Abstract
Entomopathogenic nematodes (EPNs) play a role in indirect defenses of plants under attack by root herbivores. We have tested the chemotactic responses of 4 EPN species (Steinernema feltiae, S. carpocapsae, S. kraussei, and Heterorhabditis bacteriophora) to 5 compounds ([1] α-Pinene, [2] Terpinolene, [3] Bornyl acetate, [4] 2-Ethyl-hexanol, and [5] 2, 4-Di-tert-butylphenol) released by damaged (3, 4, 5) and undamaged (1, 2) carrot roots. We hypothesized that the EPN directional responses to the tested volatile compounds (VOCs) could be related to foraging strategy and would vary among species, VOC, and VOC concentrations. Our results indicate that all of the tested EPN species exhibited a weak attraction or repulsion to volatiles, irrespective of their foraging strategy. Terpinolene was a repellent for EPN species classified in all three foraging groups. However, such values of chemotaxis index (CI) were reported with EPN species only when pure concentration of VOC was used. Based on our current results, we conclude that responses to distinct volatile cues are a species-specific characteristic. Our results suggest that EPNs are able to distinguish herbivore-induced chemicals from chemicals that are typical for healthy roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
VOCs have an important role in the tritrophic system consisting of a plant, a herbivore, and its natural enemy.
-
The chemotactic responses of four EPN species to five compounds released by damaged and undamaged carrot roots are reported.
-
Our results suggest that responses to distinct volatile cues are a species-specific characteristic and irrespective of their foraging strategy.
-
Current results suggest that EPNs are able to distinguish herbivore-induced chemicals from chemicals that are typical for healthy roots.
Introduction
Tritrophic interactions involving plants, herbivores, and parasites have been documented for belowground systems, where entomopathogenic nematodes (EPNs) can exploit root herbivore-induced volatile compounds to locate their hosts (Rasmann et al. 2005; Rasmann and Turlings 2008; Ali et al. 2010). Soil is the natural habitat of EPNs from the families Steinernematidae and Heterorhabditidae, and their application in pest management has been primarily against soil-dwelling insect pests (Ishibashi and Choi 1991; Kaya and Gaugler 1993; Koppenhöffer et al. 2004). In both Steinernema and Heterorhabditis, there is a single free-living stage, the infective juvenile (IJ), that carries in its gut bacteria of the genus Xenorhabdus and Photorhabdus, respectively (Boemare et al. 1993). On encountering a suitable insect, the IJ enters through the mouth, anus, or spiracles and makes its way to the haemocoel (Eidt and Thurston 1995). Some species may also penetrate through the intersegmental membranes of the insect cuticle (Peters and Ehlers 1994). In the haemocoel, the IJ releases cells of its bacterial symbiont. Bacteria multiply rapidly in hemolymph and produce toxins, which contribute to the weakening of the host’s defense mechanism. The host attacked by EPN usually dies because of poisoning or failure of certain organs in 24 to 72 h after the infection (Forst and Clarke 2002).
The behavior of EPN has been intensively studied and different EPN species behave very differently in terms of dispersal and host-finding (Lewis 2002; Campbell et al. 2003). The ability of EPN IJ to disperse actively through soil and locate a host is a key element for the success of application of certain EPN species in pest management. IJ host-finding strategies differ from species to species (Lewis 2002; Campbell et al. 2003). Foraging strategies used by IJs to find a host vary between cruise (Heterorhabditis bacteriophora, and Steinernema kraussei), intermediate (S. feltiae), and ambush (S. carpocapsae) (Lewis 2002; Campbell et al. 2003). However, researches on their behavior have not considered the natural habitat of these nematodes. Kruitbos et al. (2009) suggested that EPNs may be habitat specialists and highlighted the difficulties of studying soil-transmitted parasites in non-soil media.
