination therapy, respectively. Bars represent the interquartile ranges. Differences between all groups at 12 months were significant (p < 0.001). (Reproduced from Black et al. [66] with permission)
Therapy with strontium ranelate stimulates bone formation and hinders resorption. Changes in bone markers are significant, but small. Therefore monitoring strontium-ranelate therapy by markers seems useless [59].
Estimating the significance of marker changes by therapy is essential for an assessment of surveillance data. This can by calculated by the “least significant change”:
- LSC:
-
least significant change
- Ö:
-
square root
- CVa:
-
analytic coefficient of variation
- CVi:
-
individual (within-subject) coefficient of variation
If change of marker level (actual—previous), which is expressed as an absolute percentage from the mean of the actual and previous levels (% |(a − p)|*2/(a + p)), exceeds the least significant change, a significant difference can be assumed [67]. Least significant change is about 20–50 % depending on the marker (Table 5), meaning that changes according to this magnitude prove to have a therapeutic effect [47, 68].
Differential diagnosis
As an important aspect in differential diagnosis, elevated bone turnover markers can also be observed in patients with bone metastases. The prevalence of bone metasta with bone metastases. Especially in carcinomas of the prostate and breast the prevalence of bone metastases is about 70 %. Due to changes of bone turnover by metastatic spread to bones, bone metastases may be detected early by bone turnover markers; moreover, antiresorptive therapy normalized bone marker levels and improved survival [69–77].
Résumé
The monitoring of therapy in osteoporosis and also recently in metastatic bone diseases is the most established application of bone turnover markers. Reaction to therapy is registered rapidly in comparison to BMD measurements. We suggest control of bone markers before, at 3–6, 12 months and thereafter at biannual or annual intervals of antiresorptive therapy (with anabolic therapy controls that should be before and at 1–3 and 12 months), especially in patients with high fracture risk. The compliance of the patient can be judged and adherence to therapy is improved by marker investigations. Knowledge on least significant change is important for an interpretation of the time course of marker changes.
Selected markers of bone turnover
The following markers were tested in large clinical studies and intervention trials. Their usage is in centres specialized on bone metabolism and results from their analytical and clinical practicability and from their availability. However, there is no recommendation for products from specific companies.
It is necessary to consider that immunoassays for markers of bone turnover from different manufacturers may give different results, but typically the concentrations that are measured are very well correlated. This is due to the epitope specificity of antibodies, the test format, and of course standardization. At present there are no international standards defined, though an IFCC committee has been created to address these questions focused on a number of representative markers for bone formation and bone resorption, and to make reference materials available. A suggested nomenclature of bone markers has been published in 2000 [2].
Résumé
In follow-up controls with biochemical markers of bone turnover a change in methods (possibly by changing the laboratory) should be avoided.
Formation markers | Resorption markers |
|---|---|
Are direct or indirect products of activated osteoblasts. These markers are formed during different phases of the lifecycles of osteoblasts, representing different aspects of bone formation. Therefore dynamics of markers may differ | Are direct or indirect products of activated osteoclasts. The crosslinks of collagen molecules within the bone matrix lead to special structures, which are used in analytics |
Total N-terminal propeptide of type I procollagen (P1NP) | C-terminal crosslinked telopeptide of type I collagen (b-CrossLaps, CTX) |
Bone-specific alkaline phosphatase (BALP) Osteocalcin (OC) | Tartrat-resistant acid phosphatase type 5b (TRAP 5b) |
Preanalytics
Preanalytic variability of biochemical markers of bone turnover includes both biological variation of individuals tested and inconsistencies due to sample collection and handling [67]. Biological variation can be divided broadly into two categories [2]: (a) Uncontrollable sources enclose age (compared with adult levels, markers are high during childhood and puberty and elevated during menopause), gender, ethnicity, fractures, pregnancy and lactation [78], physical exercise [79, 80] and various other influences including diseases and medications (see Table 4). (b) Controllable sources are adherence to therapy (hormone replacement, antiresorptive or anabolic bone therapy) and mainly seasonal and circardian variations of bone markers, insufficient storage of specimens and analytic imprecision [67].
