Abstract
Oncoidal limestones with different oncoid types are ubiquitous in back-reef open-lagoonal and, to a minor amount, in closed-lagoonal facies of the Late Jurassic Plassen Carbonate Platform of the Northern Calcareous Alps. A common feature of the oncoids from moderately to well-agitated open-lagoonal habitats are incorporated small trochospiral benthic foraminifers, tentatively assigned to trochamminids, switched between individual micritic layers. Their life style is discussed concluding a specialized feeding on cyanophytes on the outer side of the oncoids and later becoming biomurated by successive sheet formations due to oncoid growing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The non-genetic term oncoid describes rounded, millimeter- to centimeter-sized, mostly calcareous nodules composed of an usually micritic cortex of more or less concentric laminae forming around a biogenic or abiogenic nucleus (e.g., Flügel 2004). Most oncoids are typically formed in marine shallow-water environments by photosynthetic cyanobacteria and algae (e.g., Dahanayake 1977; Peryt 1981; Gaillard 1983; Riding 1983; Dromart et al. 1994; Reolid and Gaillard 2007; Védrine et al. 2007), but there are also examples of oncoid formation in deeper-water environments in the geological record (Massari 1983; Dromart and Elmi 1986; Dromart 1989, 1992; Gygi 1992; Gradzinski et al. 2004; Reolid et al. 2005). Due to the strong participation of microbes in oncoid formation, they can also be termed “microbial coated grains”, “unattached spherical stromatolites” or “microbolites of spheroidal growth” (e.g., Schmid 1996; Riding 2000). Apart from cyanobacteria and algae, there are several records of oncoid-forming benthic foraminifera, namely miliolids or agglutinating taxa (e.g., Rat 1966; Peryt and Peryt 1975; Mišik and Sucha 1997; Gaillard 1983; Reolid et al. 2005; Gradzinski et al. 2004). Besides acting as oncoid constructors, benthic foraminifera maybe found attached to the outer surface of oncoids, simply due to the availability of hard substrates within specific palaeoenvironments and their attached way of life (e.g., Riegraf 1987).
During the study of Late Jurassic shallow-water carbonates of the Northern Calcareous Alps (Fig. 1), small low-spired foraminifera were observed occurring inside the oncoids. In the present case, the foraminifera neither acted as oncoid constructors or external encrusters nor got simply trapped. The purpose of the present paper therefore is to provide a description of these findings and discuss the possible relationship/interaction between foraminifera and the oncoids, supposed as non simply accidental.
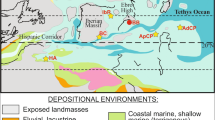
a Tectonic map showing the regional situation of the Eastern Alps after Frisch and Gawlick (2003). GPU Graz Palaeozoic Unit, GU Gurktal Unit, GWZ Greywacke Zone, RFZ Rhenodanubian Flysch Zone. b Tectonic map of the central Northern Calcareous Alps according to Frisch and Gawlick (2003) with the studied localities: B Barmsteine, H Höherstein-Plateau, J Jochwand, PL Plassen, R Rötelstein, T Trisselwand
Geological overview and material studied
The thin sections studied come from the Late Kimmeridgian to Tithonian interval of the Plassen Carbonate Platform (PCP) of the Northern Calcareous Alps (Fig. 1a). The material investigated from the PCP comes from (1) Mount Plassen near Hallstatt, the type-locality of these shallow-water platform carbonates, (2) Mount Rötelstein in the Styrian Salzkammergut area and (3) Mount Trisselwand near Altausee (Fig. 1b). At Mount Plassen the oncoids studied (Fig. 2) occur within a series of peritidal to open lagoonal (in part back-reef) cyclic carbonates of latest Kimmeridgian to Early Tithonian age (Schlagintweit et al. 2003, 2005, for details). Whether the oncoid-bearing carbonates of Mount Trisselwand are time-equivalent or may belong to a short interval of open lagoonal/back-reefal carbonates of Late Tithonian age followed by reefal carbonates at the Jurassic-Cretaceous boundary is so far not further studied. At Mount Plassen, within the Late Tithonian closed lagoonal wackestones, oncoids are also occurring showing a more diversified association of encrusting taxa with Bacinella Radoicic, Lithocodium Elliott and Thaumatoporella Pia. Foraminifera described from oncoids of moderate to well-agitated open-lagoonal to back-reefal palaeoenvironments, however, were not observed at our study site.