The rhizosphere provides a very attractive environment for a vast number of organisms (Wenke et al. 2010). Root exudates are chemically diverse, beginning with compounds such as amino acids and amides, organic acids, phenols, sugars, as well as a wide variety of secondary metabolites, polysaccharides, and proteins of higher molecular mass (Wenke et al. 2010). However, volatile compounds (VOCs) can also be detected in the rhizosphere of several plant species (Bais et al. 2004; Bais et al. 2006; Rasmann et al. 2005; Erb et al. 2013; Hiltpold et al. 2013). Ali et al. (2010) have demonstrated that citrus roots upon feeding by the root weevil Diaprepes abbreviatus emit several terpenes in the surrounding soil. Rasmann and Turlings (2008) reported that roots of cotton (Gossypium herbaceum) after feeding by the larvae of the chrysomelid beetle Diabrotica balteata emit >10 compounds, among which at least seven terpenoid volatiles were observed. Rasmann et al. (2005) reported that maize roots damaged by larvae of Diabrotica virgifera virgifera [Coleoptera: Chrysomelidae, known commonly as western corn rootworm (WCR)] emit a key attractant for EPNs. The compound in question (E)-β-caryophyllene proved to be a weak attractant for H. megidis, one of the most infectious nematode against WCR. Volatile metabolites emitted underground enable plants to directly and indirectly influence the community of soil-dwelling organisms (Bais et al. 2006; Erb et al. 2013). Using volatile metabolites plants can defend themselves against herbivores and plant pathogenic fungi and bacteria, support beneficial symbiosis, and combat competitive plant species (Bais et al. 2006). Chemotaxis is the main sensory mode nematodes use to orient themselves to their hosts. IJs have been shown to respond to both CO2 and other cues (Hallem et al. 2011; Dillman et al. 2012; Turlings et al. 2012). There are reports that IJs move toward or away from host excretory products, changes in pH, temperature, bacterial symbionts, electrical fields, and various plant volatile compounds (Burman and Pye 1980; Grewal et al. 1993; Rasmann et al. 2005; Shapiro-Ilan et al. 2012).
Wireworms, the soil-dwelling larval stages of the click beetle (Coleoptera: Elateridae), are a serious pest problem worldwide (Kuhar and Alvarez 2008). As polyphagous insects, they attack a number of important crops (e.g., potato, carrots, sugar beet, and occasionally cereals) (Parker and Howard 2001). White grubs are the root-feeding larvae of scarab beetles (Coleoptera: Scarabaeidae), and they are among the most destructive pests of horticultural plants, pastures, and turfgrass in many parts of the world (Laznik and Trdan 2015). Both species damage crops by feeding on their root systems after planting, which can significantly reduce crop quality (Jackson and Klein 2006; Johnson et al. 2008).
Here, we describe our study of the chemotactic behavior of Steinernema feltiae (Filipjev), Steinernema carpocapsae Weiser, Steinernema kraussei (Steiner), and Heterorhabditis bacteriophora Poinar toward α-Pinene, Bornyl acetate, 2, 4-Di-tert-butylphenol, 2-Ethyl-hexanol, and Terpinolene; VOCs released from insect (wireworms and grubs)-damaged carrot roots (Weissteiner and Schütz 2006; Weissteiner 2010). The aims of our research were (1) to study the effect of different EPN foraging strategies (ambush, intermediate, or cruise) toward the tested VOCs (2) to determine whether chemotaxis is species specific, and (3) to assess whether the VOCs from damaged and undamaged carrot roots have any effect on the tested EPNs behavior, and (4) if these VOCs are a part of an indirect plant defense.