Measuring bone markers in serum or plasma samples is well established in routine laboratories because manual analyses from urine of e.g. bone resorption markers (like deoxypyridinoline or N-terminal collagen fragments) are more prone to analytical errors and are more expensive. Although desirable, a 24-h collection did not force through, because of a lack of compliance of patients and reliability of collection. As an alternative, the results from the second void urine have to be related to creatinine excretion. But mostly an additional blood sample is demanded for the analyses of formation marker and other laboratory parameters [81].
Distinct circadian rhythm is a frequent observation with most of these markers [82, 83]. It is strongly recommended, that especially the blood collection for CTX be taken from overnight fasting patients between 7 and 9 a.m., to reduce individual oscillations [84]. In addition, the collagen resorption markers decrease within minutes in respond to a raise of glucose [85]. Therefore, only the drinking of water or unsweetened tea or coffee is allowed prior to blood collection. If it is doubtful whether the patient indeed had fasted especially for reporting collagen resorption marker concentrations, glucose and triglycerides may be measured additionally. Thus, preanalytic cautions are similar to that of lipid-analyses.
Blood samples should be collected before dialysis from dialysis patients with a loss of renal elimination and circadian rhythm. One must acknowledge that the reference values from bone turnover markers, which are cleared via the kidney, are unusable in kidney insufficiency. For these patients a monitoring of BALP and TRAP 5b is in favour. These markers are cleared via the liver and not the kidney [2, 63, 86] while in liver diseases osteocalcin, P1NP and CTX are in favour.
Recent bone fractures or surgical intervention on bones limit the usage of bone turnover markers, because they increase due to the remodelling processes. Depending on the site and size of fractures, marker levels might return to initial values up to 6–12 months after the event [87–90].
Résumé
For a correct estimation of bone turnover, blood should be collected between 7 and 9 a.m. The patient has to fast overnight and must not drink sweetened beverages prior to blood collection.
Concerning the route of clearance, appropriate bone markers have to be selected for dialysis patients and for patients with severe liver diseases. Blood has to be collected prior to dialysis.
CAVE: Reference values are unusable in kidney insufficiency. For these patients BALP and TRAP 5b are in favour, because they are cleared via the liver and not the kidney.
CAVE: Bone markers may be elevated up to a year following fractures.
Characteristics of formation markers
Bone-specific alkaline phosphatase (BALP) [86, 91, 92]
Alkaline phosphatases belong to a family of ubiquitous, membrane-associated enzymes with a molecular weight of about 140 kDa. The different isoforms are coded by one gene, but differ in degree of glycosylation and sialylation. Then most abundant are isoforms from the liver and bone. BALP, a tetrameric glycoprotein is anchored in the plasma membrane of osteoblasts and is released as a dimer into circulation by phospholipase cleavage during bone formation [90].
Analytical methods are enzyme electrophoresis, lectin binding, selective inhibition of other isoforms by heat or urea, immunoassays and by immuno-extraction with subsequent measurement of enzyme activity. Relevant cross-reactions to the liver isoform are possible with some methods. Because of the different analytical procedures and units, the reference values differ extremely. BALP slightly increases during the luteal phase and is elevated in Paget’s disease, hyperthyroidism, primary hyperparathyroidism, acromegaly and bone metastases.
Sample material: Serum, heparin plasma.
Stability: Up to 48 h at room temperature, 1 week at 4 °C, 1 year at − 70 °C.
Interferences: Haemolysis, lipidemia, incorrect high in liver disease due to cross-reaction of antibodies with the liver enzyme.
Clearance: Predominantly by the liver.
Total N-terminal propeptide of type I procollagen (P1NP) [86, 92–97]
The bone matrix is composed of about 90 % of type I collagen, which is synthesized by osteoblasts. Sinews, skin and cartilage are other sources of type I collagen. The monomer procollagen chains are secreted and drilled to helical triplets extracellularly. Thereby the terminal procollagen regions are eliminated into circulation. These fragments (N-terminal and C-terminal, molecular weight 100 kDa and 35 kDa, respectively) represent the newly synthesis of type I collagen and are early markers of osteoblast activity. P1NP concentrations show a wide dynamic range and markedly rise during puberty. P1NP exhibits low circadian variation. In circulation P1NP is heterogeneous, where the trimeric form partly dissociates at 37 °C, which may have an impact on several assays. P1NP is well suited for the monitoring of anabolic bone therapy, because of a high dynamic range.
Sample material: EDTA plasma preferred, serum or heparin plasma.
