Abstract
The possible effects of soil microbial community structure on organic matter decomposition rates have been widely acknowledged, but are poorly understood. Understanding these relationships is complicated by the fact that microbial community structure and function are likely to both affect and be affected by organic matter quality and chemistry, thus it is difficult to draw mechanistic conclusions from field studies. We conducted a reciprocal soil inoculum × litter transplant laboratory incubation experiment using samples collected from a set of sites that have similar climate and plant species composition but vary significantly in bacterial community structure and litter quality. The results showed that litter quality explained the majority of variation in decomposition rates under controlled laboratory conditions: over the course of the 162-day incubation, litter quality explained nearly two-thirds (64 %) of variation in decomposition rates, and a smaller proportion (25 %) was explained by variation in the inoculum type. In addition, the relative importance of inoculum type on soil respiration increased over the course of the experiment, and was significantly higher in microcosms with lower litter quality relative to those with higher quality litter. We also used molecular phylogenetics to examine the relationships between bacterial community composition and soil respiration in samples through time. Pyrosequencing revealed that bacterial community composition explained 32 % of the variation in respiration rates. However, equal portions (i.e., 16 %) of the variation in bacterial community composition were explained by inoculum type and litter quality, reflecting the importance of both the meta-community and the environment in bacterial assembly. Taken together, these results indicate that the effects of changing microbial community composition on decomposition are likely to be smaller than the potential effects of climate change and/or litter quality changes in response to increasing atmospheric CO2 concentrations or atmospheric nutrient deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic matter decomposition controls the size of soil C pools, helps regulate the rate and magnitude of CO2 fluxes from the biosphere to the atmosphere, and acts as a dominant control over the recycling of plant nutrients in terrestrial ecosystems (Swift et al. 1979; Chapin et al. 2002). As such, identifying the controls over decomposition is critical for understanding terrestrial ecosystem function, both now and into the future. An extensive body of research has shown that decomposition rates are regulated by climate, litter quality and decomposer organisms (Daubenmire and Prusso 1963; Meentemeyer 1978; Swift et al. 1979; Couteaux et al. 1995; Aerts 1997; Moorhead et al. 1999; Gholz et al. 2000; Cornwell et al. 2008), but that the relative importance of those factors varies with scale. Climatic differences often explain the largest proportion of variation in decomposition rates at regional to global scales (Meentemeyer 1978; Hobbie 1996; Aerts 1997; Gholz et al. 2000), but at smaller spatial scales (over which climate remains relatively constant), litter quality—commonly assessed by examining concentrations of litter lignin or lignin to nutrient ratios—explains much of the variation in decomposition rates (Daubenmire and Prusso 1963; Melillo et al. 1982; Aerts 1997; Cornwell et al. 2008).
The relationships between climate, litter quality and decomposition are well represented in a range of contemporary ecosystem models (Parton et al. 1994; Moorhead et al. 1999), but few models explicitly consider the effects of variations in microbial community composition on terrestrial C cycling in detail (Treseder et al. 2012). Uncertainty about the identity and role of microbes confounds predictions of how changes in microbial community composition may drive decomposition responses to global environmental change (Castro et al. 2010; Nemergut et al. 2010; Ramirez et al. 2010; Wang et al. 2012). In addition, our understanding of how variations in microbial community structure and diversity could alter litter decomposition dynamics within an ecosystem type with a similar climate is poor, largely because it is difficult to experimentally isolate and/or manipulate microbial community composition in a controlled and realistic way (Reed and Martiny 2007; McGuire and Treseder 2010). However, variations in microbial community composition and diversity have been shown to correlate with variations in rates of multiple ecosystem processes (Schimel and Gulledge 1998; Zak et al. 2003; Reed et al. 2010; Philippot et al., submitted), leading some to suggest that decomposer community composition and physiology should be explicitly included in C cycle models (e.g., Allison et al. 2010; McGuire and Treseder 2010; Wang et al. 2012).
On the other hand, many studies have revealed strong correlations between microbial community structure and an array of environmental factors, including pH and C quantity and quality (Fierer and Jackson 2006; Lozupone and Knight 2005; Logue and Lindström 2010; Nemergut et al. 2010; Legg et al. 2012). The fact that many of these same parameters influence ecosystem processes (Paul and Clark 1996; Chapin et al. 2002) prompts the question, how much added value is provided by detailed information on microbial community structure (e.g., Schimel 2001)? Certainly, the degree to which microbial community assembly is niche based—or driven by environmental parameters—will affect the degree to which an understanding of microbial community structure is important for accurate predictions of community function (Nemergut et al. 2013). Likewise, the amount of functional redundancy within a community will have a strong influence on the relationship between structure and function—the more redundancy, the less valuable structural data may be in predicting process (e.g., Schimel and Gulledge 1998). Further, the degree to which communities are assembled at the functional level rather than at the phylogenetic level (e.g., Burke et al. 2011) will affect how much additional information is provided by community composition data for understanding processes.