Schematic profile of the Plassen Carbonate Platform at Mount Plassen (based on Schlagintweit et al. 2003, 2005 and Gawlick and Schlagintweit 2006) with indication of the intervals with the studied oncoids, autochthonous (PCP, ?Latest Kimmeridgian-Early Tithonian) and allochthonous (Barmstein Limestones, Late Tithonian). At Mount Trisselwand and Mount Rötelstein, equivalent oncoids seem to belong to the back-reef/open lagoonal facies of the second reefal interval of Late Tithonian age. The source area of the resedimented oncoids in the Barmstein Limestones is today eroded (not to scale)
Huge quantities of carbonate material of the PCP were transported into the adjacent hemipelagic basins as mass-flows and calciturbidites known as Barmstein Limestones intercalated in Late Tithonian-Early Berriasian calpionellid limestones (Steiger 1981, 1992; Gawlick et al. 2005, for details). Besides (1) the type-locality Mount Barmsteine, additional material comes from (2) Mt. Jochwand and (2) the Höherstein Plateau (Fig. 1b). The original source area or facies belt of the Late Tithonian PCP was eroded due to younger erosional processes and can be only reconstructed by the analysis of the mass-flow deposits (=Barmstein Limestones) in basinal sediments.
From each locality of the PCP and the Barmstein Limestones about 100 to more than 500 thin sections were studied.
Description of the material
Oncoids
Observed sizes of the oncoid vary between some millimeters up to 1.5 cm, and can therefore be described as meso-oncoids according to Flügel (2004, p. 123). In older classifications, for instance that one provided by Richter (1983), the oncoids would belong to both pisoids (2–10 mm) and macroids (>10 mm). As we have both within the same samples grown under the same palaeoenvironmental conditions, a nomenclatorial splitting would not make a sense. Shapes of the oncoids are spherical to elliptical, elongated specimens depending on shape of nucleus, such as shell debris, and thicknesses of the cortices (Fig. 3a, b, d). In the Barmstein limestones, nuclei are often represented by rounded clasts of lagoonal pack- and wackestones (Fig. 3e, f). Concentrically growth laminae are mostly well developed (=type C of Logan et al. 1964). Some oncoids show uniformly laminated cortices (e.g., Fig. 4a), others multi-stage sequences (e.g., Fig. 3d) obviously reflecting physical changes during oncoid growth. Differences are expressed by either more-dense micritic microfabrics or more open with a peloidal microfabric and irregularly distributed sparitic patches. In that way, oncoids exhibiting two stages, a micritic inner and a more loosely packed outer part, are fairly common (e.g., Fig. 3d, f). Differences of the peloid packing also generate the appearance of darker and lighter layers. Dark micritic layers (envelopes) of older oncoid stages are likely to represent growth interruptions before ongoing formation of laminae in a following younger stage. The microfacies of the oncoid-bearing PCP are packstones with varying amounts of interparticle cements and internal sediments (Fig. 3c, d). It should be mentioned, however, that there is no participation of microencrusters in the oncoid laminae, a characteristic of many Late Jurassic records (e.g., Schmid 1996). Accompanying biota are dasycladalean algae such as Clypeina sulcata (Alth) or Salpingoporella annulata Carozzi, rivulariacean algae, benthic foraminifera (e.g., lituolids, miliolids) and debris of molluscs, gastropods occasionally also corals and stromatoporoids. They account for an open-lagoon to back-reef depositional environment (Schlagintweit et al. 2003, 2005, for details).