Stability: Twenty-four hours at room temperature, 5 days at 4–8 °C, 6 months at −20 °C. No influence of five freezing-thawing cycles.
Interferences: Liver diseases (significant higher levels in liver cirrhosis).
Clearance: Liver and kidney.
Osteocalcin [86, 98–107]
Osteocalcin, the most abundant noncollagenous protein of bone, is synthesized solely by osteoblasts. The synthesis is regulated by 1,25 dihydroxy vitamin D. Osteocalcin is formed from 49 amino acids, the carboxylation of three N-terminal glutamine residues depends on vitamin K and is critical for the high affinity of osteocalcin to hydroxylapatite. The majority of osteocalcin is integrated into the bone matrix, but 20–30 % arrives in circulation. Osteocalcin represents a late marker of osteoblast activity and is formed during differentiation subsequent to BALP and type I collagen.
Osteocalcin circulates in a heterogeneous and partly fragmented manner. In vitamin K deficiency a part of osteocalcin is imperfectly carboxylated, which increases the risk of hip fractures. Undercarboxylated osteocalcin can be measured with special assays and is involved in glucose haemostasis, insulin control and probably androgen formation. Apparently, there is an endocrine regulation of energy metabolism by the skeleton.
During bone resorption short osteocalcin fragments are liberated, however osteocalcin (1–34) and/or osteocalcin (1–49) predominantly reflect bone formation.
Sample material: EDTA-plasma preferred, serum or heparin plasma.
Stability: The data are inconsistent because of the epitope specificity of immunoassays. In practical use, direct immunoassays which recognize the mid-fragment osteocalcin (1–43) seem advantageous. Proteolytic cleavage of the 6 C-terminal amino acids is fast but individually different. Preanalytics are more sensitive when using intact osteocalcin (1–49) assays.
Stability N-Mid osteocalcin: EDTA plasma 2 days at room temperature, 3 days at 4 °C, 3 months at -20 °C. Serum or heparin plasma: 8 h at room temperature. Avoid haemolysis, freeze only once.
Interferences: Liver diseases, chronic kidney insufficiency.
Clearance: Mainly by kidney, less by liver.
Characteristics of resorption markers
C-terminal cross-linked telopeptide of type I collagen (CTX, Crosslaps) [86, 108–113]
The proteolytic degradation of bone matrix forms a variety of type I collagen fragments. The structures of these cleavage products are more or less bone-specific. This results from the primary structure of protein chains and the characteristic modifications during collagen fibril formation, maturation and ageing of the matrix. Optimal bone specificity is known for the peptide bound crosslink structures of the N- or C-terminal teleopeptide regions (NTX, CTX) of type I collagen. A b-isomer of C-telopeptide is formed spontaneously by slow isomerization of a peptide-bond; analysis of “b-CrossLaps” is specific for elderly tissue—and therefore for bone and its proteolytic resorption. CTX is well suited for monitoring the efficacy of antiresorptive drugs including the different bisphosphonates.
Sample material: EDTA-plasma preferred, serum or heparin plasma, CAVE preanalytics!
Stability: EDTA-plasma 24 h at room temperature. Three months at -20 °C. Avoid haemolysis, freeze only once.
Interferences: Liver diseases, chronic kidney insufficiency.
Clearance: Mainly by kidney, less by liver.
Tatrate-resistant-acid phosphatase Type 5b (TRAP 5b) [86, 114–117]
TRAP is expressed by osteoclasts, macrophages and dendritic cells. The isoenzyme TRAP 5b lacks sialic acid and works at a higher pH optimum than isoenzyme 5a. TRAP 5b levels resemble the number of activated osteoclasts. Kathepsin K activates TRAP 5b by elimination of a propeptide domain. The enzyme is formed from two subunits, which are connected by two disulfide bonds. Although its high protein-tyrosin-phospatase activity is known, the substrate has not been identified yet. Another function of the enzyme may be the formation of free oxygen radicals which supplement the degradation of the organic bone matrix.
In circulation about 90 % of TRAP is fragmented. Analysis is done by immune-extraction with TRAP 5b-specific antibodies and measurement of bound enzyme activity at pH 6.1. Alternatively, the enzyme activity can be measured by inhibition of TRAP 5a by tartrate and heparin.