The goal of this work was to quantify the relative effects of litter quality versus microbial community structure in regulating litter decomposition rates (assessed as CO2 respired). Recently, experimental approaches (e.g., reciprocal transplants) have been used to directly explore the potential effects of microbial community composition on ecosystem processes (e.g., Balser and Firestone 2005; Reed and Martiny 2007; Ayres et al. 2009), and we took a similar approach here. For example, Strickland et al. (2009a) assessed the effects of microbial community composition on the decomposition of rhododendron, pine, and grass litter sampled from three different temperate ecosystems by incubating litters with a soil inoculum obtained from each ecosystem type in a full-factorial design. The authors concluded that microbial community composition explained a significant proportion of the variation in C mineralization rates (with community explaining 22.3–86.2 % of rate variation), and that soil communities decomposed litter from a common site more rapidly than litter from a foreign site (i.e., home-field advantage). However, while reciprocal transplants of microbial communities between such different ecosystem types may illustrate the maximum likely importance of variations in decomposer community composition on C mineralization rates, they may not reflect the effects of more subtle changes in biogeochemical properties (e.g., litter quality) or microbial community composition (Henry et al. 2005; Finzi et al. 2006; Castro et al. 2010; Nemergut et al. 2010) of the sort that could occur within a single ecosystem type. Many such within-system changes are predicted for terrestrial ecosystems across the globe in the next few decades (e.g., Luo et al. 2006), with potentially important effects on C cycling.
In light of previous results (e.g., Strickland et al. 2009a) and in an attempt to assess how changes to litter quality and decomposer community within a single ecosystem type could affect decomposition, we conducted a laboratory incubation experiment. Specifically, we performed reciprocal transplants with soil inoculum and litter from three sites occupying an ecosystem age gradient in the Hawaiian Islands. Our overall goal was to quantify the relative effects of litter quality versus soil inoculum in regulating litter decomposition rates. In doing so, we implicitly assumed that potential differences in decomposition with varying inoculum types would most strongly reflect differences in microbial community composition among inoculum types (e.g., Strickland et al. 2009a). However, variations in litter decomposition with different inoculum types may have also been influenced by variation in edaphic characteristics of the inoculum types (e.g., Strickland et al. 2009b). Thus, to specifically examine the relationship between bacterial community structure and respiration rates, we also used pyrosequencing of 16S rRNA genes at each time point. This approach allowed us to not only examine the relationships between the starting communities and ecosystem function, but also to examine any subsequent changes in bacterial community composition that may have occurred through assembly processes.
Materials and methods
Study sites
We collected samples from three sites spanning a 4-million-year chronosequence in the Hawaiian Islands (Vitousek 2004). This long substrate age gradient (LSAG) includes sites that vary in soil fertility due to substantial differences in substrate age, but in which other ecosystem state factors (e.g., climate, parent material, and topography) remain constant (Crews et al. 1995; Vitousek 2004; Reed et al. 2011; Supplemental Table 1). In addition, the tree species Metrosideros polymorpha is dominant at all of the sites, but its foliar chemistry and leaf litter quality vary significantly along the gradient (Hobbie and Vitousek 2000; Supplemental Table 1), allowing us to quantify the effects of varying leaf litter chemistry (of a single tree species) versus varying bacterial community composition on decomposition. The “young” site (Thurston) on the island of Hawaii was initiated ~300 years ago by eruptions from the Kilauea volcano, the “intermediate-aged” site (Laupāhoehoe), also on the island of Hawaii, was initiated ~20,000 years ago in tephra deposits following eruptions of Mauna Kea, and the “old” site (Kokee) on the island of Kauai was initiated ~4.1 million years ago.
Soil and litter collection and analysis
At each site, M. polymorpha litter was collected by deploying four 40 × 40-cm litter traps (1-mm mesh) in random locations on the forest floor around trees inside a set of unamended (control) plots nested within a larger set of long-term nutrient manipulation plots (Vitousek et al. 1997; Vitousek 2004). Within 14 days of deploying the traps, M. polymorpha litter was collected, air dried and ground to a fine powder (40 mesh) using a Wiley Mill (Thomas Scientific, Swedesboro, NJ) and mixed to form a single, homogeneous litter source per site. Litter was ground in an attempt to create homogeneous samples and to minimize the possible influence of variations in physical litter structure on decomposition rates, as M. polymorpha has very tough and variably sized leaves. Ground litter was sterilized using an autoclave [two cycles, each for 1 h, 120 °C, 0.10 MPa (15 lb in.−2)] to maximize the chance that decomposer communities were introduced only via the inoculum sources, but we acknowledge that autoclaving samples may have altered the original litter chemistry. Sterilized litter subsamples from each site were dried for 72 h at 65 °C, and analyzed for total C and N using an elemental analyzer (CE Elantech model EA1110, Milan, Italy). Ash-free litter lignin concentrations were determined using acidified detergent dissolution (Association of Official Analytical Chemists official method 973.18).
At each site, three surface (0–10 cm) soil samples were collected from an area adjacent to the litter traps using a hand bulb corer (7 × 10 cm) after removing the litter (Oi) layer from the soil surface. Within 24 h of collection, soil samples were shipped on ice to the laboratory at the University of Montana, hand homogenized and gently sieved to ~4 mm to remove rocks and large organic debris. Samples were bulked to form a single soil sample per site, and these composited samples were used as the inoculum sources in the incubation experiment (see below). Soil moisture content and water holding capacity (WHC) of the composited samples were assessed gravimetrically, and total soil C and N contents were determined on dry soil samples using an elemental analyzer. All fresh soil samples were refrigerated for 24 h until the start of the incubation experiment.