Oncoids from the Late Jurassic Plassen Carbonate Platform (a–d) and the Barmstein Limestones (e–f). a Well washed-out packstone with intraclasts and oncoids. Mount Plassen. Scale bar 2 mm. b Oncoid with concentric cortex laminae growing around a nucleus made up of pelecypod debris. Note different thicknesses of cortex on two opposite sides, indicating some stable conditions during growth before becoming overturned. Mount Plassen. Scale bar 0.5 mm. c Packstone with internal sediment between closely attaching oncoids (sizes between 5 and 9 mm). Mount Trisselwand. Scale bar 5 mm. d Large elongated ellipsoidal oncoid with nucleus of pelecypod shell. Note two different types of cortex fabrics (white dashed line): an inner more densely layered part and an outer part with more open fabric and poorly developed laminar fabric. Mount Trisselwand. Scale bar 5 mm. e Oncoid with core of lagoonal wackestone/packstone. Locality Mount Barmsteine. Samples B 123. Scale bar 5 mm. f Oncoid with gastropod shell as core and two trochospiral foraminifera (white circles, for details see Fig. 4f, h). Note two stages of oncoid growth (white dashed line), a micritic inner part and an outer stage more loosely packed with irregularly distributed sparitic patches. Locality Mount Trisselwand. Sample MT 481. Scale bar 5 mm
Oncoid-dwelling foraminifera from the Late Jurassic Plassen Carbonate Platform (a, b, d–f, h locality Mount Trisselwand, i–l: locality Mount Rettenstein) and the Barmstein Limestones (c, g localities Höherstein Plateau and Jochwand). a Concentrical-laminated oncoid (diameter about 8.5 mm) with foraminifer (arrow). Sample MT 164. Scale bar 2 mm. b Detail from a showing low trochospiral benthic foraminifer (axial section). Scale bar 0.5 mm. c–h Variously oriented sections of trochospiral benthic foraminifera within oncoids. Samples D 645, MT 164, MT 164, MT 481, D 47, MT 481. Scale bars 0.5 mm. Specimens shown in (b, c, e, g, h) and i belong to the same taxon, d, f most likely represent phenotypic adaptations of the former. i Concentrical laminated oncoid (diameter about 8 mm) with foraminifer (arrow). Sample BS 90. Scale bar 2 mm. j Detail from i showing low trochospiral benthic foraminifer (axial section). Scale bar 0.5 mm. k Oncoid with trapped lithoclasts (e.g., peloids) and foraminifera (larger lituolid and small Quinqueloculina). Sample BS 90. Scale bar 0.5 mm. l Trochospiral foraminifera occurring free within the sediment; same taxon as occurring in the oncoids. Sample D 432. Scale bar 0.5 mm
Foraminifera
The enclosed benthic foraminifers are found between individual fine-peloidal, micritic oncoid layers with an orientation of the ventral side towards the nucleus, meaning that the dorsal side faces the direction of oncoid growth (e.g., Figs. 3f, 4a). They are found mostly in the middle, more seldom in the outer part of the oncoids, never attached to the core or the outer side. A change of the microfabric related to the position where the foraminifera occur is not detectable. Noteworthy, no specimens were observed within the inner dense micritic oncoid stage. The foraminifera observed show a low trochospiral, convexo-concave test with 2–4 whorls (e.g., Fig. 4b, c, h); oblique sections pretend a more high conical test arrangement (Fig. 3g). Chamber periphery is rounded. The width of the tests ranges from 0.30 to 0.90 mm (most values between 0.50 and 0.75 mm), height is 0.29–0.40 mm; the ratio test height/test width is 0.39–0.55. The wall is microgranular micritic, finely agglutinating structure cannot be excluded. Taking into account the observable features, we tentatively assign our specimens to the Trochamminidae Schwager (see Loeblich and Tappan 1988, p. 120, for details). Noteworthy, presumably the same taxon (taxa?) that occurs within oncoids can be observed also “free” within the surrounding sediment (Fig. 4l).
Discussion and interpretation
First of all, we should start discussion as to whether the occurrence of the foraminifers within the oncoid cortices is simply accidental or not. The observations that the foraminifers occur (1) only with the same trochospiral taxa, (2) always with a test orientation of the ventral (umbilical) side facing towards the interior part of the oncoid, (3) occurring only in one specific oncoid type and (4) other incorporated biogenic groups or abiogenic particles are lacking, point to a causality (e.g., biological interaction or active microhabitat selection) rather than accidental observations. Because of the afore-mentioned reasons, we therefore exclude a simple trapping of the foraminiferan tests by microbial biofilms. Such oncoids with a multitude of trapped and incorporated sediment particles or biogens show a poorly developed laminar fabric with a certain degree of open interparticle porosity (e.g., Flügel 2004, p. 128) different from the studied Alpine Late Jurassic oncoids with the enclosed foraminifera. Nonetheless, only very few such examples with lithoclasts such as peloids and different foraminifera (e.g., lituolids, miliolids) were found trapped within oncoid laminae (Fig. 4k) in the PCP.