Materials and methods
Source and maintenance of entomopathogenic nematodes
Four EPN species were tested in the experiment. The commercial preparations of Nemasys (a.i. S. feltiae), Nemasys C (a.i. S. carpocapsae), Nemasys L (a.i. S. kraussei), and Nemasys G (a.i. H. bacteriophora) were obtained from Becker Underwood. All EPN species were reared using the last instar larvae of Galleria mellonella (L.) (Lepidoptera: Pyralidae) (Bedding and Akhurst 1975). G. mellonella was reared in a controlled environment at 28 ± 2 °C, 60 % relative humidity (RH) with a 12-h photoperiod (Woodring and Kaya 1988; Parra 1998). The IJs were stored at 4 °C at a density of 2000 IJ ml−1. We only used IJs that were less than 2-week old (Laznik and Trdan 2013). The concentration of the EPN suspension was calculated according to Laznik et al. (2010). Nematode viability was determined prior to initiation of the chemotaxis experiment (Laznik and Trdan 2013), and only nematode stocks with >95 % survival were used (De Nardo and Grewal 2003).
Tested volatile compounds
The choice of VOCs used in our investigation was based on the research of Weissteiner (2010). Organically cultivated carrots (Daucus carota ssp. sativus) were used in their investigation. Larvae of Melolontha hippocastani and Agriotes sp. were used in their experiment in order to damage carrot roots. Gas chromatography–Mass spectrometry (GC–MS) analysis of VOCs released by undamaged and damaged roots was used in order to show different feeding-induced volatile pattern when infested by Melolontha or Agriotes larvae. Results of their investigation showed that undamaged carrot roots release several VOCs and among them were also (1) α-Pinene and (2) Terpinolene. Wireworm-damaged carrot roots release (3) Bornyl acetate and (4) 2-Ethyl-hexanol. Grub-damaged carrot roots release VOCs (3), (4), and (5) 2, 4-Di-tert-butylphenol. In order to perform our investigation, we used synthetic-produced compounds (Sigma Aldrich). The VOCs in the experiment were tested at two concentrations: (1) at pure concentration (O’Halloran and Burnell 2003; Laznik and Trdan 2013) and (2) at 0.03 ppm (the average concentration of VOCs in soil, 10 cm from the root system) (Weissteiner et al. 2012).
Chemotaxis assay
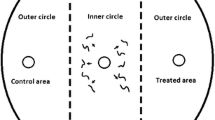
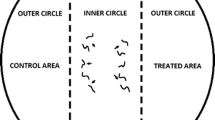
The chemotaxis assay was based on an assay developed by Ward (1973) and O’Halloran and Burnell (2003) and modified by Laznik and Trdan (2013). The assay plates used were Petri dishes, 9 cm in diameter containing 25 ml of 1.6 % technical agar (Biolife, Milano, Italy), 5 mM potassium phosphate (pH 6.0), 1 mM CaCl2, and 1 mM MgSO4. Three circular marks (1 cm in diameter) were made on the bottom of the plate: first in the center, then on the right and lastly on the left side of the Petri dish, 1.5 cm from its edge. A 10 μl drop of tested substance was placed on the right side of the agar surface (treated area), and 10 μl of distilled water (control area) (Laznik and Trdan 2013) was placed on the left side of the agar surface (both parts represent outer circles). The VOCs were immediately applied to the agar plates before the application of the nematodes (Bargmann and Horvitz 1991). A 50 μl drop of 100 IJs was placed in the center of the agar surface (inner circle). In control treatment 10 μl of distilled water was applied in control and treated area, and a 50 μl drop of 100 IJs was placed in the center of the agar surface. Each treatment included five replicates. All of the experiments were repeated 3 times. The Petri dishes were placed in a rearing chamber (RK-900 CH, Kambič Laboratory equipment, Semič, Slovenia) at 22 °C and 75 % RH, without light. The nematodes were allowed to move freely for 2 or 24 h, and the Petri dishes were then placed in a freezer at −20 °C for 3 min to immobilize the nematodes. The number of nematodes in the treatment and control areas was counted using a binocular microscope (Nikon C-PS) at ×25 magnification. The specific chemotaxis index (CI) (Bargmann and Horvitz 1991) was calculated as follows:
The CI varied from 1.0 (perfect attraction) to −1.0 (perfect repulsion). In the experiments reported here, compounds with a CI are classified as follows: ≥0.2, as attractive; from 0.2 to 0.1, as a weak attractant; from 0.1 to −0.1, no effect; from −0.1 to −0.2, as a weak repellent and ≤−0.2, as a repellent to EPNs (Laznik and Trdan 2013).