Litter incubation experiment
We used a reciprocal transplant design to simultaneously test the effects of bacterial community composition and litter quality on decomposition. Reciprocal transplant approaches can partition the relative importance of microbial community and litter quality, as well as detect the interactive effects on decomposition (Reed and Martiny 2007). We manipulated litter and soil inoculum types from each of the three sites in a full-factorial design. The four litter and inoculum types consisted of M. polymorpha litter or soil collected from each of the three LSAG sites and a set of “mixed” treatments that consisted of equal proportions of litter or inoculum from the three sites. Thus, the experiment included a total of 16 treatments (four litter types × four inoculum types). We began the experiment with a total of 12 replicates per treatment, but on days 1, 22 and 162 we destructively harvested three replicates for chemical and bacterial community analyses. In addition to the 16 experimental treatments, we also incubated “litter-only” and “inoculum-only” samples (n = 3 per group) to assess possible background respiration rates in litter or soil alone. Finally, a set of empty tubes served as blanks for each of the 18 unique incubation types.
In an attempt to minimize other potential non-microbial community effects of the soil additions on decomposition rates (e.g., nutrients), we used a higher litter to inoculum ratio than has been used in previous similar experiments (e.g., Strickland et al. 2009a, b). However, we acknowledge that other inoculum characteristics could also influence decomposition dynamics. We added 0.25 g of soil inoculum to 1.0 g of litter in the incubation vessels, and sample mixtures were adjusted to 50 % WHC and well mixed on day 1 using a sterile spatula. Tubes were weighed every 7 days to determine moisture loss, and as necessary, 24 h prior to sampling events, sterile deionized water was added to return samples to the initial soil moisture content using a micropipette. All tubes were incubated uncapped but covered with aluminum foil in the dark in a closed plastic cooler at 20 °C. Moist paper towels in the cooler were replaced weekly to maintain humidity and minimize water loss.
We determined C mineralization rates across the 162-day experiment using a static incubation procedure (Fierer et al. 2003). Prior to sampling, tubes were flushed with room air and sealed with airtight plastic caps equipped with rubber-septa for gas sampling. After 6 h, the headspaces of the tubes were mixed with a syringe and a 10-ml sample was removed from each tube. C mineralization rates were calculated as CO2 produced per gram of dry soil per hour of incubation using a gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector. C mineralization rates were measured 14 times throughout the experiment. Initially, all 12 replicates were sampled, but destructive sampling of three replicates per time point (on days 1, 22 and 162) ultimately reduced the number of replicates per treatment to six replicates per treatment by the end of the experiment. Following the C mineralization assays, replicates were harvested, frozen and stored at −80 °C for bacterial community analyses. CO2 mineralization rates in the inoculated samples were adjusted to account for background respiration of the soil inoculum or the uninoculated litter by subtracting the sum of CO2 production rates in the litter/inoculum-only samples from the rates measured in the experimental treatments. However, C mineralization rates in the litter-only and soil-only samples were consistently an order of magnitude lower than rates in inoculated litter samples. To approximate total CO2 respired over the incubation, we extrapolated rates by interpolating between sampling events. Briefly, we used linear interpolation between each measurement time and then summed across all times to estimate the total CO2 produced over the course of the incubation.
Bacterial community analyses
Bacterial DNA was extracted from 0.15-g samples of the litter + inoculum treatments three times (on day 1, 22, and 162 of the incubation period) using the PowerSoil DNA isolation kit (MO BIO Laboratories, Carlsbad, CA) following the manufacturer’s instructions. DNA samples were then bulked to form a single DNA sample per treatment per time point, for a total of 48 unique DNA samples.
Error-corrected bar-coded pyrosequencing of bacterial 16S rRNA genes was performed using the 27F and 338R primers described in Fierer et al. (2008) modified with a six nucleotide adapter for titanium chemistry. Polymerase chain reaction (PCR) amplifications were performed in triplicate and consisted of 10 μL H2O, 10 μL HotMasterMix (5 PRIME, Gaithersburg, MD), 2 μL template DNA, and 1.2 μL of each primer. Thermocycling consisted of an initial denaturation step of 3 min at 94 °C followed by 35 cycles of 45 s at 94 °C, 90 s at 50 °C, and 30 s at 72 °C. The reaction was terminated after an elongation step of 10 min at 72 °C. Triplicate PCR products were pooled and cleaned using the Ultra Clean htp PCR Clean-up Kit (MO BIO Laboratories, Carlsbad, CA). Cleaned amplicons were quantified using the Quant-iT Pico Green DNA Assay Kit (Invitrogen, Carlsbad, CA) and were pooled in equal concentrations. Samples were analyzed at the Engencore Environmental Genomics Core Facility at the University of South Carolina using titanium chemistry. Pyrosequencing data were screened against a minimum quality score of 25, maximum homopolymer of five, maximum ambiguous bases of zero, and maximum mismatched bases in the primer of zero in QIIME (Caporaso et al. 2010). Operational taxonomic units (OTUs) were selected at the 97 % identity level using UCLUST (Edgar 2010) in QIIME. Sequences were denoised using Denoiser implemented in QIIME (Reeder and Knight 2010).
