Abstract
Few studies have analyzed how tree species within a mixed natural forest affect the dynamics of soil chemical properties and soil biological activity. This study examines seasonal changes in earthworm populations and microbial respiration under several forest species (Carpinus betulus, Ulmus minor, Pterocarya fraxinifolia, Alnus glutinosa, Populus caspica and Quercus castaneifolia) in a temperate mixed forest situated in northern Iran. Soil samplings were taken under six individual tree species (n = 5) in April, June, August and October (a total of 30 trees each month) to examine seasonal variability in soil chemical properties and soil biological activity. Earthworm density/biomass varied seasonally but not significantly between tree species. Maximum values were found in spring (10.04 m−2/16.06 mg m−2) and autumn (9.7 m−2/16.98 mg m−2) and minimum in the summer (0.43 m−2/1.26 mg m−2). Soil microbial respiration did not differ between tree species and showed similar temporal trends in all soils under different tree species. In contrast to earthworm activity, maximum microbial activity was measured in summer (0.44 mg CO2–C g soil−1 day−1) and minimum in winter (0.24 mg CO2–C g soil−1 day−1). This study shows that although tree species affected soil chemical properties (pH, organic C, total N content of mineral soils), earthworm density/biomass and microbial respiration are not affected by tree species but are controlled by tree activity and climate with strong seasonal dynamics in this temperate forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Hyrcanian vegetation zone, also called Caspian forest, is a green belt stretching over the northern slopes of the Alborz mountain ranges and covers the southern coast of the Caspian Sea (Talebi et al. 2014). These mixed forests are one of the last remnants of natural deciduous forests in the world (Kooch et al. 2014). While the Caspian coastal areas enjoy a milder climate, the inland plateau experiences extremes of temperature in summer and winter (Talebi et al. 2014). These mixed forests are one of the most diverse in the area and are composed of several species. The overstory composition of a forest affects soil biological and physical–chemical functioning (Augusto et al. 2015). Understanding how tree species affect soils is important because soils are a fundamental component of ecosystems, and changes in soil properties are likely to change future vegetation development at a given site (Frouz et al. 2013). In order to obtain a broader insight into how single forest species affect soils, aspects such as biological activity and soil physicochemical properties should be taken into consideration (Neirynck et al. 2000). Tree species affect physical, chemical and biological characteristics of soils in several ways depending on leaf and root traits (Wang et al. 2009). Additionally, spatial and temporal variability are crucial to understand soil functionality in forest ecosystems. Up to now, most studies have examined physical and chemical properties of soils, but its biological activity is rarely considered (Frouz et al. 2013). The effect of trees on soil physical and chemical properties can be mediated by soil fauna, which may substantially affect topsoil properties as a consequence of bioturbation (Frouz et al. 2013). Recent studies recognize soil fauna as one of the important factors for evaluating soil quality and health (Holdsworth et al. 2012; Frouz et al. 2013). Among these, macrofauna plays a crucial role in food and energy cycles, affecting mainly soil organic matter dynamics (Barrios 2007). Among soil macrofauna, earthworms account for the greatest invertebrate biomass (Tondoh et al. 2007; Blouin et al. 2013). Previous studies (Suthar 2012; Sackett et al. 2013; Crumsey et al. 2014) have described the main factors influencing earthworm activity, including tree species, soil and litter characteristics (such as soil pH and litter palatability) and environmental factors (such as soil temperature and moisture). Meanwhile, earthworms exert a key role in nutrient cycling through their own metabolism increasing carbon and nitrogen, stimulation of microbial activity and bioturbation or physical mixing of organic and mineral soil (Suthar 2012).