Statistical analysis
For all of the treatments and controls, preferential movement of nematodes from the inner to the outer circle of the Petri dish (i.e., a directional response) was determined using a paired t test comparing the number of IJs in the inner versus the outer circle (Statgraphics Plus for Windows 4.0; Shapiro-Ilan et al. 2012; α = 0.05). Additionally, to compare response levels among the foraging strategies, the average number of IJs that moved to the outer circle or stayed in the inner circle was calculated for each dish, and average numbers were compared through an analysis of variance (ANOVA, α = 0.05). Additionally, an analysis of variance (ANOVA) was performed on the CI to compare the level of response to the tested volatile compounds among the different EPN species depending on the exposure time and concentration, the means were separated by Duncan’s multiple range test with a significant level of p < 0.05. The data are presented as the mean ± SE. All of the statistical analyses were performed using Statgraphics Plus for Windows 4.0 (Statistical Graphics Corp., Manugistics, Inc., Rockville, MD, USA).
Results
Diversity of movement among EPN species and their foraging strategies
Analysis of the results showed that directional movement in response to volatile compounds from the inner (central part of the petri dish) to outer test circles (control and treated area) was influenced by different factors and their interactions (Table 1). Based on the t test results (t = 56, 73; p < 0.0001; α = 0.05), statistically significant differences were observed among the average number of IJs in the inner (83.0 ± 1.2) and outer (17.0 ± 0.6) circles after 24 h. There were significant differences in the average number of IJs in the outer circles among ambushers (12.9 ± 1.4), intermediates (16.4 ± 1.3), and cruisers (23.1 ± 0.9). Furthermore, after only 2 hours, an average of 3.1 ± 1.0 IJs moved to the outer circle, whereas after 24 h an average of 31.8 ± 2.1 IJs moved. Among the tested concentrations, there was a significantly higher number of IJs in the outer circles at pure concentration (23.5 ± 1.3) while an average of only 11.4 ± 0.8 IJs moved at 0.03 ppm. In the terpinolene treatment, we found a significantly higher number of EPNs in the outer circles (16.3 ± 1.2). There were also differences among cruisers (21.5 ± 1.0), intermediates (14.9 ± 0.5), and ambushers (12.6 ± 0.7) in movement toward the outer circles in the Terpinolene treatment. Foraging strategy did not affect the movement of IJs toward the other tested volatile compounds or the control. Among the tested EPN species, a significantly higher number of IJs in the outer circles was confirmed for S. kraussei (29.4 ± 0.2) and H. bacteriophora (29.2 ± 1.3). The number of IJs in the outer circles was significantly lower for S. feltiae (20.8 ± 2.4) and S. carpocapsae (16.3 ± 3.0).
Chemotaxis index
The analyses of the results showed that CI values were influenced by the species of EPN (F = 3.62; df = 3, 478; p = 0.0131), concentration of the volatile compound (F = 6.84; df = 1, 478; p = 0.0092), volatile compound (F = 2.58; df = 6, 478; p = 0.0183), time of exposure (F = 12.62; df = 1, 478; p = 0.0004), and interaction between EPN species and time of exposure (F = 9.21; df = 7, 478; p < 0.0001). Foraging strategy (F = 0.50; df = 2, 478; p = 0.6055); interaction between volatile compounds and foraging strategy (F = 0.79; df = 12, 478; p = 0.6571); interaction between volatile compounds and time of exposure (F = 1.03; df = 6, 478; p = 0.4071); and interaction between volatile compounds, foraging strategy, and time of exposure (F = 0.90; df = 12, 478; p = 0.5462) did not have a statistically significant influence on the CI values.