Consequently, we have to face the possibilities of an active boring life style within and into the oncoids or attaching and anchoring of the foraminifera with their umbilical sides at the outer part of already existing oncoids followed by subsequent oncoid laminae overgrowth. Boring into the oncoid can be excluded, as we never observed a boring cavity or gallery that started from the oncoid surface (e.g., Fig. 4a, i). Finally, we did not observe any larger cavity around the foraminifera (domichnion). However, we never found these trochospiral foraminifera attached on the outer side of the oncoids in the thin sections studied, most probably also as these would easily become abraded and thus having no potential becoming conserved. The attached specimens obviously were overgrown by successively formed laminae of cyanobacterial activity, thus becoming enclosed and fixed and therefore observable. It is inferred that the motivation of the foraminifera to become attached to the oncoids was not their usage as simple hard substrate, but supposedly because of selectively feeding on microalgae (cyanophytes) and/or bacteria that trigger carbonate precipitation and thus cortex laminae formation. Such an interpretation, already envisaged by Gradzinski et al. (2004), would explain their test orientation with the pseudopodia extruding from the umbilical region and capturing food particles. It is unclear whether the foraminifera were still able to live after becoming overgrown (biomurated) or they died. The missing of a spar-filled cavity, where the foraminifera could live and the close fixation into the cortex is not suitable for continued living as movements and thus further feeding became impossible.
Conclusions
Small trochospiral benthic foraminifera were detected in oncoid cortices switched between individual layers. This observation is not interpreted as simple trapping, but as a biological interaction, namely a specialized selective feeding behavior of the foraminifera with microalgae or bacterial biofilms acting as main food resource, later becoming biomurated by successive cortex growth. As specific food preferences of fossil benthic foraminifera are far from being understood, such an interpretation deserves special interest. These foraminifera were observed within oncoids from moderate to well-agitated palaeoenvironments and so far not detected from oncoids showing a more diversified participation of microencrusters (e.g., Bacinella, Lithocodium) occurring in lagoonal wackestones of reduced water energy. Such micropalaeontological features therefore seem to allow a better characterization and differentiation of Late Jurassic oncoid-bearing shallow-water palaeoenvironments.
References
Dahanayake K (1977) Classification of oncoids from the Upper Jurassic carbonates of the French Jura. Sediment Geol 18:337–353. doi:10.1016/0037-0738(77)90058-6
Dromart G (1989) Deposition of Upper Jurassic fine-grained limestones in the Western Subalpine Basin, France. Palaeogeogr Palaeoclimatol Palaeoecol 69:23–43. doi:10.1016/0031-0182(89)90154-5
Dromart G (1992) Jurassic deep-water microbial biostromes as flooding markers in carbonate sequence stratigraphy. Palaeogeogr Palaeoclimatol Palaeoecol 91:219–228. doi:10.1016/0031-0182(92)90068-G
Dromart G, Elmi S (1986) Développement de structures cryptalgaires en domaine pélagique au cours de l’ouverture des bassins jurassiques (Atlantique Central, Tethys occidentale). C R Acad Sci Paris 303(II, 4):311–316
Dromart G, Gaillard C, Jansa LF (1994) Deep-marine microbial structures in the Upper Jurassic of western Tethys. In: Bertrand-Sarfati J, Monty C (eds) Phanerozoic stromatolites II. Kluwer, Dordrecht, pp 295–318
Flügel E (2004) Microfacies of carbonate rocks—analysis interpretation and application. Springer, Berlin Heidelberg New York, p 976
Frisch W, Gawlick HJ (2003) The nappe structure of the central Northern Calcareous Alps and its disintegration during Miocene tectonic extrusion—a contribution to understanding the orogenic evolution of the Eastern Alps. Int J Earth Sci 92:712–727
Gaillard C (1983) Les biohermes à spongiaires et leur environment dans l’Oxfordian du Jura méridional. Doc Lab Géol Lyon 90:515
Gawlick HJ, Schlagintweit F (2006) Berriasian drowning of the Plassen carbonate platform at the type-locality and its bearing on the early Eoalpine orogenic dynamics in the Northern Calcareous Alps (Austria). Int J Earth Sci 95:451–462. doi:10.1007/s00531-005-0048-4