The presence of different tree species having different above- and belowground functional traits may result in distinct soil microbial communities with different abilities to resist and recover after a disturbance (Rivest et al. 2015). Soil respiration is a major process controlling carbon (C) loss from terrestrial ecosystems (Huang et al. 2014; Makita and Fujii 2015; Rey 2015) and a good indicator of total belowground C allocation and ecosystem productivity (Davidson et al. 2000). How tree species impact soil microbial respiration is still limited, but is crucial to guide selection of species for reforestation and carbon management purposes (Makita and Fujii 2015). The activity of soil biota is strongly driven by environmental factors such as soil temperature, soil moisture, bulk density and pH. Moreover, soil community composition regulates soil carbon and nitrogen dynamics (Huang et al. 2014; Sherman et al. 2015). Soil temperature and soil water content are the main environmental factors controlling the temporal variation in CO2 emission from soils (Davidson et al. 2000; Rey et al. 2002). However, the direct effect of tree species on both soil autotrophic and heterotrophic respiration is less well understood, because of the strong interaction between abiotic (e.g., soil moisture, temperature and texture) and biotic (e.g., quality and quantity of organic matter input, soil functioning) factors (Huang et al. 2014). In temperate forest ecosystems, soil temperature is the most important factor controlling the spatial and temporal variation of soil heterotrophic respiration, while soil moisture is secondary, only important under drought conditions (Huang et al. 2014). Tree species can influence the composition and functioning of the underlying soil microbial communities (Garbeva et al. 2006) through differences in tree canopy cover, litter quality and quantity (Gruselle and Bauhus 2010). On the other hand, many studies have revealed strong correlations between microbial community structure and an array of environmental factors, including pH, quantity and quality of C and N (e.g., Cleveland et al. 2014). The variability of environmental conditions, temperature and moisture regimes can strongly affect soil biological activity (Qiu-Hui et al. 2012) and biochemical properties (Guicharnaud et al. 2010; Zhu and Cheng 2013), nutrient cycling (Mikha et al. 2005) and site productivity (Sajedi 2010). In order to gain better understanding on the spatial and temporal distribution of earthworm populations and microbial activity, we carried out a study in a mixed forest located in Northern Iran. The aim of this study was to investigate: (1) how tree species affect soil physicochemical properties and, in turn, earthworm density/biomass and microbial activity, (2) seasonal changes in earthworm and microbial activity under different forest species within the forest and (3) which factors determine earthworm and microbial activity in these temperate forests.
Materials and methods
Site characteristics
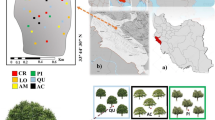
The study area is located at the experimental forest station of Tarbiat Modares University, north of Iran (51°46”E, 36°47″N) (Fig. 1a, b). The experimental plots were located at an altitude of 15 m above sea level. The area is flat and uniform (slope 0–3%). Mean annual temperature is 17 °C, and mean annual rainfall is 803.4 mm with a dry season between May and August (Fig. 2). The parent material is dolomite limestone that belongs to upper Jurassic and lower Cretaceous periods. The soil is of the order Alfisols, with a silty clay loam texture (Kooch et al. 2012). The natural forest vegetation is temperate deciduous forests containing broadleaved species dominated by oak (Quercus castaneifolia C. A. M. macranthera F. and M.), hornbeam (Carpinus betulus L.), elm (Ulmus minor Mill., Ulmus glabra Huds.), Caucasian wingnut (P. fraxinifolia), Persian poplar (Populus caspica Bornm.) with some associated species such as maple (Acer velutinum Boiss., Acer cappadocium Gled.), ash (Fraxinus excelsior L.), alder (Alnus subcordata C. A. M., Alnus glutinosa Gaertn.), wild cherry (Prunus avium L.), wild service tree (Sorbus torminalis Crantz) and lime tree (Tilia platyphyllus Scop.) (Mirzaei et al. 2007). According to a previous report (Anonymous 2015), the variations in soil physical characteristics [i.e., soil bulk density (1.35–1.56 g cm−3) and texture (20.40–25.20% for sand; 38.60–48.20% for silt and 29.20–36.40% for clay)] were not significantly different among tree species in the study area. However, litter thickness and C/N ratio were significantly higher under oak (15.72 cm and 61.16, respectively) and the least were found under alder (6.29 cm and 21.35, respectively) species.
