Abstract
Aims
This research investigated the effects of woody plant identity and season on soil physicochemical properties and microbiological function in the semi-arid Zagros forest, one of the old-growth semi-arid oak forests in the world.
Methods
Soil sampling was conducted beneath the canopy of six woody (tree and shrub) species in spring and winter. Microbial variables analysed included soil basal respiration (BR), microbial biomass C and N (MBC and MBN), microbial entropy index (MIE), substrate induced respiration (SIR) and enzymatic activities (i.e., urease and alkaline phosphatase). Soil physicochemical properties were also analysed and included pH, electrical conductivity (EC), available calcium and magnesium (Ca2+ and Mg2+), organic carbon (OC), total nitrogen (Ntot), lime, water content (WC), bulk density (BD), clay, silt and sand.
Results
Results demonstrated significant differences among the woody species (Pseudo-F = 56.31; p = 0.001), season (Pseudo-F = 97.37; p = 0.001) and their interaction (Pseudo-F = 2.96; p = 0.005) for the matrix of microbiological soil parameters. Differences were species-specific for shrubs and trees with a marked effect for tree species such as Quercus brantii. Microbial parameters were consistently higher in spring when higher temperature and lower moisture were recorded. Soil OC, Ntot, BD, and WC were important drivers of the soil microbial function.
Conclusions
Our results evidenced a strong effect of season and plant species on soil physicochemical and microbiological soil properties in a semi-arid forest ecosystem. Higher values of microbiological soil parameters, including urease and phosphatase activities, were recorded for tree species during spring season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant diversity and vegetation type can largely influence the soil microbial community structure and function because of the differences in litter quality, root exudates and nutrient uptake (Dai et al. 2018; Xia et al. 2019; Wu et al. 2019; Estruch et al. 2020). Soil microorganisms play a key role in the functioning of plant communities, e.g. promoting plant-microbe associations and releasing soil nutrients for plant development. In turn, plant remains provide the main carbon and energy source for sustaining microbial activity (Rezende et al. 2017; Di Sabatino et al. 2020). Microbial communities in the soil are directly related to the turnover of carbon, nitrogen, and phosphorus in terrestrial ecosystems (Sandoval-Pérez et al. 2009; Zhang et al. 2018). These organisms are responsible for both organic matter degradation by mineralization and organic matter stabilization by humification, through a wide range of enzymatic activities (Shahbaz et al. 2017; Li et al. 2020).
Soil enzymes such as urease and phosphatase are involved in the decomposition of litter components (Adetunji et al. 2017). These enzymes play important roles in the soil system, e.g. catalysing biological and chemical reactions and promoting the mineralization of soil organic phosphorus and the decomposition of soil organic matter and nitrogen (Zaman et al. 2009; Jarosch et al. 2019). On the other hand, soil microorganism can access the energy and nutrients in complex substrates through the activity of these enzymes (Allison and Vitousek 2005; Avazpoor et al. 2019). Enzymatic activities related to cycles of carbon, nitrogen or phosphorus and microbial indicators such as microbial biomass and respiration, have been proposed as reliable indicators for assessment or soil quality and function (Yang et al. 2012; Hedo et al. 2015; Muñoz-Rojas 2018). Soil respiration reflects the microbial activity level (Rey et al. 2011; Oyonarte et al. 2012), which is sensitive to soil environmental or physicochemical soil variables, such as temperature and humidity, texture, pH, electrical conductivity and soil nutrients (Hursh et al. 2017; Heydari et al. 2019). Furthermore, these soil microbial parameters can be useful for characterizing the extent of aboveground plant influence in soil biological properties (Lucas-Borja et al. 2012a).
Overall, the microbial community is a critical component of sustainable soil-plant systems in forests, including those located in semi-arid areas (Bastida et al. 2008; Ushio et al. 2008; Merilä et al. 2010). Semi-arid regions cover ~15% of terrestrial earth lands and play an important role as potential carbon sinks under elevated CO2 (Safriel and Adeel 2005; Ahlström et al. 2015). The area occupied by arid and semi-arid ecosystems is increasing due to global warming and advanced desertification (Teimouri et al. 2018). In these ecosystems, climatic conditions, forms of vegetation and low levels of organic matter may promote erosion processes, reducing soil fertility (Khalyani and Mayer 2013; Bracken et al. 2019). Thus, plants and soil feedbacks in these semi-arid regions need to be thoroughly understood in order to preserve and promote higher soil and ecosystem functionality in current and future global change scenarios (Ostle et al. 2009; Sardans and Peñuelas 2013). Different woody species can create diverse microclimate conditions (Ayres et al. 2009; Lucas-Borja et al. 2012b; Scheibe et al. 2015; Heydari et al. 2017b), and generate a varied quantity and quality of litter inputs and root exudates which are major carbon source for microorganisms (Grayston et al. 1997; Cheng et al. 2013; Wan et al. 2015). These differences can affect the spatially dependent soil enzymatic activities together with the soil physicochemical and biological characteristics (Mungai et al. 2005). Despite the importance of these processes, the ability of soil enzymes to reflect nutrient and microbial hotspots under different tree and shrub species and climatic conditions has not been thoroughly studied (Lucas-Borja et al. 2012a; Yao et al. 2019; Zuccarini et al. 2020).
The effects of season on the soil and ecosystem functioning can be critical, particularly in arid and semi-arid ecosystems (Jia et al. 2020). Contrasting effects in the degradation of soil organic compounds through soil moisture and temperature regulation, can simultaneously affect the soil microbial community response and enzymatic activities (Kang et al. 2009; Huang et al. 2016). Previous studies have shown that seasonality significantly affects soil enzymatic activity. For example, Zuccarini et al. (2020) showed a significant decrease in soil enzyme activities during summer due to warming and limited soil moisture whereas Wallenstein et al. (2009) reported a decrease of soil enzyme activity due to winter cold temperatures in semi-arid climate areas. Overall, seasonal variation may generates different patterns of microbial processes depending on the availability of different substrates, variation in soil temperature and moisture (Kaiser et al. 2011). In semi-arid ecosystems, the availability of water, plant biomass, litter and soil nutrients is modulated by the occurrence of precipitation, which mainly occurs in autumn and winter (Snyder and Tartowski 2006). Despite an increase of studies focused on the impacts on climate on semi-arid forests, our understanding regarding the effects of season on soil microbial and physicochemical characteristics for specific woody plant species is still limited in semi-arid ecosystems.