Serum TRAP 5b activity is less influenced by kidney function than the resorption markers derived from type I collagen. Therefore, main indications of TRAP 5b are patients with reduced kidney function, such as dialysis patients.
Sample material: Only serum, transport should be done in dry ice.
Stability: Serum should be obtained within 4 h after blood collection. Samples have to be stored at -20 °C or below. Transport in dry-ice. Freeze only once.
Interferences: Haemolysis. Formation of TRAP 5b complexes with alpha-2-macroglobulin might reduce the measured concentration.
Clearance: Liver.
Some selected parameters which regulate bone metabolism
Parathyroid hormone (PTH)
Time of sample collection: In the morning, optimal before 9 a.m., the patient must fast overnight. As an exception, blood is sampled from dialysis patients prior to dialysis for convenience, but before the long dialysis interval. Although this results in slightly higher PTH and phosphate, the dangers of elevated phosphate and PTH may be reflected more sensitively.
Selection of assay: The test results should be compatible with the recommendations given in the guideline of National Kidney Foundation [118, 119]. The test should correlate well with the PTH (1–84) assays.
Sample material: EDTA-plasma preferred, serum or heparin plasma.
CAVE: Serum is unusable in pancreatitis due to a fast degradation of PTH!
Stability: EDTA plasma 24 h at room temperature, 72 h at 4° C, 12 month at -20 °C.
Serum 4 h at room temperature, 24 h at 4 °C, 6 month at − 20 °C.
Interferences: Inadequately filled EDTA tubes may cause a pH shift, which may cause false low PTH in certain assays.
25-hydroxy vitamin D (25OHD, “calcidiol”)
Time of sample collection: In the morning, the patient must fast overnight. Analyses of 25OHD should be carried out every 5 years starting with the age of 50. Samples should be collected between January and April. During this timeframe the 25OHD levels are lower and those of PTH are higher [120].
Sample material: Serum preferred, plasma.
Stability: Avoid direct sunlight; shipping is possible without refrigeration for 48 h.
Interferences (depend on the assay in use): Lipemia, cross-reactions with hydroxylated metabolites of vitamin D3, Intoxication with dihydrotachysterol (AT10®, Tachystin®).
Analytical methods: Immunoassays, protein binding analyses, HPLC and HPLC-mass-spectrometry. A co-measurement of 25OHD3 (metabolite of cholecalciferol) and 25OHD2 (metabolite of ergocalciferol) is desirable, although ergocalciferol is not available from Austrian pharmacies, but may be purchased via the internet. 25OHD assays do not measure 1,25-dihydroxy vitamin D (calcitriol, Rocaltrol®, Bocatriol®), or a-calcidiol (Bondiol®, Doss®, Etalpha “Leo”®)
Résumé
The optimal 25OHD concentration for the bone and the ability to avoid a variety of diseases was defined by a consensus conference of experts to be 30–40 mg/l (75–100 nmol/l) [33, 121]
Recently another expert group (Institute of Medicine) recommended at least 20 ng/ml (50 nmol/l) 25OHD, but less than 50 mg/l (125 nmol/l) to avoid adverse effects [122].
Recommendations for monitoring a therapy and check for an optimal vitamin D substitution
Parameter | Frequency | Target |
|---|---|---|
Bone resorption marker | Before, 3–6 months after start of antiresorptive therapy. Yearly controls to check compliance | Significantly lowered or near low limit of reference range |
Bone formation marker | Basal and after 1, (if no response: 3–6), 12, 18 month of anabolic therapy | Significantly increased or near upper reference range |
25OHD | At least once in winter during substitution | 20–40 mg/l (50–100 nmol/l) |
PTH | At least once in winter | Middle reference range |
Synopsis
Remarkable features of biochemical markers of bone turnover and a proposal of their use are summarized in Table 6 as a shortcut.
Conclusion
A detailed anamnesis in combination of an evaluation of individual risk factors e.g. by the FRAX® tool of WHO may raise suspicion of clinically relevant bone loss, which would be confirmed by clinical investigations, conventional X-ray and BMD measurements. Due to its strong impact on therapeutic measures, it is important to differentiate between primary and secondary osteoporosis which is achieved by a “basal laboratory” and by extended laboratory investigations, if necessary. 25OHD and bone turnover markers support the assessment of fracture risk and the markers enable the estimation of the bone turnover rate. An underlying disease must be treated appropriately and the therapeutic success must be proved by disease-specific parameters.