Data analysis
C mineralization rate data and cumulative C losses were analyzed separately. Rate data were first assessed using a repeated measures general linear model (GLM), with litter type and inoculum type as between-subject factors. Next, we used a univariate GLM (with C mineralization rate as the dependent variable) to compare the effect of varying litter type versus inoculum type at given sampling times. To explore the effect of litter type for a given inoculum type and the effect of inoculum type for a given litter type, we used one-way ANOVA to explicitly assess the effect of litter type or inoculum type alone. Multiple comparisons of specific treatments were made using Tukey’s post hoc analyses. The effect of varying litter type and inoculum type on cumulative C lost was assessed using ANOVA, and multiple comparisons were conducted using Tukey’s post hoc analysis. We initially analyzed the data with all treatments included (i.e., including the effects of litter type and inoculum type in the same analyses), and subsequently by assessing the effects of a single treatment while holding the other variable constant (e.g., assessing the relationship between inoculum type and decomposition for each litter type individually; Table 1). Sum of squares values were obtained from ANOVAs. For all data, significance was determined when P < 0.05. These statistical analyses were performed with SPSS 15.0 (Chicago, IL).
We used UniFrac (Lozupone et al. 2011) to test for significant differences in the bacterial community composition of the inoculum types. Briefly, a phylogenetic tree of a representative set of OTUs was generated in QIIME using FastTree (Caporaso et al. 2010). QIIME was also used to convert our OTU matrix into a UniFrac environment file and the online UniFrac distance metric was used to compare community composition (Lozupone et al. 2011). We used QIIME to generate UniFrac distance matrices and used permutational multivariate ANOVA executed in Primer v.6 to test the significance of time, inoculum and litter type in structuring the bacterial community (Anderson 2001). Finally, we examined the specific relationships between respiration and bacterial community composition by performing a Mantel-like test (RELATE function in Primer v.6) comparing the significance of the relationship between the UniFrac distance matrix and a Euclidian distance matrix of ln-transformed respiration values.
Results
Multiple indices of litter chemistry varied significantly between sites: bulk litter lignin concentrations decreased with site age, and litter N content was lowest at the old site (4,100 k years; Kokee), intermediate at the young site (300 years; Thurston), and highest at the intermediate-aged site (20 k years; Supplemental Table 1). Litter lignin:N ratios were highest at the young site, lowest at the intermediate-aged site, and intermediate at the old site. In addition, the UniFrac significance test (Lozupone et al. 2011) used to compare bacterial community composition confirmed that soil bacterial communities in the initial inoculum types from the three sites were significantly different from one another (Bonferroni-corrected P < 0.05). Alphaproteobacteria, Acidobacteria, and Actinobacteria dominated the starting bacterial community.
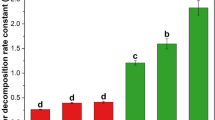
Over the course of the 162-day incubation experiment, decomposition patterns (i.e., CO2 production rates) were generally similar among all litter and soil inoculum type combinations (Fig. 1). Within the first 3 weeks, CO2 mineralization rates peaked in all samples, then gradually declined (by day 60) to low rates, where they remained for the duration of the experiment (Fig. 1). Consistent with that observation, across all data, the repeated measures ANOVA showed that time was a significant (P < 0.001) factor explaining variations in C mineralization rates. In addition, there were both significant time × litter type and time × soil inoculum type interactions (P < 0.001 for both), suggesting temporal variation in the relative importance of litter quality versus inoculum type (Table 1). By day 162, cumulative CO2 respired among the different treatments ranged from 22.5 ± 0.05 mg CO2-C g−1 dry soil in Thurston inoculum/Thurston litter samples to 32.0 ± 0.35 mg CO2-C g−1 dry soil in Kokee inoculum/Laupāhoehoe litter samples (Fig. 2).
Decomposition rates (CO2 fluxes) measured over the course of the 162-day decomposition experiment. Top panels represent one of four inoculum treatments decomposing each litter type: Thurston (“young”) soil inoculum decomposing each litter type; Laupāhoehoe (“intermediate”) soil inoculum decomposing each litter type; Kokee (“old”) soil inoculum decomposing each litter type; and mixed soil inoculum decomposing each litter type. Lower panels represent one of four litter types with each inoculum source: Thurston (young) litter decomposed by each inoculum type; Laupāhoehoe (intermediate) litter decomposed by each inoculum type; Kokee (old) litter decomposed by each inoculum type; mixed litter decomposed by each inoculum type. For top and bottom panels, the litter or inoculum variables are shown in the first panel (circle Thurston, triangle Laupāhoehoe, diamond Kokee, square mixed)
Cumulative C losses (mg CO2-C g−1 dry soil) over the course of the 162-day decomposition experiment. The treatments show inoculation source (first letter)/litter source (second letter), where T Thurston, L Laupāhoehoe, K Kokee, M mixed. Bars labeled with dissimilar letters are significantly different at α = 0.05, and error bars are ±1 SE
To explore the possible effects of differences in litter and inoculum type on decomposition over the course of the experiment, we calculated cumulative CO2 mineralized over the 162-day incubation, analyzing data with all litter and inoculum types together. This analysis suggested that both litter and soil inoculum type were important in explaining C mineralization over time (Table 2): litter type explained the majority (64 %) of the variation in C mineralization, and soil inoculum type explained 25 % of the variation in CO2 produced over the course of the experiment (Table 2). However, when assessing the role of inoculum type on C mineralization within a litter type, we found that the relative importance of inoculum type on total C mineralized over the incubation varied with litter quality (Table 1; Supplemental Table 1). The inoculum type had a significant effect (P < 0.05) on decomposition of litter from the old site (Kokee, lignin:N = 109) and the young site (Thurston, lignin:N = 119), and a marginally significant effect (P = 0.054) on the decomposition of litter from the intermediate-aged site (Laupāhoehoe, lignin:N = 47) (Table 1 and Supplemental Table 1). As well, inoculum type explained a nearly identical proportion of the variation in total C mineralized from litter from the old (Kokee) and young site (Thurston, 94 and 96 %, respectively) (Table 1; Supplemental Table 1). By contrast, inoculum type explained only 77 % of the variation in total C mineralized from the litter from the intermediate-aged site (Laupāhoehoe). Respiration rates in the mixed litter and mixed inoculum treatments fell between rates in the individual litter treatments.