None of tested EPNs in our investigation showed any behavior response to tested VOCs at a concentration of 0.03 ppm after 2 h (Table 2). IJs of S. feltiae, S. carpocapsae did not show any behavior response to tested VOCs at a concentration of 0.03 ppm after 24 h (Table 3). The analysis of the CI values of different VOCs after 24 h at a concentration of 0.03 ppm showed that 2, 4-Di-tert butylphenol was a weak repellent (CI = −0.15 ± 0.03) for S. kraussei (Table 3). For other cruisers nematode species in our investigation (H. bacteriophora), the same VOC proved to be a weak attractant (CI = 0.17 ± 0.07). Similar findings were confirmed also in response of H. bacteriophora to α-Pinene (CI = 0.18 ± 0.07) (Table 3). Terpinolene proved to be a weak repellent (CI = −0.17 ± 0.07) of H. bacteriophora after 24 h, at a concentration of 0.03 ppm (Table 3). IJs of S. feltiae, S. carpocapsae, and H. bacteriophora did not show any behavior response to tested VOCs at pure concentration after 2 h (Table 4). The analysis of the CI values of different VOCs after 2 h at pure concentration showed that Terpinolene was a repellent (CI = −0.21 ± 0.02) for S. kraussei (Table 4). VOC α-Pinene, at a pure concentration after 24 h, proved to be a weak repellent for S. carpocapsae (CI = −0.11 ± 0.03), S. kraussei (CI = −0.14 ± 0.02), and H. bacteriophora (CI = −0.10 ± 0.02) (Table 5). Furthermore, VOCs 2-Ethyl-1-hexanol (CI = −0.11 ± 0.06) and Bornyl acetate (CI = −0.16 ± 0.04) proved to be a weak repellents for S. carpocapsae in our investigation (Table 5). In a contrast, VOC Bornyl acetate was a weak attractant (CI = 0.13 ± 0.05) for S. kraussei in our investigation at a pure concentration after 24 h (Table 5). The analysis of the CI values of different VOCs after 24 h at a pure concentration showed that Terpinolene was a repellent for S. carpocapsae (CI = −0.23 ± 0.04) and S. feltiae (CI = −0.23 ± 0.03) (Table 5).
Discussion
Our results show that the chemosensation of IJs toward and away from insect-induced carrot root volatile compounds (Weissteiner and Schütz 2006; Weissteiner 2010) varied depending on the EPN species, volatile compound, concentration of volatile compound, time of exposure, and interaction between EPN species and time of exposure. Our results indicate that all tested EPN species exhibited very low chemotaxis to volatiles irrespective of their foraging strategy. The highest value of CI −0.23 ± 0.03) was in our investigation reported when the IJs of S. feltiae were exposed to Terpinolene. In several related studies (O’Halloran and Burnell 2003; Hallem et al. 2011; Dillman et al. 2012), authors report many CIs above 0.5 for H. bacteriophora and S. carpocapsae, species which were included also in our investigation. In a related research (Laznik and Trdan 2013), authors used the same strain of EPN species as in our current investigation. The values of CI of EPNs toward β-caryophyllene, linalool, and α-caryophyllene were similar low as in our current investigation toward other VOCs. One possible explanation of low chemotaxis to volatiles can be a strain-specific characteristic of EPNs. Laznik and Trdan (2013) suggested that the response to different volatile cues is more a strain-specific characteristic than a different host-searching strategy. Since the strains used in other related studies (O’Halloran and Burnell 2003; Hallem et al. 2011; Dillman et al. 2012) were different to ours, we could confirm our previous results. The second possible explanation of low chemotaxis to volatiles in our investigation in comparison with related studies can be explained with the use of different VOCs as were used in our current study. Anyway, our results demonstrate that EPNs have evolved specialized olfactory system that is able to distinguish herbivore-induced chemicals from chemicals that are typical for healthy carrot roots. Similar conclusions were also reported in the recent research from Ali et al. (2011), in which the cruiser Heterorhabditis indica (Lewis 2002), the ambusher S. carpocapsae (Lewis 2002), and the intermediate S. diaprepesi and S. riobrave (Lewis 2002) were all attracted to root weevil Diaprepes abbreviatus-damaged roots of the Swingle rootstock. Our current results suggest that responsiveness to different volatile cues is a species-specific characteristic.