In this study, we aimed to investigate these processes in the Zagros forest in western Iran, which is one of the oldest semi-arid oak forests in the world influenced by the Mediterranean climate. Zagros has lost abundant forest area over the last 30 years due to economic expansion but these forests are critical because they supports several ecosystem services such as food, timber, water, carbon storage, air purification, wildlife habitat and social and cultural benefits (Heidarlou et al. 2019). Our main objective was to evaluate the effects of plant species and season on soil physicochemical properties and microbial function, e.g. soil respiration, microbial biomass and enzymatic activities. For this study, six woody species representative of the semi-arid Zagros forest were selected. We hypothesized that tree and shrub growth forms will have different effects on soil patterns, which can be attributed to mass action effect (e.g. differences in quantity and quality of plant remains and organic matter) and the availability of water, plant biomass, litter and soil nutrients during winter. In addition, trees and shrubs may generate patchy microclimate conditions which may modulate the microbial functional response in semi-arid forest. These heterogeneous conditions would have a relevant response in the activity and function of soil microbial communities.
Material and methods
Study area
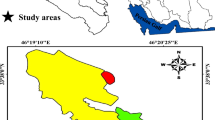
This study was conducted in a 60-ha area in the central part of the Zagros Mountain (Sirvan city, western Iran, 33° 44′ 55“ N; 46° 25’ 19” E, Fig. 1) with homogeneous physiographic conditions (slope < 10% and altitude 1900–2000 m.a.s.l.). Forests in this area are typically formed by relatively spare woody overstorey (basal area 11 ± 5.2 m2 ha−1) with patchy distribution. Tree and shrub species in the area include Quercus brantii Lindl., Pistacia atlantica Desf., Acer monspessulanum L. subsp. cinerascens (Boiss.) Yaltirik., Crataegus puntica C. Koch., L., Amygdalus scoparia Spach., and Lonicera nummularifolia Jaub & spach. In the study area, the age of shrub and tree species is approximately 30–70 and more than 150 years old, respectively. The understory vegetation is relatively dense and comprised of annual and perennial grasses and forbs such as Alyssum marginatum Steud. ex Boiss., Astragalus adscendens Boiss., Geranium lucidum L., Medicago radiata L., Valerianella vesicaria Moench, Neslia apiculata Fisch., Hordeum bulbosum L., Gundelia turneffortii L. and Bromus tectorum L. Mean annual rainfall and temperature are 428.8 mm and 18.55 °C respectively (Sarableh climate station, 2009–2018). Precipitation usually occurs during October–May with a maximum during December to April. During June to September, effective precipitation is very sparse and rarely recorded (Fig. 2). Soil in the Zagros Mountains is generally calcareous shallow (Jazirehi and Ebrahimi 2003). Soil across our study area was classified as a sandy clay loam. The soil type in the study area is Calcisols based on FAO classification.
The study area is located in the Southern Zagros forests in western Iran (a) and Ilam province, Sirvan County (b). For each woody species [QU: Quercus brantii), AC: Acer monspessulanum L. and PI: Pistacia atlantica Desf. and three shrub species, CR: Crataegus puntica C. Koch., AM: Amygdalus scoparia Spach. And LO: Lonicera nummularifolia Ja ub & spach.] five patches (colored squares) were selected in the study site (c). Soil sampling was done in spring and winter 2018 in each patch under the canopy of a central woody species (d) at 0–25 cm depth
Average annual temperature and precipitation regime in the study area for comparison between conditions during the experiment (light black line, January 2018 to December 2018) and historic records from the local meteorological station (thick black line, from 2009 to 2017). The difference is represented as a grey shadow (deviation)
Experimental design and soil sampling
This study focused on three tree species, i.e. Quercus brantii (hereinafter indicated as QU), Acer monspessulanum L. (AC) and Pistacia atlantica Desf. (PI), and three shrub species, i.e. Crataegus puntica C. Koch. (CR), Amygdalus scoparia Spach. (AM) and Lonicera nummularifolia Ja ub & spach. (LO). Five patches were sampled for each species (total of 30 patches), which consisted in small groups of individuals of the same species and same size (Bayranvand et al. 2017). The minimum distance between two adjacent patches was 40 m. Vegetation patches (≈ 55–240 m2 canopy cover) included 4–5 individuals of the same woody species, and the size between patches was kept as less variable as possible. One composite soil sample (n = 30 × 2 seasons) was randomly collected beneath the canopy of the central woody species for each patch from the uppermost 25 cm layer using a cylindrical extractor with an area of 314 cm2 in spring (May) and winter (December) in 2018 (Bayranvand et al. 2017; Heydari et al. 2017b). We focus on this soil depth as soil chemical and biological conditions in the semi-arid environments is restricted to this uppermost soil layer (Yao et al. 2019). Soils were placed in hermetic boxes and immediately taken to the laboratory. Soil samples were then divided into two subsamples; one was kept in the refrigerator (+4 °C) for one 30 days before measuring microbial and biochemical properties, and the other was air-dried and passed through a 2 mm sieve to measure the physical and chemical properties. Additional undisturbed soil cores were taken for the determination of bulk density (BD) in the 0–25 cm mineral layer (Blake and Hartge 1986).