Bone treatment depends on fracture risk assessment and the severity of the impairment. Clearly, high-risk patients must be treated and monitored more efficiently compared to patients of low risk. Today a plurality of bone-specific medications exists with different mechanisms of action. The drugs are dosed daily or as a depot and operate in antiresorptive and/or anabolic fashion. Understandably, the therapy must be optimized to the patient’s individual responsiveness. Monitoring drug treatment of osteoporosis is an established application of bone turnover markers, because markers allow an early judgement of therapeutic success or failure within months compared to late decisions obtained from BMD reports. This has an important economical aspect. Taking a costly daily medication and detecting its failure about 1–2 years later by BMD examination will waste health budget, frustrate the patient, diminish his confidence in the prescribing physician and the compliance for a further therapy. Osteoporosis therapy monitoring by bone turnover markers in patients with high fracture risk most likely improve economic perspectives, not only by the enhanced efficacy in the early detection of a response, but also by the well-documented adherence of patients to the prescriptions and a better relationship with the physician. By a reasonable application of bone turnover markers in the monitoring of bone therapy, BMD will not lose relevance in the surveillance of bone disease, but its importance can be restricted to controls in larger time intervals, which may also help to save costs.
References
Seibel MJ. Biochemical markers of bone remodelling. Endocrinol Metab Clin North Am. 2003;32:83–113.
Delmas PD, Eastell R, Garnero P, et al. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000;11(Suppl 6):S2–17.
WHO Technical report series—assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: WHO; 1994.
Kanis J, Melton L, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–41.
Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):S3–7.
Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–9.
Cooper C, Cole ZA, Holroyd CR, et al. and IOF CSA Working Group on Fracture Epidemiology. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–88.
Dimai HP, Svedbom A, Fahrleitner-Pammer A, et al. Epidemiology of hip fractures in Austria: evidence for a change in the secular trend. Osteoporos Int. 2011;22:685–92.
Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–61.
Kanis JA, Borgstrom F, De Laet C, Johansson H, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–9.
Kanis JA, Johnell O, Oden A, et al. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–97.
Anderson F, Francis R, Selby P, et al. Sex hormones and osteoporosis in men. Calcif Tissue Int. 1998;68:185–8.
Ludwig H, Fritz E, Friedl H. Epidemiologic and age dependent data on multiple myeloma in Austria. J Nat Cancer Inst. 1982;68:729–33.
McFarlane X, Bhalla A, Reeves D, et al. Osteoporosis in treated adult coeliac disease. Gut. 1995;36:710–4.
Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008;19:1683–704.
Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M. Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess. 2007;11:1–231.
Takkouche B, Montes-Martinez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30:171–84.
Christiansen C, Baastrup PC, Transbol I. Osteopenia and dysregulation of divalent cations in lithium-treated patients. Neuropsychobiology. 1975;1:344–54.
Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44:331–4.
Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319–26.
Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–9.
Jamal SA, Browner WS, Bauer DC, et al. Warfarin use and risk for osteoporosis in elderly women. Study of osteoporotic fractures research group. Ann Intern Med. 1998;128:829–832.
Rezaieyazdi Z, Falsoleiman H, Khajehdaluee M, et al. Reduced bone density in patients on long term warfarin. Int J Rheum Dis. 2009;12:130–5
Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1117–41.
Kanis JA, Johnell O, Oden A, et al. Risk of hip fracture according to the World Health Organization criteria for osteoporosis and osteopenia. Bone. 2000;27:585–90.
Cummings S, Black D, Nevitt M. for the study of osteoporotic fractures research group. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341,72–5.
Cooper C, Atkinson E, Jacobsen S, et al. Population based study of survival following osteoporotic fractures. Am J Epidemiol. 1993;137:1001–5.
Kudlacek S, Schneider B, Resch H, et al. Die lumbale BMD – Risikofaktor für Wirbelkörperfrakturen bei der Frau. Dtsch Med Wschr. 1998;123:651–7.
Dobnig H. A review of the health consequences of the vitamin D deficiency pandemic. J Neurol Sci. 2011 Sept 21. Epub ahead of print.
Sahota O, Mundey MK, San P, et al. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004;35:312–9.
Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006 Jul;84:18–28.