Location of the study site in the Hyrcanian zone, the Central Caspian region of northern Iran (a, b). Study site with 50 ha area including clumps of tree species [C. betulus (CB), U. minor (UM), P. fraxinifolia (PF), A. glutinosa (AG), P. caspica (PC) and Q. castaneifolia (QC)] (c, d). Five replications for each tree species with same diameter class of ≈65 cm were considered (n = 30); soil samples with 0.5 m2 to a 20 cm depth (e) were taken under tree canopy cover in April, June, August and October (N total = 120). These species were surrounded by same tree species (the schematic design not in scale)
Soil sampling and analyses
In this study, the effect of individual tree species on soil chemical and biological features in mixed Caspian plain temperate forests with area of 50 hectares was investigated. For all species, weather conditions, topography (elevation and slope), geology and soil types were identical. All tree species were identified in the study area. Samplings were performed under six species of C. betulus (CB), U. minor (UM), P. fraxinifolia (PF), A. glutinosa (AG), P. caspica (PC) and Q. castaneifolia (QC). Five individuals of each tree species (with same diameter class of ≈65 cm) were considered (in total 30 trees each month), and soil samples underneath each tree (n = 120) were taken in April, June, August and October. The selected trees were surrounded by individuals of the same tree species (Fig. 1c, d). Earthworms were collected by a combination of hand sorting and mustard extraction (on the whole sampling area of 0.5 m2 to a 20 cm depth; Fig. 1e), followed by hand sorting of a soil sample (Hlava and Kopecký 2013). Sampling of earthworms was conducted in the soil volume after the removal of litter (Scullion et al. 2014). Then, whole of soils related to one sample were completely mixed in the field (0.5 m2 to a 20 cm depth), and just some parts (≈500 g) were taken to the laboratory. Soil samples for microbial respiration determination were immediately transferred to a cooled, insulated container for transport to the laboratory and were stored at 4 °C until they were processed. Soils for chemical analyses were air-dried and passed through a 2-mm sieve (aggregates were broken). We determined the following soil properties: soil water content by drying soil samples at 105 °C for 24 h, soil pH using an Orion Analyzer Model 901 pH meter in a 1:2.5, soil/water solution, soil organic C using the Walkley–Black technique (Allison 1975) and total N using a semi-micro-Kjeldahl technique (Bremner and Mulvaney 1982). The collected earthworms were washed and weighed to milligram precision after oven drying at 60 °C for 24 h for earthworm biomass determination (Kooch et al. 2014). Species of earthworms were identified (epigeic, anecic and endogeic) by external characteristics using the key of Edwards and Bohlen (1996). Soil microbial respiration was determined by measuring the CO2 evolved in a 3-day incubation experiment at 25 °C, in which 50 g of each soil sample (remoistened to 55% its water holding capacity) was placed in a glass jar. A glass vial holding 10 mL of 0.5 M NaOH was placed in a glass jar to trap the evolved CO2. The excess alkali, after precipitating the CO3 2− with 0.5 M BaCl2 solution, was titrated with standard 0.5 M HCl to a phenolphthalein end point (Alef 1995).
Statistical analysis
Three-way analysis of variance (ANOVA) with repeated measures on one factor (time) were used to compare soil properties between tree species over time. When factors were significant (P < 0.05), post hoc tests were done using a Duncan’s multiple comparison test. All statistical analyses were conducted using the SPSS v. 20 statistical software package. Data were checked to meet the assumptions of normality by the Kolmogorov–Smirnov test and homogeneity of variances by the Levene’s test. Relationships between earthworm density, biomass (earthworm communities) and chemical properties of soils and microbial activity under the six tree species across different seasons (months) were analyzed by principle component analysis (PCA) using PC-Ord version 5.0 (McCune and Mefford 1999).
Results
Soil water content and soil chemical properties
Overall, tree species affected soil chemical properties (Table 1). All soil properties showed statistically significant differences among different tree species: soil water content (P = 0.006), pH (P = 0.001), soil organic C content (P < 0.001), total N (P < 0.001) and C/N ratio (P < 0.001) (Table 1). Soil organic C was significantly higher in all months under QC when compared with the other tree species (Table 2). Total N was significantly higher in soils under AG, and soil C/N ratio was significantly higher under the QC when compared with the other studied tree species (Table 2). Except for C/N ratio (P > 0.05), all soil physicochemical properties varied with season (Table 1). Maximum soil organic C (P = 0.003) and total N (P < 0.001) were measured in August (Tables 1, 2). Water content (P < 0.001) and pH (P < 0.001) were significantly higher in April compared with values measured in the other months (Tables 1, 2).