Laboratory measurements
Soil texture was measured using a hydrometer (Bouyoucos 1962) and soil water content (WC) was determined using the gravimetric method (Famiglietti et al. 1998). Soil pH was measured electrometrically (in soil: water suspension), while electrical conductivity (EC) was determined with a conductivity probe in filtered extracts (Kalra and Maynard 1991). Dichromate oxidation followed by rapid titration was used for soil organic carbon (OC) determination (Walkley and Black 1934). Lime content as the total neutralizing value (TNV) were determined by titration with NaOH (Black 1986). Available calcium and magnesium were determined using atomic absorption and complexometric titration (Botha and Webb 1952). Soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were analysed by dichromate digestion in both chloroform-sprayed and non-fumigated samples (Vance et al. 1987). Soil MBC was estimated from the carbon concentration (μgC g−1 of dried soil) of 0.5 M of K2SO4 soil extracts using the equation (Vance et al. 1987): MBC = 2.64 (A); where A is the difference in carbon from fumigated and non-fumigated soils. To obtain the MBN, fumigated and non-fumigated soil samples were extracted with K2SO4 and the filtered extract was measured for total Nitrogen using the Kjeldahl digestion method (Brookes et al. 1985). Soil basal respiration (BR) was determined by trapping and measuring emitted CO2 over a 5-day period (Alef and Nannipieri 1995). Substrate-induced respiration (SIR) was measured using glucose (1%) as substrate and evolved CO2 was measured after eight hours of incubation. Evolved CO2 was adsorbed by 1 M NaOH and measured by titration of 0.1 M HCl (Anderson and Domsch 1978). The total N discharge (N extracted by the K2SO4 from the non-fumigated soil subtracted from the fumigated soil) was divided by the KN value (N fraction of the biomass extracted after chloroform fumigation) of 0.54 (Brookes et al. 1985). Urease activity was measured according to Tabatabai and Bremner (1972) using a water-urea solution as a substrate. This activity was determined by the NH4+ released after a 2-h incubation at 37 °C. The soil alkaline phosphatase was measured based on the detection of p-nitrophenol (PNP) released after incubation (37 °C, 1 h) (Tabatabai and Bremner 1969).
Statistical analyses
Resemblance-based methods were used to analyse multivariate data in the context of more complex sampling structures and experimental designs (Clarke and Gorley 2015). Since microbiological soil parameters used in this study, i.e. (soil enzymes, soil respiration, SIR, MIE, MBC and MBN) did not meet the assumption of normality, we analysed the data using a resemblance matrix for environmental variables (physicochemical soil properties) and biological data (microbiological soil parameters). Factor studied were woody species (QU, AC, PI, CR, AM, LO) and season (spring and winter). The routines used were first PERMANOVA (Permutational MANOVA) in which biological data (urease and phosphatase activities, BR, MBC, MBN, MIE and SIR) were square root transformed and the resemblance matrix was built using the Bray-Curtis distance. The sums of squares type were type III (partial) and woody species and season were considered as fixed effects. The permutation method used was unrestricted permutation of raw data and the number of permutations was 999. Secondly, physicochemical soil data was analysed using non-metric Multi-Dimensional Scaling (MDS) and the Kruskal stress formula (minimum stress: 0.01) for visualizing the level of similarity of individual cases of a dataset. Previously to MDS, an analysis of similarities (ANOSIM), described by Clarke (1993), was developed for soil physicochemical properties, and multivariate resemblances were analysed according to the factors woody species and season. Thirdly, we made a Similarity Percentages (SIMPER) routine for identifying the physicochemical soil variables primarily discriminating between woody species. Fourthly, a comparative (Mantel-type) test on both physicochemical soil properties and soil microbiological parameters similarity matrices (RELATE) was developed to identify statistical relationships between environmental and biological data. Finally, Distance-based linear models (DISTLM) and distance-based redundancy analysis (dbRDA) were applied to the biological data being the criterion AICc used for selection of best model flowing the step-wise procedure. Statistical analyses were made using PRIMER V6 software (Clarke and Gorley 2015; Anderson et al. 2008).
Results
Permanova analyses showed significant statistical differences among the different woody species (Pseudo-F = 56.31; p = 0.001), season (Pseudo-F = 97.37; p = 0.001) and their interaction (Pseudo-F = 2.96; p = 0.005) for the matrix of microbiological soil parameters (urease and alkaline phosphatase activities, BR, MBC, MBN, MIE and SIR) (Table 1). Most microbial soil parameters varied among woody species and seasons and higher values of microbiological soil parameters were generally observed for tree species, i.e. QU, AC and PI, and during the spring season (Figs. 3 and 4). Similarly, soil microbial factors were higher during the spring season under shrub species (AM, CR and LO) although differences between seasons were lower compared to tree species (Figs. 3 and 4). Specifically, BR was significantly different between seasons across all species with significant larger values in spring (P < 0.05, Fig. 3). Similarly, SIR was higher in spring, but differences were only significant for tree species (P < 0.05, Fig. 4). Overall, the effect of season on the microbial parameters was stronger compared to the plant identity (Table 1).
Boxplots for each microbiological soil parameters according to factors (woody species and season). Urease activity (μgNH4+ –N g−1 2 h−1), phosphatase activity (μg PNP g−1 h−1) and basal soil respiration (mg CO2-C kg−1 soil day−1) among woody species (QU: Quercus brantii, AC: Acer monspessulanum, PI: Pistacia atlantica, CR: Crataegus pontica, AM: Amygdalus scoparia, LO: Lonicera nummularifolia) and season (S: spring, W: winter). Asterisk indicating significant statistical differences between seasons among species (P(perm) <0.05). A blue and a green boxplot with the meaning of spring and winter, respectively
Boxplots for microbiological soil parameters according to factors (woody species and season). SIR: substrate-induced respiration (mg CO2-C kg−1 soil day−1), MIE: microbial entropy, MBC: microbial biomass carbon (mg kg soil−1), MBN: microbial biomass nitrogen (mg kg soil−1); QU: Quercus brantii, AC: Acer monspessulanum, PI: Pistacia atlantica, CR: Crataegus pontica, AM: Amygdalus scoparia, LO: Lonicera nummularifolia and season (S: spring, W: winter). Asterisk indicating significant statistical differences between seasons among species (P(perm) < 0.05). A blue and a green boxplot with the meaning of spring and winter, respectively
The ANOSIM analyses showed statistically significant differences in soil physicochemical properties among woody species levels (Global R: 0.86, significance level of sample statistic: 0.1%) and between seasons for each woody specie (Global R: 0.43, Significance level of sample statistic: 0.1%) (Table S1). The NMDS analyses clearly separated all woody species (Fig. 5a) and season levels within species (Fig. 5b). Tree species QU and AC clustered and were largely explained by variation in total N, OC and nutrients. Shrub species such as CR were driven mostly by soil texture parameters, e.g. clay, silt and sand, C:N, lime and BD (Fig. 5).