Kudlacek S, Schneider B, Peterlik M, et al. And Austrian study group on normative values of bone metabolism. Assessment of vitamin D and calcium status in healthy adult Austrians. Eur J Clin Invest. 2003;33:323–31.
Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–9.
Garnero P, Munoz F, Sornay-Rendu E, et al. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716–22.
Meier C, Woitge HW, Witte K, et al. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open-label prospective trial. J Bone Miner Res. 2004;19:1221–30.
Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64.
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. and Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J Bone Miner Res. 1996;11:1531–8.
Garnero P, Sornay-Rendu E, Duboeuf F, et al. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res. 1999;14:1614–21.
Garnero P, Sornay-Rendu E, Claustrat B et al. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women. The OFELY study. J Bone Min Res. 2000;15:1526–36.
Garnero P, Delmas PD. Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact. 2004;4:50–63.
Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–7.
Obermayer-Pietsch BM, Bonelli CM, Walter DE, et al. Genetic predisposition for adult lactose intolerance and relation to diet, bone density, and bone fractures. J Bone Miner Res. 2004;19:42–7.
Gugatschka M, Dobnig H, Fahrleitner-Pammer A, et al. Molecularly defined lactose malabsorption, milk consumption and anthropometric differences in adult males. Quart J Med. 2005;12:857–63.
Ji GR, Yao M, Sun CY, et al. Association of collagen type I alpha1 (COLIA1) Sp1 polymorphism with osteoporotic fracture in Caucasian post-menopausal women: a meta-analysis. J Int Med Res. 2009;37:1725–32.
Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–49.
Srivastava AK Vilet EL, Lewiecki EM, et al. Clinical use of serum and urine bone markers in the management of osteoporosis. Curr Med Res Opin. 2005;21:1015–26.
Chesnut CH 3rd, Bell NH, Clark GS, et al. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med. 1997;102:29–37.
Delmas PD. The role of markers of bone turnover in the assessment of fracture risk in postmenopausal women. Osteoporosis Int. 1998;8(Suppl 1):S32–6.
van Daele PL, Seibel MJ, Burger H, et al. Case-control-analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. Brit Med J. 1996;312:482–3.
Pfeilschifter J, Kann PH. Diagnostik der Osteoporose. Z Gastroenterol. 2002;40:46–56.
Stepan JJ. Clinical value of the biochemical markers of bone remodelling in the assessment of bone metabolic diseases. Jugoslov Med Biohem. 2006;25:241–8.
Bauer DC, Garnero P, Hochberg MC et al. Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture Intervention trial. J Bone Miner Res. 2006;21:292–9.
Kreihuber A. Osteoporosetherapie – aktueller Datenüberblick zu Raloxifen. J Miner Stoffwechs. 2008;15:220–2.
Maricic M, Adachi JD, Sarkar S, et al. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med. 2002;162:1140–3.
Cummings SR, San Martin J, McClung MR, et al. the FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65.
Jiang Y, Zhao JJ, Mitlak BH, et al. Recombinant human parathyroid hormone (1–34) (teriparatide) improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–41.
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–68.
Christgau S, Cloos PA. Current and future applications of bone turnover markers. Clin Lab. 2003;49:439–46.
Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2000;85:3109–15
Hochberg MC, Greenspan S, Wasnich RD, et al. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87:1586–92.
Bergmann P, Body JJ, Boonen S, et al. and Members of Advisory Board on Bone Markers. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract. 2009;63:19–26.
Bauer DC, Garnero P, Bilezikian JP, et al. Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrin Metab. 2006;91:1370–9.
Dobnig H, Sipos A, Jiang Y, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab. 2005;90:3970–7.
Black DM, Greenspan SL, Ensrud KE, et al. and PaTH Study Investigators. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15.
H, Cosman F, Endres DB, et al. Application of biochemical markers of bone turnover in the assessment and monitoring of bone diseases; Approved Guideline. In: NCCLS document C48-A (ISBN 1-56238-539-9) 2004; 24 (22). NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087–1898 USA.
Bieglmayer C, Clodi M, Kudlacek S. Biomarker in der Osteologie: Aktueller Stand. J Miner Stoffwechs. 2006;13:82–7.
Ganero P. Markers of bone turnover in prostate cancer. Cancer Treatm Rev. 2001;27:187–92.
Jung K, Lein M, Stephan C, et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. In J Ca. 2004;111:783–91.