In our investigation, Terpinolene was a repellent for EPN species classified in all three foraging groups. However, such values of CI were reported with the EPN species S. feltiae, S. kraussei, and S. carpocapsae only when pure concentration of VOC was used. Off course, such high concentration of VOCs is unrealistic (Köllner et al. 2004) and probably toxic to the EPNs. With lower concentration of VOCs (0.03 ppm), which is the average concentration of VOCs found in soil, 10 cm away from the root system (Weissteiner et al. 2012), only nematode species H. bacteriophora responded to Terpinolene (as a weak repellent). Terpinolene is a VOC, which is released by undamaged carrot roots (Weissteiner 2010). Our results suggest that EPN are able to distinguish herbivore-induced chemicals from chemicals that are typical for healthy roots.
In our investigation, two distinct VOCs concentrations were used. A pure concentration, which does not reflect a concentration found near plant roots (Köllner et al. 2004), had a bigger influence on IJ movement than a concentration of 0.03 ppm. However, we are aware that such laboratory studies do not reflect a nematode’s true behavior in nature because of exposure to different conflicting chemical signals (Bais et al. 2006). Kruitbos et al. (2009) suggested that EPNs may be habitat specialists and highlighted the difficulties of studying soil-transmitted parasites in non-soil media.
Plant roots emit an incredible variety of compounds, which are known to affect interactions between plants (Erb et al. 2013; Hiltpold et al. 2013) and other organisms (Bonkowski et al. 2009). The active role plants play in recruiting natural enemies, like belowground herbivores, has been recently demonstrated in a few plant species (Rasmann et al. 2012). EPN host-finding is mediated by both long-range cues that facilitate root zone finding, as well as shorter-range cues, that facilitate host localization within the root zone (Hiltpold et al. 2011; Turlings et al. 2012; Demarta et al. 2014). Recently, Hallem et al. (2011) reported positive chemotaxis of the two EPN species H. bacteriophora and S. carpocapsae to several VOCs such as methyl salicylate, hexanol, heptanol, undecyl acetate, and 4, 5-dimethylthiazole. Interestingly, they showed that several volatiles repelled the nematodes. Dillman et al. (2012) reported that EPN host-seeking behavior is stimulated by a wide range of host-derived odorants. Similar effects of VOCs on the behavior of EPNs were also observed in our investigation. Terpinolene repelled both Steinernema and Heterorhabditis species in our investigation. Weissteiner and Schütz (2006) reported that Terpinolene is a VOC released from the undamaged roots of cultivated carrots. Our results suggest that EPN are able to distinguish herbivore-induced chemicals from chemicals that are typical for healthy roots. Our findings could support the theory of Ali et al. (2011). Ali et al. (2011) suggest that selection of an herbivore-induced signaling response should be directionally stronger toward channeling resources for production of a distress signal only when necessary because a constant release would likely carry a high physiological cost (Heil 2008; van Dam 2009; Degenhardt et al. 2009; Robert et al. 2013). Our conclusion is also supported by the VOC α-pinene (released from undamaged carrot roots) (Weissteiner and Schütz 2006), which was a weak repellent of S. carpocapsae and S. kraussei. The other tested VOCs in our investigation (Bornyl acetate, 2, 4-Di-tert-butylphenol, and 2-Ethyl-hexanol) acted inconsistently (as a weak repellents or weak attractants).
Most VOCs that are involved in belowground tritrophic interactions remain unknown but an increasing effort is being made in this field of research. Understanding more of these complex interactions would not only allow a better understanding of the rhizosphere but could also offer ecologically sound alternatives in pest management of agricultural systems (Hiltpold et al. 2010; Turlings et al. 2012).
Author contribution
ŽL: designed and performed the experiments and wrote the manuscript. ST: analyzed the data. All authors read and approved the manuscript.