The Similarity Percentages analyses showed the most important soil properties contributing to the average square distance for each woody species level (Table 2). The average square distance for QU plots was 3.74 and the soil physicochemical properties that most contributed to similarity of QU plots were BD, Mg2+, pH, sand and clay properties. In the case of AC plots, the average square distance was 3.44 and the soil properties that most contributed to similarity of AC plots were BD, silt, Mg2+, pH and clay properties. In the case of PI plots, average squared distance was 13.44 and the soil properties that most contributed to similarity of PI plots were pH, clay, sand, silt and EC. Average square distance was 3.25 in case of CR plots, being BD, lime, WC, Mg2+ and Ca2+ the most important variables; and 3.42 for the LO plots, being sand, pH, clay, Mg2+ and Ca2+ the most important variables. Finally, average square distance was 2.21 in case of AM plots, being BD, lime, WC, Mg2+ and Ca2+ the most important variables (Table 2).
The matched resemblance matrices test (RELATE test) indicated that soil physico-chemical properties significantly correlated with microbiological soil parameters (Rho: 0.43; significance level of sample statistic: 0.1%). According to the distance based linear models (DistLM), marginal test and sequential test for physicochemical soil variables indicated that almost all physicochemical soil variables influenced soil microbiology when considered isolated (Table 3).
The best model for predicting microbiological soil parameters was composed by BD, OC, WC, lime, Mg2+, pH, Ca2+ and Clay % (R2 = 0.75, AICs = 173.56; Table 4). The RDA analysis for the microbiological soil parameters showed that the percentage of variation explained by axis1 was 72.0% out of the fitted model and 54.2% out of total variation whereas the percentage of variation explained by the axis 2 was 16.8% out of the fitted model and 12.6% out of total (Fig. 6). The dbRDA1 axis clearly discriminated trees from shrub species. There was a positive correlation between microbial parameters, e.g. BR, SIR, MBC and MBN which accounted for a large variation in the distribution of samples along axis 1 (Fig. 6a). Samples did not show a clear clustering for season (Fig. 6b).
Redundancy analysis (RDA) analysis graph for microbiological soil parameters matrix according to microbiological soil matrix (a) and different seasons (b); MIE: microbial entropy, BR: basal respiration, MBC: microbial biomass carbon, MBN: microbial biomass nitrogen, SIR: substrate-induced respiration; QU: Quercus brantii, AC: Acer monspessulanum, PI: Pistacia atlantica, CR: Crataegus pontica, AM: Amygdalus scoparia, LO: Lonicera nummularifolia
Discussion
Overall, our results showed that woody species had a strong influence on the soil microbial and physicochemical properties, which confirmed our first hypothesis. Specifically, we found a clear contrast in the enzymatic activities (i.e. urease and phosphatase), BR and SIR between trees and shrubs. Woody plants have an undeniable role in soil processes and generates different microclimates conditions that facilitate processes such as surface runoff reduction and seed trapping efficiency (Aerts et al. 2006). All these processes promote the formation of ‘fertile islands’, assisting the establishment and survival of seedlings in degraded sites (Heydari et al. 2017b; Avendaño-Yáñez et al. 2018; Urza et al. 2019). Although the effects of particular plant species on soil properties have been broadly reported (Ayres et al. 2009; Waring et al. 2015) few studies have examined the factors that control these aspects. (Bayranvand et al. 2017; Heydari et al. 2017a).
Our results highlight the effect that woody species identity can exert on important soil physicochemical and microbiological parameters and point to the factors driving these differences. Firstly, the distribution of soil nutrient is influenced by plant structure, as shown by the progressive increase in litter content with tree age or forest management (Lucas-Borja et al. 2016). The higher organic matter accumulation in unmanaged and older forests in comparison with younger plant ecosystems may allow higher soil microbiological activity (Lucas-Borja et al. 2019a). Secondly, a stronger effect of plant identity can be expected as a consequence of the irregular distribution of soil nutrients nearby plants in arid and semi-arid ecosystems (Koch et al. 2007; Allison et al. 2008; Li et al. 2014). This effect has been attributed to the diverging carbon and nitrogen enrichments beneath shrub and tree canopies (via litter and rhizodeposition) driven by the diverse species (Perroni-Ventura et al. 2006; Chen et al. 2020). In this study, tree species, i.e. Quercus brantii, and Acer monspessulanum and Pistacia atlantica showed higher rates of enzymatic activity, SIR and MBC compared to shrubs. These microbial parameters were strongly correlated to nutrient levels, and particularly related to total nitrogen, organic carbon and the C:N, in agreement with other studies (Thompson et al. 2006). Soil water content and bulk density were among the most significant physicochemical variables driving the activity of microbes and enzymes in the Zagros forests. Specifically, we found a negative correlation between bulk density and carbon and nitrogen, and higher bulk density was found in shrubs compared to trees. Our findings agree with recent studies that also found a significant connection between bulk density and microbial diversity and activity (Muñoz-Rojas et al. 2016; Liu et al. 2018; Ramírez et al. 2020). Moreover, the higher BD under the shrubs is certainly caused primarily by the lower SOC (Lucas-Borja et al. 2019b). These relationships reflect the positive effect of an improved soil structure and associated lower rates of surface runoff and soil erosion, on the soil microbial communities (Xiao et al. 2017). Although several studies have pointed to pH as a regulator of microbial activity and composition (Rousk et al. 2010), we did not find a strong relationship between pH and the soil microbial parameters in tree species. This can be explained by the calcareous nature of the substrate found at the study site, so that soils are very well buffered and any acidification by organic activity is not detectable (Certini 2005).