Soil biological activity
Soil biological activity was not significantly different among tree species (P > 0.05), but changed seasonally in the same way in all tree species (Table 1). Earthworm density/biomass (P < 0.001), epigeic density/biomass (P < 0.001), anecic density/biomass (P < 0.001), endogeic density/biomass (P < 0.001) and microbial respiration (P < 0.001) varied significantly over time (Table 1). The amount of earthworm density/biomass was maximum in April and October and minimum in August (Figs. 3, 4). Similarly, soil microbial respiration was maximum in April and minimum in October (Fig. 5). From the PCA output, the first PC explained more than 60% of variance in soil properties under studied tree species in different seasons (Fig. 6). The right PC1 shows that under conditions with more water content, pH favored higher activity of earthworms (April and October), while the left PC1 shows that water content, pH and earthworms activity were minimum over the summer (June and August) under different studied tree species (Fig. 6). In the case of soil microbial respiration, the PC2 explained less than 20% of the variance, with soil water content, pH, organic C and total N explaining microbial activity in August (as significant under AG tree species) and April (Fig. 6).
Temporal variability in earthworm population density (epigeic, anecic and endogeic) (m−2) under the six tree species studied [C. betulus (CB), U. minor (UM), P. fraxinifolia (PF), A. glutinosa (AG), P. caspica (PC) and Q. castaneifolia (QC)] in the 4 months. Different letters (a–c) indicate a significant difference under the six tree species and month (analysis of variance (ANOVA) and Duncan’s test; P < 0.05). Values are the mean (n = 5 ± 1 SE for each tree species, N total = 30; n = 30 ± 1 SE for each month, N total = 120)
Temporal variability in earthworm population biomass (epigeic, anecic and endogeic) (mg m−2) under the six tree species studied [C. betulus (CB), U. minor (UM), P. fraxinifolia (PF), A. glutinosa (AG), P. caspica (PC) and Q. castaneifolia (QC)] in the 4 months. Different letters (a, b and c) indicate a significant difference under the six tree species and month (analysis of variance (ANOVA) and Duncan’s test; P < 0.05). Values are the mean (n = 5 ± 1 SE for each tree species, N total = 30; n = 30 ± 1 SE for each month, N total = 120)
Temporal variability in soil microbial respiration (mg CO2–C g dry soil−1 day−1) under the six tree species studied [C. betulus (CB), U. minor (UM), P. fraxinifolia (PF), A. glutinosa (AG), P. caspica (PC) and Q. castaneifolia (QC)] in the 4 months. Different letters (a, ab, b, abc, bc and c) indicate a significant difference under the six tree species and month (analysis of variance (ANOVA) and Duncan’s test; P < 0.05). Values are the mean (n = 5 ± 1 SE for each tree species, N total = 30 for all trees; n = 30 ± 1 SE for each month, N total = 120 for all month)
PCA based on the correlation matrix of the tree species, soil water content, chemical and biological properties in different months. The studied tree species were the C. betulus (CB), U. minor (UM), P. fraxinifolia (PF), A. glutinosa (AG), P. caspica (PC) and Q. castaneifolia (QC). PC1: Eigen value = 6.20, percent of variance = 68.94, cumulative percent of variance = 68.94; PC2: Eigen value = 1.36, percent of variance = 15.12, cumulative percent of variance = 84.06
Discussion
Earthworm activity
Our main objective was to investigate whether tree species within a forest affect earthworm diversity and activity. Since forest tree species affect soil physicochemical properties, we expected changes in earthworm activity. In contrast to other studies that have found that earthworm population are affected by tree species (e.g., differences in litter quality) and/or soil features (e.g., pH, nutrient availability) (Kooch et al. 2012; Crumsey et al. 2014), our study did not show significant statistical differences in earthworm density/biomass among tree species. Instead, our results showed temporal changes in earthworm density/biomass driven by seasonal changes in environmental conditions. Indeed, environmental conditions play an important role in the functional categories of earthworms present in an ecosystem (Chaudhuri and Paliwal 2008; Suthar 2012) causing spatial and temporal heterogeneity in earthworm populations. Soil moisture and soil temperature are usually inversely correlated in this climatic zone and act synergistically to influence earthworm activity (Day and Chaudhur 2014). Indeed, maximum earthworm activity was recorded in April and October, when soil moisture and soil temperature are favorable, whereas in August, the driest and warmest period, earthworm population was reduced (see Fig. 2). Crumsey et al. (2013) also showed that soil water content had significant effects on the relative abundance of earthworm species and was a stronger driver of earthworm species richness than pH or soil organic carbon. Overall, earthworm population density and biomass values are similar to those reported by Ewing et al. (2015). Furthermore, we also observed differences in earthworm diversity with more abundance of epigeic compared to anecic and endogeic earthworms. This may be related to the deep burrowing ability of anecic and endogeic earthworms, a hypothesis supported by previous studies (Jégou et al. 2001; Uvarov 2009). This could also partly explain the low dispersal rates observed of these anecic and endogeic earthworms in the topsoil (0–20 cm) under different tree species and seasons, because of their activity in the lower layers of soil.