Brown JE, Thomson CS, Ellis SP, et al. Bone resorption predicts for skeletal complications in metastatic bone disease. Brit J Ca. 2003;89:2031–37.
de la Piedra C, Castro-Errecaborde NA, Traba ML, et al. Bone remodelling markers in the detection of bone metastases in prostate cancer. Clin Chem Acta. 2003;331:45–53
Koizumi M, Takahashi S, Ogata E. Bone metabolic markers in bisphosphonate therapy for skeletal metastases in patients with breast cancer. Breast Cancer. 2003;10:21–7.
Brasso K, Christensen IJ, Johansen JS, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–13.
Beke D, Kudlacek S, Meran JG. Klinische Relevanz von Biomarkern bei der Skelettmetastasierung von Malignomen. Wien Med Wochenschr. 2007;157:375–80.
Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201.
Lipton A, Chapman JA, Demers L, et al. Elevated bone turnover predicts for bone metastasis in postmenopausal breast cancer: results of NCIC CTG MA.14. J Clin Oncol. 2011;29:3605–10.
Naylor KE, Iqbal P, Fledelius C, et al. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15:129–37.
Woitge HW, Friedmann B, Suttner S, et al. Changes in bone turnover induced by aerobic and anaerobic exercise in young males. J Bone Miner Res. 1998;13:1797–1804.
Maïmoun L, Manetta J, Couret I, et al. The intensity level of physical exercise and the bone metabolism response. Int J Sports Med. 2006;27:105–111.
Huber F, Traber L, Roth HJ, et al. Markers of bone resorption—measurement in serum, plasma or urine? Clin Lab. 2003;49:203–7.
Schmidt-Gayk H, Huber F, Traber L, et al. Vitamin-D-Versorgung und Marker des Knochenabbaus (b-CrossLaps) bei prä- und postmenopausalen Frauen. Osteoporose Rheuma aktuell. 2003;4:36–42.
Garnero P, Mulleman D, Munoz F, et al. Long-term variability of markers of bone turnover in postmenopausal women and implications for their clinical use: the OFELY study. J Bone Miner Res. 2003;18:1789–94.
Christgau S, Bitsch-Jensen O, Hanover Bjarnason N, et al. Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone. 2000;26:505–11.
Bjarnason NH, Henriksen EEG, Alexandersen P, et al. Mechanism of circadian variation in bone resorption. Bone. 2002;30:307–13.
Schmidt-Gayk H, Roth HJ, Becker S, et al. Noninvasive parameters of bone metabolism. Curr Opin Nephrol Hypertens. 1995 Jul;4:334–8.
Ohishi T, Takahashi M, Kushida K, et al. Changes of biochemical markers during fracture healing. Arch Orthop Trauma Surg. 1998;118:126–30.
Ingle BM, Hay SM, Bottjer HM, et al. Changes in bone mass and bone turnover following distal forearm fracture. Osteoporos Int. 1999;10:399–407.
Akesson K, Käkönen SM, Josefsson PO, et al. Fracture-induced changes in bone turnover: a potential confounder in the use of biochemical markers in osteoporosis. J Bone Miner Metab. 2005;23:30–5.
Veitch SW, Findlay SC, Hamer AJ, et al. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int. 2006;17:364–72.
Garnero P, Delmas PD. Assessment of the serum levels of bone alkaline phosphatase with a new immunoradiometric assay in patients with metabolic bone disease. J Clin Endocrinol Metab. 1993;77:1046–53.
Alvarez L, Guañabens N, Peris P, et al. Usefulness of biochemical markers of bone turnover in assessing response to the treatment of Paget’s disease. Bone 2001;29:447–52.
Melkko J, Kauppila S, Niemi S, et al. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clin Chem. 1996;42:947–54.
Bornstein P. The NH. (2)-terminal propeptides of fibrillar collagens: highly conserved domains with poorly understood functions. Matrix Biol. 2002;21:217–26.
Tahtela R, Seppanen J, Laitinen K, et al. Serum tartrate-resistant acid phosphatase 5b in monitoring bisphosphonate treatment with clodronate: a comparison with urinary N-terminal telopeptide of type I collagen and serum type I procollagen amino-terminal propeptide. Osteoporos Int. 2005;16:1109–16.
Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–70.