Our results also evidenced the critical role of season as a factor modulating soil microbial properties and enzymatic activities, which corroborated our second hypothesis. Both urease and phosphatase activities were significantly different between spring and winter. Specifically, we found higher values in spring where higher temperatures and lower precipitations were recorded. Nevertheless, differences among shrubs and trees were species-specific with a marked effect for tree species. Several studies have documented seasonal variations in soil enzymatic activity, attributed mostly to patterns in temperature and/or moisture (Bloem et al. 2005; Brockett et al. 2012). Temperatures in different seasons can largely affect organic matter, decomposition of litter and soil acidity and thus enzymatic activity (Fekete et al. 2011; Baldrian et al. 2013). However, variation reported in the overall enzymatic activities across seasons has been largely inconsistent among enzyme types, soil properties and ecosystems (Salazar et al. 2011; Veeraragavan et al. 2018). Specific woody species producing different amounts of plant residues and tree canopy cover (Geisseler et al. 2011; Maillard et al. 2019) can in fact change the microclimate conditions such as understory temperature and humidity (Koch et al. 2007). Moreover, these differences in litter can result in different microbial carbon biomass that in turn will affect the soil enzymatic activity (Singh et al. 2017).
Basal respiration was consistently higher in spring (with higher temperature and lower moisture compared to winter) across all the studied species. In semi-arid ecosystems, water availability is a crucial factor regulating soil respiration and moderating the influence of other factors on soil respiration such as temperature and substrate availability (Yan et al. 2014; Muñoz-Rojas et al. 2016). The intra- and inter-annual variation of this water availability is directly connected to the intensity and frequency of precipitations (Fang et al. 2017). Projected climate scenarios in dryland regions such as the Zagros forests in Iran, predict extended dry periods and more irregular rainfall events (Vaghefi et al. 2019). From our results we can then assume that this increased warming would have large impacts on the enzymatic activity and soil respiration in these semi-arid ecosystems. In fact, an increase in soil temperature and a decrease in soil moisture would negatively impact soil microbial activity (Muñoz-Rojas et al. 2016).
Overall, our results highlighted the importance of soil microbial and enzyme activities to assess the variation of soil characteristics and functions and soil-plant associations across woody plant species. Soil microbial parameters and enzyme activities have been proposed as relevant indicators to assess ecosystem recovery following restoration (Muñoz-Rojas 2018). In our study region, the use of criteria evaluation and relevant indicators has been identified as a priority for a sustainable forest management (Nazariani et al. 2017). Thus, the outcomes of this study may have practical significance for restoration and conservation management for the Zagros forest. Our study evidence that enzyme activities and soil microbial parameters can serve as effective indicators of soil function at landscape or ecosystem scale, but they are also sensitive to intra-annual changes and plant-specific rhizospheres. We found that both urease and phosphatase activities could be useful to identify hotspots of biological activity and soil nutrient in semi-arid ecosystems, which can assist ecosystem recovery (Hu et al. 2016). This is particularly relevant in areas like the Zagros forest (Arekhi et al. 2010; Komprdová et al. 2016), where high dependence of human population on forest resources has caused severe land degradation through deforestation processes (Heydari et al. 2014; Amiraslani and Dragovich 2011).
Adequate forest management strategies need to be implemented in arid and semi-arid forests, including our study area in western Iran. These forests present a high ecological heterogeneity in terms of woody species composition and canopy structure, which guarantees diverse ecosystem services and a high biodiversity level (Assal et al. 2016; Heydari et al. 2019). However, the high level of human pressure due to the strong livelihood dependence of people on forest resources generates different anthropogenic disturbances (i.e. alteration on vegetation composition or excessive grazing pressure), which are accentuated by climatic and land-use changes (Plieninger et al. 2011; Heydari et al. 2019). Having solid information about plant-soil interactions can help to effectively manage these arid and semi-arid environments.
Conclusion
Our results evidenced a strong effect of season and plant species on soil physicochemical and microbiological soil properties in semi-arid forest ecosystems. Soil nitrogen, carbon and bulk density were the most significant variables driving the activity of microbes and enzymes in the Zagros forests. Higher values of microbiological soil parameters, e.g. microbial biomass and enzymatic activities were generally observed for tree species, particularly Quercus brantii, and Acer monspessulanum. Soil microbial function parameters, including urease and phosphatase activities were higher during the spring season under shrub species although differences between seasons were lower compared to tree species. This study highlights the importance of soil microbial parameters and enzyme activities to assess soil function and soil-plant interactions. Furthermore, it provides essential information for maintaining ecosystems’ health and quality through appropriate restoration and conservation management of semi-arid ecosystems such as the Zagros forests.
References
Adetunji AT, Lewu FB, Mulidzi R, Ncube B (2017) The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: a review. J Soil Sci Plant Nutr 17(3):794–807
Aerts R, Maes W, November E, Behailu M, Poesen J, Deckers J, Hermy M, Muys B (2006) Surface runoff and seed trapping efficiency of shrubs in a regenerating semiarid woodland in northern Ethiopia. Catena 65(1):61–70