We also explored the relationships among time, litter type and inoculum type on C mineralization rates using a GLM. We found that the relative importance of litter versus inoculum type on decomposition rates varied over the course of the experiment (Table 1). Early in the experiment (day 22), differences in litter type explained nearly all (~90 %) of the variation in C mineralization rates, and the effect of inoculum type was not significant [although there was a significant interaction between litter type and inoculum type (P < 0.001)] (Table 1). By contrast, on day 162 of the experiment, both litter and inoculum type had significant effects on C mineralization rates (P < 0.001 each), with litter type and inoculum type explaining 25 and 43 % of variation in C mineralization, respectively (Table 1).
Finally, we used molecular phylogenetics to assess the relationship between bacterial community composition and respiration in each of the flasks at the different time points. This allowed us to specifically examine the potential role of bacterial community structure in the observed differences in respiration. As well, this approach allowed us to examine the role of litter quality vs. inoculum source in bacterial community assembly. We found that the bacterial communities in the litter incubations were markedly different from the initial soil inoculum bacterial communities, being more dominated by taxa from the Alphaproteobacteria (Fig. 3). Over the course of the incubations, beta diversity decreased (communities became more similar) while alpha diversity, or the diversity within individual communities, increased. A PERMANOVA revealed that bacterial community composition in the individual litter samples was best explained by time (Table 3; Fig. 3). Both litter type (Fig. 3a) and soil inoculum type (Fig. 3b) were also significant factors in explaining litter bacterial community structure, each accounting for 16 % of the variation in community composition (Table 3). Additionally, we observed significant time × litter type and time × soil inoculum type interactions (Table 3). Analysis of the individual litter samples revealed that bacterial community composition explained 32 % of the variation in respiration values (RELATE test, ρ = 0.571, P < 0.001). In addition, the relative importance of bacterial community composition on respiration increased over the course of the experiment (RELATE test, ρ = 0.467, P < 0.001, T1; ρ = 0.514, P < 0.001, T2).
Non-metric multidimensional scaling ordination of bacterial community composition based on pairwise, unweighted UniFrac distances. Different symbols represent different time points: triangles day 1, squares day 22, circles day 162. a Colors represent different litter sources: green Thurston (young), maroon Laupāhoehoe (intermediate), purple Kokee (old), orange mixed litter, gray no litter (starting soil communities). b Colors represent different inoculum sources: green Thurston, maroon Laupāhoehoe, purple Kokee, orange mixed inoculum
Discussion
We collected samples from a set of sites where climate and plant species composition are similar but where litter chemistry varies, and designed an experiment in which litter quality and soil inoculum type represented the primary potential sources of variability in decomposition rates. Litter quality of the dominant species (M. polymorpha) varied between the three sites (Hobbie and Vitousek 2000; Vitousek 2004), as did the soil bacterial community composition of the inoculum types (Fig. 3; Supplemental Table 1). Despite these measured differences, as well as other potential differences, among inoculum types (e.g., fungal community composition and/or soil chemistry), patterns of decomposition were remarkably similar across all treatments. In general, we observed an initial and rapid increase in soil respiration, followed by a slow decline in CO2 mineralization rates (Fig. 1), which is consistent with previous conceptual models and empirical observations suggesting a multi-stage model of decomposition (Moorhead and Sinsabaugh 2006; Strickland et al. 2009a). Time was the most important factor explaining variations in CO2 production and litter bacterial community composition (Fig. 3; Table 1).
Previous research has shown that in some ecosystems, microbial communities decompose litter from a “home” site more rapidly than they decompose litter from foreign sites (i.e., microbial communities have a “home-field advantage” when decomposing their native litter) (Strickland et al. 2009a; Ayres et al. 2009). However, we saw no evidence of a home-field advantage in our experiment (Supplemental Table 2). This inconsistency may reflect the fact that most evidence of a home-field advantage from similar experiments has emerged following major (i.e., species-level) differences in litter type between samples, or movements of unique litter types into non-native ecosystems, rather than the more subtle changes in intraspecific litter quality mimicked in this experiment. Instead, our data suggest that litter type explains the majority of variation in decomposition, and we infer that differences among litter types reflect differences in litter quality between sites. Over the course of the 162-day incubation experiment, litter type explained 64 % of the variability in decomposition rates, while inoculum type explained 25 % (Table 1). While other factors may have contributed to difference in decomposition rates among different inoculum types (e.g., soil chemistry, differences in fungal community composition), the pyrosequencing results revealed that bacterial community composition explained 32 % of the variation in respiration rates in the different flasks. In turn, equal portions (16 %) of the variation in bacterial community composition were explained by inoculum type and litter quality, reflecting the importance of both the meta-community and the environment in bacterial assembly processes.