Similar to our findings, Suthar (2012) showed that maximum earthworm abundance occurs in autumn and declines sharply in winter. Low physiological activity and winter temperatures are likely to limit earthworm activity. In subsequent spring, earthworms reappeared and showed a moderate population density, which again declined in early summer and virtually disappeared in winter. In the rainy season, earthworms reappeared in topsoil layers and newly hatched subadults were also recorded during the period. The population abundances observed during rainy season showed continuous increase up to autumn months (Suthar 2012). Among the factors responsible for earthworm distribution in this study, soil moisture content seems to be of major importance as it has been observed in other studies (Edwards and Bohlen 1996; Crumsey et al. 2014). Kaushal and Bisht (1994) found that in northern part of India, the population density of earthworm was much higher during the wet period of the year than dry months. In Indian subtropical climate in plains, the abundance of earthworm population has been recorded during the rainy season leading to maximum densities in September–October and least during the hot summer (May–June) (Suthar 2012). In agreement with our results, Suthar (2012) indicated that earthworm population increased during spring and autumn but decreased in summer and winter. Besides moisture content, some other soil properties affected earthworm population at our study site. The differences in earthworm density/biomass in the present study can be also related to differences in soil fertility (Emmerling and Strunk 2012; Sigurdsson and Gudleifsson 2013; Sackett et al. 2013). In addition, in April, higher soil pH may have favored earthworm population according to previous studies (Whalen and Costa 2003; Chaudhuri and Paliwal 2008; Valckx et al. 2009). As suggested by Nurhidayati et al. (2012), earthworm abundance in the soil in spring is greatly related to food availability and soil physicochemical properties such as soil moisture, soil temperature and soil pH.
Soil microbial respiration
Soil respiration is a good indicator of total belowground allocation of carbon and of ecosystem productivity (Davidson et al. 2000). Similar to earthworm density/biomass, soil microbial respiration was not affected by tree species and showed similar temporal trends in all soils under different tree species with maximum activity in spring and summer, particularly under AG tree species. According to the findings of Mo et al. (2004) and Kooch et al. (2016), soil total N content is the main factor affecting decomposition of plant litter, and litter decomposition rate is a major source of soil CO2 emission (Rey et al. 2002) in the summer with high temperatures. Furthermore, the activities of these microorganisms can continue for a short time in the casts because of the suitable amount of soluble carbon and nutrient resources (Zirbes et al. 2012). Greatest values of total N and microbial respiration were found under AG species compared to other tree species. Parallel to our study, Cao et al. (2011) reported that the soil N content promoted the release of CO2 from soils since better litter quality promotes microbial litter decomposition. Indeed, (in spring season with suitable soil moisture and temperature), the survival of microorganisms in the earthworm gut depends on their capacity to resist digestive enzymes of microbial or earthworm origins, or to bacteriostatic and microbial substances and transit time. For example, epigeic species, which feed on rich substrates, need a complex enzymatic system (Zirbes et al. 2012). Zirbes et al. (2012) have shown that some microorganisms of soil increase in abundance through the gut tract of earthworms.
In our study, environmental variables controlled soil microbial respiration activity with no differences between tree species. Similar to our findings, previous studies (Schlesinger and Andrews 2000; Liu et al. 2012; Luo et al. 2014) have observed higher values of soil microbial respiration in the summer than autumn season in temperate regions where most favorable environmental conditions occur. In our study area, temperature increases during summer season (see Fig. 2) favoring microbially mediated processes in soils (Kooch et al. 2015). Accelerated turnover of soil organic compounds in summer is supported by increased organic C and total N, as well as by microbial respiration. Particularly, strong increases were observed for soil microbial respiration with increasing pH (in April), organic C and total N (in August). Variability in soil chemical properties over the season as well as changes in soil moisture can be responsible for temporal changes in soil microbial respiration. In August and April months, increased pH (Cookson et al. 2006), higher C (Kuzyakov et al. 2000; Huang et al. 2014; Makita and Fujii 2015) and N (Mo et al. 2004; Werner et al. 2007; Wang et al. 2013) and more available nutrients due to greater earthworms mass (Ponge 2003; Zirbes et al. 2012) can contribute to microbial respiration.