Ahlström A, Raupach MR, Schurgers G, Smith B, Arneth A, Jung M, Reichstein M, Canadell JG, Friedlingstein P, Jain AK, Kato E (2015) The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 348(6237):895–899
Alef K, Nannipieri P (1995). Methods in applied soil microbiology and biochemistry (No. 631.46 M592ma). Academic Press
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37(5):937–944
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Chang Biol 14(5):1156–1168
Amiraslani F, Dragovich D (2011) Combating desertification in Iran over the last 50 years: an overview of changing approaches. J Environ Manag 92(1):1–13
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10(3):215–221
Anderson, M., Gorley, R.N.R.N., Clarke, K., Anderson, M.J., Gorley, R.N., Clarke, K.R., Anderson, M., Gorley, R. and Andersom, M.J. (2008) Permanova+ for primer. Guide to software and statistical methods
Arekhi S, Heydari M, Pourbabaei H (2010) Vegetation-environmental relationships and ecological species groups of the Ilam oak forest landscape, Iran. Caspian Journal of Environmental Sciences 8(2):115–125
Assal TJ, Anderson PJ, Sibold J (2016) Spatial and temporal trends of drought effects in a heterogeneous semi-arid forest ecosystem. For Ecol Manag 365:137–151
Avazpoor Z, Moradi M, Basiri R, Mirzaei J, Taghizadeh-Mehrjardi R, Kerry R (2019) Soil enzyme activity variations in riparian forests in relation to plant species and soil depth. Arabian Journal of Geosciences 12(23):708
Avendaño-Yáñez MDLL, López-Ortiz S, Perroni Y, Pérez-Elizalde S (2018) Leguminous trees from tropical dry forest generate fertility islands in pastures. Arid Land Res Manag 32(1):57–70
Ayres E, Steltzer H, Berg S, Wallenstein MD, Simmons BL, Wall DH (2009) Tree species traits influence soil physical, chemical, and biological properties in high elevation forests. PLoS One 4(6):e5964
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V (2013) Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem 56:60–68
Bastida F, Barberá GG, García C, Hernández T (2008) Influence of orientation, vegetation and season on soil microbial and biochemical characteristics under semiarid conditions. Appl Soil Ecol 38(1):62–70
Bayranvand M, Kooch Y, Rey A (2017) Earthworm population and microbial activity temporal dynamics in a Caspian Hyrcanian mixed forest. Eur J For Res 136:447–456
Black CA (1986) Methods of soil analysis, part l. ASA, Madison, WI, pp 545–566
Blake GR, Hartge KH (1986) Bulk density. Methods of soil analysis: Part 1 Physical and mineralogical methods 5:363–375
Bloem J, Hopkins DW, Benedetti A (2005) Microbiological methods for assessing soil quality. CABI
Botha, C.R. and Webb, M.M. (1952). The versenate method for the determination of calcium and magnesium in mineralized water containing large concentrations of interfering ions. Institute of Water Engineers Journal, 6
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils 1. Agron J 54(5):464–465
Bracken A, Coburn C, Staenz K, Rochdi N, Segl K, Chabrillat S, Schmid T (2019) Detecting soil erosion in semi-arid Mediterranean environments using simulated EnMAP data. Geoderma 340:164–174
Brockett BF, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44(1):9–20
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143(1):1–10
Chen X, Chen HY, Chen C, Ma Z, Searle EB, Yu Z, Huang Z (2020) Effects of plant diversity on soil carbon in diverse ecosystems: a global meta-analysis. Biol Rev 95(1):167–183
Cheng F, Peng X, Zhao P, Yuan J, Zhong C, Cheng Y, Cui C, Zhang S (2013) Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling Mountains. PLoS One 8(6)
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18(1):117–143
Clarke KR, Gorley RN (2015) PRIMER v7: user manual/tutorial, 3rd edn. Primer-E Ltd., Plymouth, United Kingdom
Dai X, Fu X, Kou L, Wang H, Shock CC (2018) C: N: P stoichiometry of rhizosphere soils differed significantly among overstory trees and understory shrubs in plantations in subtropical China. Can J For Res 48(11):1398–1405
Di Sabatino A, Cicolani B, Miccoli FP, Cristiano G (2020) Plant detritus origin and microbial–detritivore interactions affect leaf litter breakdown in a central Apennine (Italy) cold spring. Aquatic Ecology:1–10
Estruch C, Macek P, Armas C, Pistón N, Pugnaire FI (2020) Species identity improves soil respiration predictions in a semiarid scrubland. Geoderma 363:114153
Famiglietti JS, Rudnicki JW, Rodell M (1998) Variability in surface moisture content along a hillslope transect: Rattlesnake Hill, Texas. J Hydrol 210(1–4):259–281
Fang Y, Michalak AM, Schwalm CR, Huntzinger DN, Berry JA, Ciais P, Piao S, Poulter B, Fisher JB, Cook RB, Hayes D (2017) Global land carbon sink response to temperature and precipitation varies with ENSO phase. Environmental Research Letters 12(6):064007
Fekete I, Varga C, Kotroczó Z, Tóth JA, Várbiró G (2011) The relation between various detritus inputs and soil enzyme activities in a central European deciduous forest. Geoderma 167:15–21
Geisseler D, Horwath WR, Scow KM (2011) Soil moisture and plant residue addition interact in their effect on extracellular enzyme activity. Pedobiologia 54(2):71–78
Grayston SJ, Vaughan D, Jones D (1997) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5(1):29–56
Hedo, J., Lucas-Borja, M.E., Wic, C., Andrés-Abellán, M. and De Las Heras, J., 2015. Soil microbiological properties and enzymatic activities of long-term post-fire recovery in dry and semiarid Aleppo pine (Pinus halepensis M.) forest stands. Solid Earth, 6(1)
Heidarlou HB, Shafiei AB, Erfanian M, Tayyebi A, Alijanpour A (2019) Effects of preservation policy on land use changes in Iranian northern Zagros forests. Land Use Policy 81:76–90
Heydari M, Prévosto B, Naji HR, Mehrabi AA, Pothier D (2017a) a. Influence of soil properties and burial depth on Persian oak (Quercus brantii Lindl.) establishment in different microhabitats resulting from traditional forest practices. Eur J For Res 136(2):287–305
Heydari M, Prévosto B, Abdi T, Mirzaei J, Mirab-Balou M, Rostami N, Khosravi M, Pothier D (2017b) b. Establishment of oak seedlings in historically disturbed sites: regeneration success as a function of stand structure and soil characteristics. Ecol Eng 107:172–182