In addition, our data suggest that as decomposition proceeded, bacterial community composition converged (i.e., beta diversity decreased from day 1 to day 22 and from day 22 to day 162; P < 0.001; Fig. 3) while alpha diversity increased (P < 0.01). The convergence in litter bacterial community composition to a more diverse community among samples through time is also consistent with the notion that as decomposition proceeds, overall microbial decomposer community composition shifts towards a functionally diverse group of microorganisms that specialize on cellulose and lignin (e.g., Moorhead and Sinsabaugh 2006). Interestingly, we noted that decomposition rates were negatively correlated with alpha diversity at 22 days (r 2 = 0.72) but positively correlated at day 162 (r 2 = 0.45), suggesting that as time proceeds, there is a positive correlation between alpha diversity and ecosystem function. This pattern is consistent with Philippot et al. (2013) who documented a positive correlation between diversity and denitrification in a laboratory incubation experiment.
Overall, decomposition patterns were similar among the experimental treatments, yet there were some differences (Figs. 1, 2). First, the relative effect of inoculum type varied as a function of litter quality (Table 1). Among all data, inoculum type explained a higher proportion of the variation in CO2 production in low-quality litter (Table 1), again supporting that interactions between litter quality and community composition may be more important in regulating decomposition rates than starting bacterial community composition alone. As stated above, we cannot rule out the possible effects of differences in inoculum beyond those in bacterial community composition. However, the statistical analyses with the pyrosequencing data and the soil respiration data revealed that bacterial community structure actually explained more of the variation in respiration rates than overall inoculum type (i.e., 33 vs. 25 %). This supports the role of the bacterial community in the observed variation in soil respiration. As well, this suggests that the changes in bacterial community over the course of the incubation were driven to some degree by environmental factors, an assertion supported by relationship between litter quality and bacterial community composition (Table 3).
Additionally, the relative importance of inoculum type on decomposition varied over the course of the experiment. Early (when respiration rates were high; Fig. 1), variations in litter quality explained almost 90 % of the variation in CO2 produced, but after 162 days, litter type explained less than 25 % of the variation in CO2 and inoculum type explained nearly 44 % (Table 1). The increasing importance of inoculum type combined with the observed convergence in bacterial composition through time (Fig. 3) is consistent with the negative relationship between the importance of soil inoculum type and litter quality (Table 1). Taken together, these data suggest that declines in litter quality may explain the increase in the effect of the soil bacterial community through time. Gradual declines in litter quality during decomposition may favor a subset of the bacterial community that specializes on relatively low-quality C compounds (Schimel and Gulledge 1998), and the presence/absence of these organisms in the initial inoculum types may have had an increasing effect on C mineralization with time. Finally, when compared to most other species, the M. polymorpha litter collected from all sites could be considered “low quality”, with higher lignin:N ratios than those seen for many common temperate forest species (e.g., Melillo et al. 1982). Thus, given that the importance of community composition in explaining decomposition appeared to increase with lignin:N ratios, the effects of community composition observed here may be more pronounced than effects observed in most ecosystems with higher quality litter. That said, although Strickland et al. (2009a) did not report litter-quality metrics, two of the three communities they assessed suggested a much larger role of microbial decomposer inoculum than observed here.
In sum, we found a significant but relatively low effect of the inoculum type in explaining decomposition. While we focused on bacterial community composition and did not assess fungi, assessing the overall effects of “inoculum type” would implicitly account for the possible effects of varying fungal community composition among sites. Interestingly, the functional similarity among bacterial communities decreased with time as the degree of compositional similarity increased, and we interpret this as a community response to decreasing litter quality. This is noteworthy given recent evidence suggesting that multiple aspects of global change can drive changes in litter quality. For example, litter C:N ratios may change with increasing atmospheric CO2 concentrations (Finzi et al. 2006), increasing N deposition (Carreiro et al. 2000), or both. The results suggest that if environmental change drives overall declines in litter and organic matter quality, the functional importance of the soil microbial community in any particular site may increase.