References
Ali JG, Alborn HT, Stelinski LL (2010) Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol 36:361–368
Ali JG, Alborn HT, Stelinski LL (2011) Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol 99:26–35
Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9:26–32
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266
Bargmann CI, Horvitz HR (1991) Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7:729–742
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21:109–110
Boemare NE, Akhurst RJ, Mourant RG (1993) DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol 43:249–255
Bonkowski M, Villenave C, Griffiths B (2009) Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321:213–233
Burman M, Pye A (1980) Neoaplectana carpocapsae: movement of nematode populations on thermal gradient. Exp Parasitol 49:258–265
Campbell JF, Lewis EE, Stock SP, Nadler S, Kaya HK (2003) Evolution of host search strategies in entomopathogenic nematodes. J Nematol 35:142–145
De Nardo EAB, Grewal PS (2003) Compatibility of Steinernema feltiae (Nematoda: Steinernematidae) with pesticides and plant growth regulators used in glasshouse plant production. Biocontrol Sci Technol 13:441–448
Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. PNAS 106:13213–13218
Demarta L, Hibbard BE, Bohn MO, Hiltpold I (2014) The role of root architecture in foraging behaviour of entomopathogenic nematodes. J Invertebr Pathol 122:32–39
Dillman AR, Guillermin ML, Lee JH, Kim B, Sternberg PW, Hallem EA (2012) Olfaction shapes host-parasite interactions in parasitic nematodes. PNAS 109:E2324–E2333
Eidt DC, Thurston GS (1995) Physical deterrents to infection by entomopathogenic nematodes in wireworms (Coleoptera: Elateridae) and other soil insects. Can Entomol 127:423–429
Erb M, Huber M, Robert CAM, Ferrieri AP, Machado RAR, Arce CCM (2013) The role of plant primary and secondary metabolites in root-herbivore behavior, nutrition and physiology. In: Johnson SN, Hiltpold I, Turlings TCJ (eds) Advances in insect physiology. Academic Press, Oxford, pp 53–95
Forst S, Clarke D (2002) Bacteria-nematode symbiosis. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 57–77
Grewal PS, Gaugler R, Lewis EE (1993) Host recognition behaviour by entomopathogenic nematodes during contact within insect gut contents. J Parasitol 79:495–503
Hallem EA, Dillman AR, Hong AV, Zhang Y, Yano JM, DeMarco SF, Sternberg PW (2011) A sensory code for host seeking in parasitic nematodes. Curr Biol 21:377–383
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61
Hiltpold I, Baroni M, Toepfer S, Kuhlmann U, Turlings TCJ (2010) Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J Exp Biol 213:2417–2423
Hiltpold I, Erb M, Robert CAM, Turlings TCJ (2011) Systemic root signaling in a belowground, volatile-mediated tritrophic interaction. Plant, Cell Environ 34:1267–1275
Hiltpold I, Bernklau E, Bjostad LB, Alvarez N, Miller-Struttmann NE, Lundgren JG, Hibbard BE (2013) Nature, evolution and characterisation of rhizospheric chemical exudates affecting root herbivores. In: Johnson SN, Hiltpold I, Turlings TCJ (eds) Behaviour and physiology of root herbivores. Academic Press, cambridge, pp 97–157
Ishibashi N, Choi D-R (1991) Biological control of soil pests by mixed application of entomopathogenic and fungivorous nematodes. J Nematol 23:175–181
Jackson TA, Klein MG (2006) Scarabs as pests: a continuing problem. Coleopt Bull 60:102–119
Johnson SN, Anderson EA, Dawson G, Griffiths DW (2008) Varietal susceptibility of potatoes to wireworm herbivory. Agric For Entomol 10:167–174
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Ann Rev Entomol 38:181–206
Köllner TG, Schnee C, Gershenzon J, Degenhardt J (2004) The sesquiterpene hydrocarbons of maize (Zea mays) form five groups with distinct developmental and organ-specific distributions. Phytochemistry 65:1895–1902