Heydari M, Aazami F, Faramarzi M, Omidipour R, Bazgir M, Pothier D, Prévosto B (2019) Interaction between climate and management on beta diversity components of vegetation in relation to soil properties in arid and semi-arid oak forests, Iran. Journal of Arid Land 11(1):43–57
Heydari M, Poorbabaei H, Bazgir M, Salehi A, Eshaghirad, J (2014) Earthworms as indicators for different forest management types and human disturbance in Ilam oak forest, Iran. Folia Forestalia Polonica, 56(3):121–134
Hu R, Wang X-p, Zhang Y-f, Shi W, Jin Y-x, Chen N (2016) Insight into the influence of sand-stabilizing shrubs on soil enzyme activity in a temperate desert. CATENA 137:526–535
Huang X, Liu L, Wen T, Zhang J, Wang F, Cai Z (2016) Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Appl Microbiol Biotechnol 100(12):5581–5593
Hursh A, Ballantyne A, Cooper L, Maneta M, Kimball J, Watts J (2017) The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Glob Chang Biol 23(5):2090–2103
Jarosch KA, Kandeler E, Frossard E, Bünemann EK (2019) Is the enzymatic hydrolysis of soil organic phosphorus compounds limited by enzyme or substrate availability? Soil Biol Biochem 139:107628
Jia X, Mu Y, Zha T, Wang B, Qin S, Tian Y (2020) Seasonal and interannual variations in ecosystem respiration in relation to temperature, moisture, and productivity in a temperate semi-arid shrubland. Sci Total Environ 709:136210
Kaiser C, Fuchslueger L, Koranda M, Gorfer M, Stange CF, Kitzler B, Rasche F, Strauss J, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2011) Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 92(5):1036–1051
Kalra, Y.P. and Maynard, D.G., 1991. Methods manual for forest soil and plant analysis (Vol. 319)
Kang H, Kang S, Lee D (2009) Variations of soil enzyme activities in a temperate forest soil. Ecol Res 24(5):1137–1143
Khalyani AH, Mayer AL (2013) Spatial and temporal deforestation dynamics of Zagros forests (Iran) from 1972 to 2009. Landsc Urban Plan 117:1–12
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob Biogeochem Cycles 21(4)
Komprdová K, Komprda J, Menšík L, Vaňková L, Kulhavý J, Nizzetto L (2016) The influence of tree species composition on the storage and mobility of semivolatile organic compounds in forest soils. Sci Total Environ 553:532–540
Li P, Yang Y, Han W, Fang J (2014) Global patterns of soil microbial nitrogen and phosphorus stoichiometry in forest ecosystems. Glob Ecol Biogeogr 23(9):979–987
Li J, Nie M, Pendall E (2020) Soil physico-chemical properties are more important than microbial diversity and enzyme activity in controlling carbon and nitrogen stocks near Sydney, Australia. Geoderma 366:114201
Liu D, Huang Y, An S, Sun H, Bhople P, Chen Z (2018) Soil physicochemical and microbial characteristics of contrasting land-use types along soil depth gradients. Catena 162:345–353
Lucas-Borja ME, Candel Pérez D, López Serrano FR, Andrés M, Bastida F (2012a) Altitude-related factors but not Pinus community exert a dominant role over chemical and microbiological properties of a Mediterranean humid soil. Eur J Soil Sci 63(5):541–549
Lucas-Borja ME, Candel D, Jindo K, Moreno JL, Andrés M, Bastida F (2012b) Soil microbial community structure and activity in monospecific and mixed forest stands, under Mediterranean humid conditions. Plant Soil 354(1–2):359–370
Lucas-Borja ME, Hedo J, Cerdá A, Candel-Pérez D, Viñegla B (2016) Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. Ssp. salzmannii) Forest. Sci Total Environ 562:145–154
Lucas-Borja ME, de Santiago JH, Yang Y, Shen Y, Candel-Pérez D (2019a) Nutrient, metal contents and microbiological properties of litter and soil along a tree age gradient in Mediterranean forest ecosystems. Sci Total Environ 650:749–758
Lucas-Borja ME, Zema DA, Plaza-Álvarez PA, Zupanc V, Baartman J, Sagra J, González-Romero J, Moya D, de las Heras J (2019b) Effects of different land uses (abandoned farmland, intensive agriculture and forest) on soil hydrological properties in Southern Spain. Water 11(3):503
Maillard F, Leduc V, Bach C, de Moraes Gonçalves JL, Androte FD, Saint-André L, Laclau JP, Buée M, Robin A (2019) Microbial enzymatic activities and community-level physiological profiles (CLPP) in subsoil layers are altered by harvest residue management practices in a tropical Eucalyptus grandis plantation. Microb Ecol 78(2):528–533
Merilä P, Malmivaara-Lämsä M, Spetz P, Stark S, Vierikko K, Derome J, Fritze H (2010) Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl Soil Ecol 46(2):259–267
Mungai NW, Motavalli PP, Kremer RJ, Nelson KA (2005) Spatial variation of soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fertil Soils 42(2):129–136
Muñoz-Rojas M (2018) Soil quality indicators: critical tools in ecosystem restoration. Current Opinion in Environmental Science & Health 5:47–52
Muñoz-Rojas M, Lewandrowski W, Erickson TE, Dixon KW, Merritt DJ (2016) Soil respiration dynamics in fire affected semi-arid ecosystems: effects of vegetation type and environmental factors. Sci Total Environ 572:1385–1394
Nazariani N, Fallah A, Lotfalian M, Rastabi MI (2017) Introduction of sustainable management tools in central Zagros forests of Iran. J Sustain For 36(3):199–212
Ostle NJ, Smith P, Fisher R, Ian Woodward F, Fisher JB, Smith JU, Galbraith D, Levy P, Meir P, McNamara NP, Bardgett RD (2009) Integrating plant–soil interactions into global carbon cycle models. J Ecol 97(5):851–863
Oyonarte C, Rey A, Raimundo J, Miralles I, Escribano P (2012) The use of soil respiration as an ecological indicator in arid ecosystems of the SE of Spain: spatial variability and controlling factors. Ecol Indic 14(1):40–49
Perroni-Ventura Y, Montaña C, García-Oliva F (2006) Relationship between soil nutrient availability and plant species richness in a tropical semi-arid environment. J Veg Sci 17:719–728
Plieninger T, Schaich H, Kizos T (2011) Land-use legacies in the forest structure of silvopastoral oak woodlands in the eastern Mediterranean. Reg Environ Chang 11(3):603–615
Ramírez PB, Fuentes-Alburquenque S, Díez B, Vargas I, Bonilla CA (2020) Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol Biochem 141:107692
Rey A, Pegoraro E, Oyonarte C, Were A, Escribano P, Raimundo J (2011) Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain. Soil Biol Biochem 43(2):393–403