Schytte S, Hansen M, Moller S, et al. Hepatic and renal extraction of circulating type I procollagen aminopropeptide in patients with normal liver function and in patients with alcoholic cirrhosis. Scand J Clin Lab Invest. 1999;59:627–34.
Hellman J, Kakonen SM, Matikainen MT, et al. Epitope mapping of nine monoclonal antibodies against osteocalcin: combinations into two-site assays affect both assay specificity and sample stability. J Bone Miner Res. 1996;11:1165–75.
Takahashi M, Kushida K, Nagano A, et al. Comparison of the analytical and clinical performance characteristics of an N-MID versus an intact osteocalcin immunoradiometric assay. Clin Chim Acta. 2000;294:67–76.
Durham BH, Robinson J, Fraser WD. Differences in the stability of intact osteocalcin in serum, lithium heparin plasma and EDTA plasma. Ann Clin Biochem. 1995;32:422–3.
Noonan K, Kalu ME, Holownia P, et al. Effect of different storage temperatures, sample collection procedures and immunoassay methods on osteocalcin measurement. Eur J Clin Chem Clin Biochem. 1996;34:841–4.
Colford J, Sailer D, Langman C. Five osteocalcin assays compared: tracer specificity, fragment interference, and calibration. Clin Chem. 1997;43:1240–1.
Vergnaud P, Garnero P, Meunier PJ, et al. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab. 1997;82:719–24.
Holzer G, Grasse AV, Zehetmayer S, et al. Vitamin K epoxide reductase (VKORC1) gene mutations in osteoporosis: a pilot study. Transl Res. 2010;156:37–44.
Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69.
Fernández-Real JM, Izquierdo M, Ortega F, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94:237–45.
Iki M, Tamaki J, Fujita Y, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo osteoporosis risk in men (FORMEN) Study. Osteoporos Int. 2011 Mar 25. Epub ahead of print.
Bonde M, Qvist P, Fledelius C, et al. Applications of an enzyme immunoassay for a new marker of bone resorption (CrossLaps): follow-up on hormone replacement therapy and osteoporosis risk assessment. J Clin Endocrinol Metab. 1995;80:864–8.
Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the serum CrossLaps One Step ELISA, a new assay measuring the serum concentration of bone-derived degradation products from type I collagen C-telopetides. Clin Chem. 1998;44:2290–300.
Ganero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001;47:694–702.
Ravn P, Clemmesen B, Riis BJ, et al. The effect on bone mass and bone markers of different doses of Ibandronate: a new bisphosphonate for prevention and treatment of postmenopausal osteoporosis. A 1-year, randomized, double-blind, placebo-controlled dose-finding study. Bone 1996;19:527–33.
Delmas PD, Adami S, Strugula C, et al. Intravenous Ibandronate Injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838–46.
Reginster J-Y, Adami S, Lakatos P, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65:654–61.
Halleen JM, Raisanen S, Salo JJ, et al. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem. 1999;274:22907–10.
Janckila AJ, Nakasato YR, Neustadt DH, et al. Disease-specific expression of tartrate-resistant acid phosphatase isoforms. J Bone Miner Res. 2003;18:1916–9.
Halleen JM, Alatalo SL, Janckila AJ, et al. Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem. 2001;47:597–600.
Hannon RA, Clowes JA, Eagleton AC, et al. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone 2004;34:187–94.
103 K/DOQI guidelines for the management of renal osteodystrophy. Am J Kidney Dis. 2003;42(Suppl 3):S1–201.
Souberbielle J-C, Boutten A, Carlier M-C, et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006;70:345–50.
Woitge HW, Knothe A, Witte K, et al. Circannual rhythmus and interactions of vitamin D metabolites, parathyroid hormone, and biochemical markers of skeletal homeostasis: a prospective study. JBMR. 2000;15:2443–50.
Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteporos Int. 2010;21:1151–4.
Ross AC, Manson JE, Abrams SA, et al. The 2011 Report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8.
Conflict of interest
Authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
In grateful remembrance of Prof. Heinrich Schmidt-Gayk, who supplied our working group with valuable contributions.
Rights and permissions
About this article
Cite this article
Bieglmayer, C., Dimai, H., Gasser, R. et al. Biomarkers of bone turnover in diagnosis and therapy of osteoporosis. Wien Med Wochenschr 162, 464–477 (2012). https://doi.org/10.1007/s10354-012-0133-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-012-0133-9