Conclusion
This study shows that tree species in the mixed forest present the same earthworm populations (i.e., earthworm density/biomass) and microbial respiration despite affecting soil chemical properties. In this temperate mixed forest, seasonal environmental changes drive soil biological activity being highest in spring and summer when optimum environmental conditions occur in this region. The main soil properties affecting soil biological activity were soil water content, pH, total organic C and total N. Our study shows that deciduous tree species in the mixed forest affect soil chemical properties and contribute to spatial heterogeneity, but biological activity is driven by climate. Therefore, expected changes in climate will potentially have a stronger impact on soil biological activity than changes in forest diversity.
References
Alef K (1995) Estimating of soil respiration. In: Alef K, Nannipieri P (eds) Methods in soil microbiology and biochemistry. Academic, New York, pp 464–470
Allison LE (1975) Organic carbon In: Methods of soil analysis. American Society of Agronomy, Part 2, Madison, pp 1367–1378
Anonymous (2015) Planning of plain forest conservation. Experimental Forest Station of Tarbiat Modares University, p 165
Augusto L, De Vesterdal L, Smolander A, Prescott C, Ranger J (2015) Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol Rev 90:444–466
Barrios E (2007) Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285
Blouin M, Hodson ME, Delgado EA, Baker G, Brussaard L, Butt KR, Brun JJ (2013) A review of earthworm impact on soil function and ecosystem services. Eur J Soil Sci 64:161–182
Bremner JM, Mulvaney CS (1982) Nitrogen—total. In: Page AL, Miller RH, Keeney RR (eds) Methods of soil analysis, 2nd edn. American Society of Agronomy, Part 2, Madison, pp 595–624
Cao YS, Lin YB, Rao XQ, Fu SL (2011) Effects of artificial nitrogen and phosphorus depositions on soil respiration in two plantations in southern China. J Trop For Sci 23:110–116
Chaudhuri PS, Paliwal SNR (2008) Earthworm population of rubber plantations (Hevea brasiliensis) in Tripura, India. Trop Ecol 49:225–234
Cleveland CC, Reed SC, Keller AB, Nemergut DR, O’Neill SP, Ostertag R, Vitousek PM (2014) Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia 174:283–294
Cookson WR, Osmar M, Marschner P, Abaye DA, Clark I, Murphy DV, Stockdale EA, Watson CA (2006) Controls on soil nitrogen cycling and microbial community composition across land use and incubation temperature. Soil Biol Biochem 39:744–756
Crumsey J, Le Moine JM, Vogel CS, Nadelhoffer KJ (2013) Historical patterns of exotic earthworm distributions inform contemporary associations with soil physical and chemical factors across a northern temperate forest. Soil Biol Biochem 68:503–514
Crumsey JM, Le Moine JM, Vogel CS, Nadelhoffer KJ (2014) Historical patterns of exotic earthworm distributions inform contemporary associations with soil physical and chemical factors across a northern temperate forest. Soil Biol Biochem 68:503–514
Davidson EA, Verchot LV, Cattânio JH, Ackerman IL, Carvalho JEM (2000) Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 48:53–69
Day A, Chaudhur PS (2014) Earthworm community structure of pineapple (Ananas comosus) plantations under monoculture and mixed culture in West Tripura, India. Trop Ecol 55:1–17
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms, 3rd edn. Chapman and Hall, London
Emmerling C, Strunk H (2012) Active dispersal of the endo-anecic earthworm Aporrectodea longa (Ude) in an experimental box. Soil organisms 84:491–498
Ewing HA, Tuininga AR, Groffman PM, Weathers KC, Fahey TJ, Fisk MC, Suarez E (2015) Earthworms reduce biotic 15-nitrogen retention in northern hardwood forests. Ecosystems 18:328–342
Frouz J, Livečková M, Albrechtová J, Chroňáková A, Cajthaml T, Pižl V, Cepáková Š (2013) Is the effect of trees on soil properties mediated by soil fauna? a case study from post-mining sites. For Ecol Manag 309:87–95