These data simultaneously provide insight into the relative effects of litter quality and bacterial community composition and assembly on decomposition, and place the importance of community composition in a quantitative context. On one hand, the significant effect of the bacterial community on decomposition within a common litter type suggests an important role for community composition in regulating decomposition, and confirms previous research suggesting that community structure is important in regulating this fundamental process (Strickland et al. 2009a). On the other hand, the relatively small amount of variation explained by bacterial community composition (compared with relatively large amount explained litter types) suggests that, from a process-level perspective, microbial community composition plays a relatively minor role in regulating decomposition rates among different litter types at these sites. Given the strong effects of climate in regulating decomposition rates both in Hawaiian M. polymorpha (Vitousek et al. 1994; Austin and Vitousek 2000) and more generally (e.g., Meentemeyer 1978; Gholz et al. 2000), these results call into question the importance of knowledge of microbial community composition in predicting litter decomposition rates. The role of community composition may also be further diminished (relatively) under a climate change scenario that greatly alters temperature and/or precipitation dynamics, as incorporating the effects of climate change would be predicted to reduce the fraction of overall variation in decomposition that could be explained by either litter quality or microbial community composition. For example, Vitousek et al. (1994) reported nearly tenfold variation in decomposition rates for M. polymorpha leaves from a single source that were decomposed in sites that differed in precipitation and temperature, versus nearly fourfold variation for leaves from a wide variety of sites that were decomposed in a single site. In other words, under the controlled climate conditions in the laboratory experiment, inoculum type explained a substantial amount of the variation in decomposition rates (Table 2). However, given the strong role of climate in regulating decomposition, we might expect that the importance of both litter quality and community composition on decomposition would be more subtle in the natural environment.
Here, we focused on litter decomposition, an “aggregate” process that may be less sensitive to changes in community composition than are ecosystem processes carried out by a narrower suite of organisms (Schimel and Gulledge 1998; Schimel et al. 2005). For example, some N cycling processes (Carney et al. 2004; Hawkes et al. 2005; Reed et al. 2010) require enzyme systems that are limited to more specific microbial taxa and may be more sensitive to changes in microbial community composition. Reed et al. (2010) showed strong links between microbial community composition and heterotrophic N fixation rates, a process that is limited to organisms with nif genes, and Cavigelli and Robertson (2000) showed strong effects of community composition on denitrification, an anaerobic process that is more restricted phylogenetically than is aerobic respiration. Thus, environmental change-driven shifts in the composition of these narrower functional groups may have more profound impacts on processes other than decomposition. As well, we note that we did not track the changes in the fungal community composition, and these organisms are likely to be important in litter decomposition. Future work should examine their role in decomposition and the controls over fungal community assembly and diversity.
Conclusion
Most models used to predict ecosystem and C cycle responses to environmental change (e.g., climate change and rising atmospheric CO2 concentrations) do not consider the potential effects of changes in microbial community composition explicitly, and the importance of doing so is a current topic of interest in the literature (McGuire and Treseder 2010). Here, we quantified the relative effects of soil inoculum type versus litter quality in explaining variation among leaf litter decomposition rates. While the data suggest that differences in microbial community composition had significant effects on decomposition rates, the relative importance of such differences is a matter of perspective. Changes in climate and declines in litter quality are anticipated for many ecosystems (Norby et al. 2001), and our results suggest that in any given site, the effects of such changes may be more important to consider than the potential effects of changes in soil microbial community composition. However, the relative effects of community composition increased through time, suggesting that the long-term effects may not be predictable from short-term responses. For example, the decomposer community could play a larger role in regulating soil organic matter chemistry over longer timescales than it does over short-term CO2 mineralization rates (Wickings et al. 2012). Finally, environmental change is likely to elicit changes in many of the factors that affect decomposition in complex ways, complicating attempts to quantify the relative importance of any one factor.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Allison S, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639
Austin AT, Vitousek PM (2000) Precipitation, decomposition and decomposability of Metrosideros polymorpha in native forests on Hawai’i. J Ecol 88:129–138
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moor JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610
Balser T, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland andmixed-conifer forest. Biogeochemistry 73:395–415
Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S (2011) Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J 5:590–600
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger J, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Carney KM, Matson PA, Bohannan BJM (2004) Diversity and composition of tropical soil nitrifiers across a plant diversity gradient and among land use types. Ecol Lett 7:684–694
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Castro H, Classen A, Austin E, Norby R, Schadt C (2010) Soil microbial community responses to multiple experimental climate change drivers. Appl Environ Microb 76:999–1007
Cavigelli M, Robertson GP (2000) The functional significance of denitrifier community composition in a terrestrial ecosystem. Ecology 81:1402–1414
Chapin FS, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Couteaux M–M, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Crews T, Fownes J, Herbert D, Kitayama K, Mueller-Dombois D, Riley R et al (1995) Changes in soil phosphorus and ecosystem dynamics across a long soil chronosequence in Hawaii. Ecology 76:1407–1424