Gawlick HJ, Schlagintweit F, Missoni S (2005) Die Barmsteinkalke der Typlokalität nordwestlich Hallein (hohes Tithonium bis tieferes Berriasium; Salzburger Kalkalpen)—Sedimentologie, Mikrofazies, Stratigraphie und Mikropaläontologie: neue Aspekte zur Interpretation der Entwicklungsgeschichte der Ober-Jura-Karbonatplattform und der tektonischen Interpretation der Hallstätter Zone von Hallein—Bad Dürrnberg. N Jb Geol Paläont Abh 236:351–421
Gradzinski M, Tyszka J, Uchman A, Jach R (2004) Large microbial–foraminiferal oncoids from condensed Lower–Middle Jurassic deposits: a case study from the Tatra Mountains, Poland. Palaeogeogr Palaeoclimatol Palaeoecol 213:133–151
Gygi RA (1992) Structures, pattern of distribution and paleobathymetry of Late Jurassique microbialites (stromatolites and oncoids) in northern Switzerland. Eclogae Geol Helv 79(2):455–491
Loeblich AR Jr, Tappan H (1988) Foraminiferal genera and their classification, vol 2. Van Nostrand, New York, pp 1–970
Logan BW, Rezak R, Ginsburg RN (1964) Classification and environmental significance of algal stromatolites. J Geol 72:68–83
Massari F (1983) Oncoid and stromatolites in the Rosso Ammonitico Sequences (Middle-Upper Jurassic) of the Venetian Alps, Italy. In: Peryt MT (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 358–366
Mišik M, Sucha V (1997) Chlorite and chlorite–hematite oncoids from the Jurassic limestones of the western Carpathians, Slovakia. Geol Carpathica 48:85–98
Peryt TM (1981) Phanerozoic oncoids—an overview. Facies 4:197–214. doi:10.1007/BF02536588
Peryt TM, Peryt D (1975) Association of sessile tubular foraminifera and cyanophytic algae. Geol Mag 112:612–614
Rat P (1966) Nubecularia reicheli nov. sp., foraminifère constructeur de fausses oolithes dans le Bajocien de Bourgogne. Eclog geol Helv 59:73–85
Reolid M, Gaillard C (2007) Microtaphonomy of bioclasts and paleoecology of microencrusters from the Upper Jurassic spongiolithic limestones (external Prebetic, southern Spain). Facies 53(1):97–112. doi:10.1007/s10347-006-0097-6
Reolid M, Gaillard C, Olóriz F, Rodríguez-Tovar FJ (2005) Microbial encrustations from the Middle Oxfordian-earliest Kimmeridgian lithofacies in the Prebetic Zone (Betic Cordillera, southern Spain): characterization, distribution and controlling factors. Facies 50:529–543. doi:10.1007/s10347-004-0030-9
Richter D (1983) Classification of coated grains. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 3–6
Riding R (1983) Cyanoliths (cyanoids): oncoids formed by calcified cyanophytes. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 276–283
Riding R (2000) Microbial carbonates: the geological record of calcified bacterial–algal mats and biofilms. Sedimentology 47:179–214
Riegraf W (1987) Subbdelloidina luterbacheri sp. nov. (Foraminiferida) from Kimmeridgian to Tithonian (Upper Jurassic) sponge–algal facies of southern Germany. Paläont Z 61:29–40
Schlagintweit F, Gawlick HJ, Lein R (2003) Die Plassen-Formation der Typlokalität (Salzkammergut, Österreich)—neue Daten zur Fazies, Sedimentologie und Stratigraphie. Mitt Ges Geol Bergbaustud Österr 46:1–34
Schlagintweit F, Gawlick HJ, Lein R (2005) Mikropaläontologie und Biostratigraphie der Plassen-Karbonatplattform der Typlokalität (Ober-Jura bis Unter-Kreide, Salzkammergut, Österreich). Mitt Ges Geol Bergbaustud Österr 47:11–102
Schmid DU (1996) Marine Mikrobolithe und Mikroinkrustierer aus dem Oberjura. Profil 9:101–251
Steiger T (1981) Kalkturbidite im Oberjura der Nördlichen Kalkalpen (Barmstein Kalke, Salzburg, Österreich). Facies 4:215–348. doi:10.1007/BF02536589
Steiger T (1992) Systematik, Stratigraphie und Palökologie der Radiolarien des Oberjura-Unterkreide-Grenzbereiches im Osterhorn-Tirolikum (Nördliche Kalkalpen, Salzburg und Bayern). Zitteliana 19:1–188
Védrine S, Strasser A, Hug W (2007) Oncoid growth and distribution controlled by sea-level fluctuations and climate (Late Oxfordian, Swiss Jura Mountains). Facies 53:535–552. doi:10.1007/s10347-007-0114-4
Acknowledgments
This paper was funded by the FWF project P 16812-B06. Samples from Mount Trisselwand were kindly provided by Matthias Auer (Glasgow). We kindly acknowledge helpful comments by Matías Reolid (Jaén) and an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlagintweit, F., Gawlick, HJ. Oncoid-dwelling foraminifera from Late Jurassic shallow-water carbonates of the Northern Calcareous Alps (Austria and Germany). Facies 55, 259–266 (2009). https://doi.org/10.1007/s10347-008-0168-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10347-008-0168-y