Garbeva P, Postma J, Van Veen JA, Van Elsas JD (2006) Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ Microbiol 8:233–246
Gruselle MC, Bauhus J (2010) Assessment of the species composition of forest floor horizons in mixed spruce-beech stands by near infrared reflectance spectroscopy (NIRS). Soil Biol Biochem 42:1347–1354
Guicharnaud RA, Arnalds O, Paton GI (2010) The effect of season and management practices on soil microbial activities undergoing nitrogen treatments—interpretation from microcosm to field scale. Icel Agric Sci 23:123–134
Hlava J, Kopecký O (2013) Earthworm population responses to deciduous forest soil acidity and vegetation cover. Sci Agric Bohem 44:133–137
Holdsworth AR, Frelich LE, Reich PB (2012) Leaf litter disappearance in earthworm-invaded northern hardwood forests: role of tree species and the chemistry and diversity of litter. Ecosystems 15:913–926
Huang Z, Yu Z, Wang M (2014) Environmental controls and the influence of tree species on temporal variation in soil respiration in subtropical China. Plant Soil 382:75–87
Jégou D, Capowiez Y, Cluzeau D (2001) Interactions between earthworm species in artificial soil cores assessed through the 3D reconstruction of the burrow systems. Geoderma 102:123–137
Kaushal BR, Bisht SPS (1994) Population dynamics of earthworm Amynthas alenxandri (Annelida: Megascolecidae) in a Kuman Himalayan Pasture Soil. Biol Fertil Soils 17:9–13
Kooch Y, Hosseini SM, Zaccone C, Jalilvand H, Hojjati SM (2012) Soil organic carbon sequestration as affected by afforestation: the Darab Kola forest (North of Iran) case study. J Environ Monit 14:2438–2446
Kooch Y, Hosseini SM, Samonil P, Hojjati SM (2014) The effect of windthrow disturbances on biochemical and chemical soil properties in the northern mountainous forests of Iran. Catena 116:142–148
Kooch Y, Mollaei Darabi S, Hosseini SM (2015) The effects of pits and mounds following wind throw events on soil features and greenhouse gas fluxes in a temperate forest. Pedosphere 25:853–867
Kooch Y, Rostayee F, Hosseini SM (2016) Effects of tree species on topsoil properties and nitrogen cycling in natural forest and tree plantations of northern Iran. Catena 144:65–73
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Luo S, Liu G, Li Z, Hu C, Gong L, Wang M, Hu H (2014) Soil respiration along an altitudinal gradient in a subalpine secondary forest in China. IFor Biogeosci For (Early View). doi:10.3832/ifor0895-007
Makita N, Fujii S (2015) Tree species effects on microbial respiration from decomposing leaf and fine root litter. Soil Biol Biochem 88:39–47
McCune B, Mefford M (1999) Multivariate analysis of ecological data version 4.17. MJM software. Gleneden Beach, Oregon, p 233
Mikha MM, Rice CW, Milliken GA (2005) Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol Biochem 37:339–347
Mirzaei J, Tabari M, Daroodi H (2007) Early growth of Quescus castaneifolia (C.A. Meyer) seedlings as affected by weeding, shading and irrigation. Pak J Biol Sci 10:2430–2435
Mo JM, Xue JH, Fang YT (2004) Litter decomposition and its responses to simulated N deposition for the major plants of Dinghushan forests in subtropical China. Acta Entomol Sin 24:1420–1513
Neirynck J, Mirtcheva S, Sioen G, Lust N (2000) Impact of Tilia platyphyllos Scop., Fraxinus excelsior L., Acer pseudoplatanus L., Quercus robur L. and Fagus sylvatica L. on earthworm biomass and physico-chemical properties of loamy topsoil. For Ecol Manag 133:275–286
Nurhidayati N, Arisoesilaningsih E, Suprayogo D, Hairiah K (2012) Earthworm population density in sugarcane cropping system applied with various quality of organic matter. J Trop Life Sci 3:103–109
Ponge JF (2003) Humus forms in terrestrial ecosystems: a framework to biodiversity. Soil Biol Biochem 35:935–945
Qiu-Hui CH, Ying FE, Zhang YP, Zhang QC, Shamsi IH, Zhang YS, Xian-Yong LIN (2012) Short-term responses of nitrogen mineralization and microbial community to moisture regimes in greenhouse vegetable soils. Pedosphere 22:263–272
Rey A (2015) Mind the gap: non-biological processes contributing to soil CO2 efflux. Glob Change Biol 21:1752–1761