Rezende RS, Santos AM, Medeiros AO, Gonçalves JF Jr (2017) Temporal leaf litter breakdown in a tropical riparian forest with an open canopy. Limnetica 36(2):445–459
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME journal 4(10):1340–1351
Safriel U, Adeel Z (2005) Dryland systems. In: Hassan R, Scholes R, Ash N (eds) Ecosystems and human well-being, current state and trends, vol 1. Island Press, Washington, pp 625–658
Salazar S, Sánchez LE, Alvarez J, Valverde A, Galindo P, Igual JM, Peix A, Santa-Regina I (2011) Correlation among soil enzyme activities under different forest system management practices. Ecol Eng 37(8):1123–1131
Sandoval-Pérez AL, Gavito ME, García-Oliva F, Jaramillo VJ (2009) Carbon, nitrogen, phosphorus and enzymatic activity under different land uses in a tropical, dry ecosystem. Soil Use Manag 25(4):419–426
Sardans J, Peñuelas J (2013) Plant-soil interactions in Mediterranean forest and shrublands: impacts of climatic change. Plant Soil 365(1–2):1–33
Scheibe A, Steffens C, Seven J, Jacob A, Hertel D, Leuschner C, Gleixner G (2015) Effects of tree identity dominate over tree diversity on the soil microbial community structure. Soil Biol Biochem 81:219–227
Shahbaz M, Kuzyakov Y, Sanaullah M, Heitkamp F, Zelenev V, Kumar A, Blagodatskaya E (2017) Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils 53(3):287–301
Singh SB, Saha S, Dutta SK, Singh AR, Boopathi T (2017) Impact of secondary forest fallow period on soil microbial biomass carbon and enzyme activity dynamics under shifting cultivation in north Eastern Hill region, India. Catena 156:10–17
Snyder KA, Tartowski SL (2006) Multi-scale temporal variation in water availability: implications for vegetation dynamics in arid and semi-arid ecosystems. J Arid Environ 65(2):219–234
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1(4):301–307
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biol Biochem 4(4):479–487
Teimouri M, Mohamadi P, Jalili A, Dick WA (2018) Assessing soil quality through soil chemical properties and enzyme activities in Semiarid Area, Iran. In: Assessing soil quality through soil chemical properties and enzyme activities in semiarid area, Iran
Thompson TL, Zaady E, Huancheng P, Wilson TB, Martens DA (2006) Soil C and N pools in patchy shrublands of the Negev and Chihuahuan deserts. Soil Biol Biochem 38:1943–1955
Urza AK, Weisberg PJ, Chambers JC, Board D, Flake SW (2019) Seeding native species increases resistance to annual grass invasion following prescribed burning of semiarid woodlands. Biol Invasions 21(6):1993–2007
Ushio M, Wagai R, Balser TC, Kitayama K (2008) Variations in the soil microbial community composition of a tropical montane forest ecosystem: does tree species matter? Soil Biol Biochem 40(10):2699–2702
Vaghefi SA, Keykhai M, Jahanbakhshi F, Sheikholeslami J, Ahmadi A, Yang H, Abbaspour KC (2019) The future of extreme climate in Iran. Sci Rep 9(1):1–11
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707
Veeraragavan S, Duraisamy R, Mani S (2018) Seasonal variation of soil enzyme activities in relation to nutrient and carbon cycling in Senna alata (L.) Roxb invaded sites of Puducherry region, India. Geology, Ecology, and Landscapes 2(3):155–168
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15(7):1631–1639
Wan X, Huang Z, He Z, Yu Z, Wang M, Davis MR, Yang Y (2015) Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387(1–2):103–116
Waring BG, Álvarez-Cansino L, Barry KE, Becklund KK, Dale S, Gei MG, Keller AB, Lopez OR, Markesteijn L, Mangan S, Riggs CE (2015) Pervasive and strong effects of plants on soil chemistry: a meta-analysis of individual plant ‘Zinke’effects. Proceedings of the Royal Society B: Biological Sciences 282(1812):20151001
Wu H, Xiang W, Ouyang S, Forrester DI, Zhou B, Chen L, Ge T, Lei P, Chen L, Zeng Y, Song X (2019) Linkage between tree species richness and soil microbial diversity improves phosphorus bioavailability. Funct Ecol 33(8):1549–1560
Xia Z, Yu L, He Y, Korpelainen H, Li C (2019) Broadleaf trees mediate chemically the growth of Chinese fir through root exudates. Biol Fertil Soils 55(7):737–749
Xiao H, Li Z, Dong Y, Chang X, Deng L, Huang J, Nie X, Liu C, Liu L, Wang D, Liu Q (2017) Changes in microbial communities and respiration following the revegetation of eroded soil. Agric Ecosyst Environ 246:30–37
Yan L, Chen S, Xia J, Luo Y (2014) Precipitation regime shift enhanced the rain pulse effect on soil respiration in a semi-arid steppe. PLoS One 9(8)
Yang K, Zhu J, Yan Q, Zhang J (2012) Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann For Sci 69(7):795–803
Yao et al (2019) Effects of shrubs on soil nutrients and enzymatic activities over a 0–100 cm soil profile in the desert-loess transition zone. Catena 174:362–370
Zaman M, Saggar S, Blennerhassett JD, Singh J (2009) Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol Biochem 41(6):1270–1280
Jazirehi MH, Ebrahimi M (2003) Silviculture in Zagros. University of Tehran Press, 560p
Zhang W, Qiao W, Gao D, Dai Y, Deng J, Yang G, Han X, Ren G (2018) Relationship between soil nutrient properties and biological activities along a restoration chronosequence of Pinus tabulaeformis plantation forests in the Ziwuling Mountains, China. Catena 161:85–95
Zuccarini P, Asensio D, Ogaya R, Sardans J, Peñuelas J (2020) Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Glob Chang Biol 26:3698–3714
Acknowledgements
The authors acknowledge Mr. M. Kosha and Mr. A. Azemi for their tireless assistance with field sampling and also Mr. A. Chabok for his expert technical assistance at the laboratory analysis. This work was done by financial support of Ilam University, Ilam, Iran. MMR was supported by the Australian Research Council Discovery Early Career Research Award DE180100570.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Timothy J. Fahey
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Eslaminejad, P., Heydari, M., Kakhki, F.V. et al. Plant species and season influence soil physicochemical properties and microbial function in a semi-arid woodland ecosystem. Plant Soil 456, 43–59 (2020). https://doi.org/10.1007/s11104-020-04691-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04691-1