Daubenmire R, Prusso D (1963) Studies of the decomposition rates of tree litter. Ecology 44:589–592
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Fierer N, Allen AS, Schimel JP, Holden PA (2003) Controls on microbial CO2 production: a comparison of surface and subsurface soil horizons. Glob Change Biol 9:1322–1332
Fierer N, Hamady M, Lauber CL, Knight R (2008) The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA 105:17994–17999
Finzi AC, Moore DJP, DeLucia EH, Lichter J, Hofmockel KS, Jackson RB et al (2006) Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87:15–25
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6:751–765
Hawkes CV, Wren IF, Herman DJ, Firestone MK (2005) Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8:976–985
Henry HAL, Cleland EE, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland. Oecologia 142:465–473
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Legg TM, Zheng Y, Simone B, Radloff KA, Mladenov N, González A et al (2012) Carbon, metals, and grain size correlate with bacterial community structure in sediments of a high arsenic aquifer. FMICB. doi:10.3389/fmicb.2012.00082
Logue JB, Lindström ES (2010) Species sorting affects bacterioplankton community composition as determined by 16S rDNA and 16S rRNA fingerprints. ISME J 4:729–738
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–172
Luo Y, Hui D, Zhang D (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta analysis. Ecology 87:53–63
McGuire K, Treseder K (2010) Microbial communities and their relevance for ecosystem models: decomposition as a case study. Soil Biol Biochem 42:529–535
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Moorhead D, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Moorhead D, Currie W, Rastetter E, Parton W, Harmon M (1999) Climate and litter quality controls on decomposition-An analysis of modeling approaches. Global Biogeochem Cycl 13:575–589
Nemergut DR, Cleveland CC, Wieder WR, Washenberger CL, Townsend AR (2010) Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42:2153–2160
Nemergut DR, Schmidt SK, Fukami T, O’Neill SP, Bilinski TM, Stanish L, Knelman J, Darcy JL, Lynch R, Wickey P, Ferrenberg S (2013) Patterns and processes of microbial community assembly. Microbiol Mol Biol Rev 77:342–356
Norby RJ, Cotrufo MF, Ineson P, O’Neill EG, Canadell JG (2001) Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127:153–165
Parton WJ, Schimel DS, Cole CV, Ojima DS (1994) A general model for soil organic matter dynamics: sensitivity to litter chemistry, texture and management. In: Bryant RB, Arnold RW (eds) Quantitative modeling of soil forming processes. Soil Science Society of America, USA, pp 47–67
Paul EA, Clark FE (1996) Soil Microbiology and Biochemistry. Academic Press, San Diego
Philippot L, Spor A, Hénault C, Bru D, Bizouard F, Jones CM et al (2013) Loss in microbial diversity affects nitrogen cycling in soil. ISME J. doi:10.1038/ismej.2013.34
Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer N (2010) Consistent effects of nitrogen fertilization on the phylogenetic composition of soil bacterial communities in contrasting systems. Ecology 91:3463–3470
Reed H, Martiny J (2007) Testing the functional significance of microbial composition in natural communities. FEMS Microbiol Ecol 62:161–170
Reed SC, Townsend AR, Cleveland CC, Nemergut DN (2010) Microbial community shifts influence patterns in tropical forest nitrogen fixation. Oecologia 164:521–531
Reed SC, Vitousek PM, Cleveland CC (2011) Are patterns in nutrient limitation belowground consistent with those aboveground: results from a 4 million year chronosequence. Biogeochemistry 106:323–336
Reeder J, Knight R (2010) Rapid denoising of pyrosequencing amplicon data: exploiting the rank-abundance distribution. Nat Methods 7:668–669
Schimel JP (2001) Biogeochemical models: implicit vs. explicit microbiology. In: Schulze ED, Harrison SP, Heimann M, Holland EA, LLoyd JJ, Prentice IC, Schimel D (eds) Global biogeochemical cycles in the climate system, Academic Press, San Diego, pp 177–183
Schimel JP, Gulledge J (1998) Microbial community structure and global trace gases. Global Change Biol 4:745–758
Schimel JP, Bennett J, and Fierer N (2005) Microbial community composition and soil N cycling: is there really a connection? In: Bardgett, RD, Hopkins DW, and Usher MB (eds) Biological diversity and function in soils, Cambridge University Press, Cambridge, pp 171–188
Strickland M, Lauber C, Fierer N, Bradford MA (2009a) Testing the functional significance of microbial community composition. Ecology 90:441–451
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2009b) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23:627–636
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley
Treseder KK, Balser TC, Bradford MA, Brodie EL, Dubinsky EA, Eviner VT et al (2012) Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry 109:7–18
Vitousek P (2004) Nutrient cycling and limitation: Hawai’i as a model system. Princeton University Press, Princeton
Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994) Litter decomposition on the Mauna Loa environmental matrix, Hawaii: patterns, mechanisms, and models. Ecology 75:418–429
Vitousek PM, Chadwick OA, Crews TE, Fownes JH, Hendricks DM, Herbert D (1997) Soil and ecosystem development across the Hawaiian Islands. GSA Today 7:1–8
Wang G, Post WM, Mayes MA (2012) Development of microbial-enzyme-mediated decomposition model parameters through steady-state and dynamic analyses. Ecol Appl. doi:10.1890/12-0681.1
Wickings K, Grandy AS, Reed SC, Cleveland CC (2012) The origin of litter chemical complexity during decomposition. Ecol Lett. doi:10.1111/j.1461-0248.2012.01837.x
Zak DR, Holmes WE, White DC, Peacock AD, Tilman D (2003) Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84:2042–2050
Acknowledgments
We thank H. Farrington and J. Schulten for logistical and sampling support, and B. Houlton and the plant analysis lab at the University of California Davis for performing the litter lignin analyses. M. Strickland, N. Fierer and two anonymous reviewers provided valuable comments on the experiment and the manuscript. We acknowledge the generous financial support of the both the Andrew W. Mellon Foundation and the National Science Foundation (DEB—0919080). Any use of trade names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Madritch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cleveland, C., Reed, S.C., Keller, A.B. et al. Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia 174, 283–294 (2014). https://doi.org/10.1007/s00442-013-2758-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2758-9