Rey A, Pegoraro E, Tedeschi V, Di Parri I, Jarvis PG, Valentini R (2002) Seasonal variability in soil respiration and its components in a coppice oak forest in central Italy. Glob Change Biol 8:1–18
Rivest D, Paquette A, Shipley B, Reich PB, Messier C (2015) Tree communities rapidly alter soil microbial resistance and resilience to drought. Funct Ecol 29:570–578
Sackett TE, Smith SM, Basiliko N (2013) Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biol Biochem 57:459–467
Sajedi T (2010) The effects of excessive moisture on soil carbon and nitrogen mineralization and forest productivity. PhD thesis, University of British Colombia, p 160
Schlesinger WH, Andrews JA (2000) Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20
Scullion J, Smith AR, Gwynn-Jones D, Jones DL, Godbold DL (2014) Deciduous woodland exposed to elevated atmospheric CO2 has species-specific impacts on anecic earthworms. Appl Soil Ecol 80:84–92
Sherman RE, Bohlen PJ, Maerz JC (2015) Earthworms increase soil microbial biomass carrying capacity and nitrogen retention in northern hardwood forests. Soil Biol Biochem 30:1–8
Sigurdsson BD, Gudleifsson BE (2013) Impact of afforestation on earthworm populations in Iceland. Icel Agric Sci 26:21–36
Suthar S (2012) Seasonal dynamics in earthworm density, casting activity and soil nutrient cycling under Bermuda grass (Cynodon dactylon) in semiarid tropics, India. Environmentalist 32:503–511
Talebi KS, Sajedi T, Pourhashemi M (2014) Forests of Iran: `a treasure from the past, a hope for the future’. Springer, Berlin, pp 15–65
Tondoh JE, Monin LM, Tiho S, Csuzdi C (2007) Can earthworm be used as bio-indicators of land-use perturbations in semi-deciduous forest? Biol Fertil Soils 43:585–592
Uvarov AV (2009) Inter- and intraspecific interactions in lumbricid earthworms: their role for earthworm performance and ecosystem functioning. Pedobiologia 53:1–27
Valckx J, Cockx L, Wauters J, Van Meirvenne M, Govers G, Ermy M, Muys B (2009) Within-field spatial distribution of earthworm populations related to species interactions and soil apparent electrical conductivity. Appl Soil Ecol 41:315
Wang S, Ruan H, Wang B (2009) Effects of soil microarthropods on plant litter decomposition across an elevation gradient in the Wuyi Mountains. Soil Biol Biochem 41:891–897
Wang H, Liu Sh, Wang J, Shi Z, Lu L, Ji Z, Ming A, Tang J, Yu H (2013) Effects of tree species mixture on soil organic carbon stocks and greenhouse gas fluxes in subtropical plantations in China. For Ecol Manag 300:4–13
Werner C, Kiese R, Butterbach-Bahl K (2007) Soil-atmosphere exchange of N2O, CH4, and CO2 and controlling environmental factors for tropical rain forest sites in western Kenya. J Geophys Res Atmos 112:1–15
Whalen JK, Costa C (2003) Linking spatio-temporal dynamics of earthworm populations to nutrient cycling in temperate agricultural and forest ecosystems. Pedobiologia 47:801–806
Zhu B, Cheng W (2013) Impacts of drying-wetting cycles on rhizosphere respiration and soil organic matter decomposition. Soil Biol Biochem 63:89–96
Zirbes L, Thonart P, Haubruge E (2012) Microscale interactions between earthworms and microorganisms: a review. Biotechnol Agron Soc Environ 16:125–131
Acknowledgement
The authors acknowledge Mr. K. Nourmohammadi, Mr. A. Khodadust, Mr. A. Daryaei, Mr. R. Hossinpour, Mr. R. Darvand, Mr. P. Amozegar and Mr. Y. Shahrokhzade for their tireless assistance with field sampling and also Mis. M. Haghdust and Mr. S. Boor for their expert technical assistance at the laboratory analysis. This work was done by financial support of Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jarmo Holopainen.
Rights and permissions
About this article
Cite this article
Bayranvand, M., Kooch, Y. & Rey, A. Earthworm population and microbial activity temporal dynamics in a Caspian Hyrcanian mixed forest. Eur J Forest Res 136, 447–456 (2017). https://doi.org/10.1007/s10342-017-1044-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-017-1044-5