Koppenhöffer AM, Fuzy EM, Crocker R, Gelernter W, Polavarapu S (2004) Pathogenicity of Steinernema scarabaei, Heterorhabditis bacteriophora and S. glaseri to twelve white grub species. Biocontrol Sci Technol 14:87–92
Kruitbos L, Heritage S, Hapca S, Wilson MJ (2009) The influence of habitat quality on the foraging strategies of the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabdits megidis. Parasitology 137:303–309
Kuhar TP, Alvarez JM (2008) Timing of injury and efficacy of soil-applied insecticides against wireworms on potato in Virginia. Crop Prot 27:792–798
Laznik Ž, Trdan S (2013) An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp Parasitol 134:349–355
Laznik Ž, Trdan S (2015) Failure of entomopathogens to control white grubs (Coleoptera: Scarabaeidae). Acta Agric Scand Sect B 65:95–108
Laznik Ž, Tóth T, Lakatos T, Vidrih M, Trdan S (2010) The activity of three new strains of Steinernema feltiae against adults of Sitophilus oryzae under laboratory conditions. J Food Agric Environ 8:132–136
Lewis EE (2002) Behavioural ecology. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 205–223
O’Halloran DM, Burnell AM (2003) An investigation of chemotaxis in the insect parasitic nematode Heterorhabditis bacteriophora. Parasitology 127:375–385
Parker WE, Howard JJ (2001) The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the UK. Agric For Entomol 3:85–98
Parra JRP (1998) Criação de insetos para estudos com patógenos. In: Alves SB (ed) Controle microbiano de insetos. FEALQ, Piracicaba, pp 1015–1038
Peters A, Ehlers R-U (1994) Susceptibility of leather jackets (Tipula paludosa and Tipula oleracea; Tipulidae: Nematocera) to the entomopathogenic nematode Steinernema feltiae. J Invertebr Pathol 63:163–171
Rasmann S, Turlings TCJ (2008) First insights into specificity of belowground tritrophic interactions. Oikos 117:362–369
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737
Rasmann S, Hiltpold I, Ali J (2012) The role of root-produced volatile secondary metabolites in mediating soil interactions. In: Montanaro G, Cichio B (eds) Advances in selected plant physiology aspects. Tech Open Access Publisher, Croatia, pp 269–290
Robert C, Frank D, Leach K, Turlings T, Hibbard B, Erb M (2013) Direct and indirect plant defenses are not suppressed by endosymbionts of a specialist root herbivore. J Chem Ecol 39:507–515
Shapiro-Ilan D, Lewis EE, Campbell JF, Kim-Shapiro DB (2012) Directional movement of entomopathogenic nematodes in response to electrical field: effect of species, magnitude of voltage, and infective juvenile age. J Invertebr Pathol 109:34–40
Turlings TCJ, Hiltpold I, Rasmann S (2012) The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil 359:51–60
Van Dam NM (2009) Belowground herbivory and plant defenses. Ann Rev Ecol Evol Syst 40:373–391
Ward S (1973) Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA 70:817–821
Weissteiner S (2010) The effect of root volatiles on the orientation behavior of cockchafer larvae in the soil. Dissertation thesis. Georg-August-University-Göttingen, Göttingen, p 182
Weissteiner S, Schütz S (2006) Are different volatile pattern influencing host plant choice of belowground living insects? Mitt Dtsch Ges Allg Angew Ent 15:51–55
Weissteiner S, Huetteroth W, Kollmann M, Weißbecker B, Romani R, Schachtner J, Schütz S (2012) Cockchafer larvae smell host root scents in soil. PLoS ONE 7:e45827
Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506
Woodring JL, Kaya HK (1988) Steinernematid and Heterorhabditid nematodes: a handbook of biology and techniques. Agricultural Experimental Station, Arkansas: Southern Cooperative Service Bulletin 331
Acknowledgments
This work was conducted within Horticulture No P4-0013-0481, a program funded by the Slovenian Research Agency. Part of this research was funded within Professional Tasks from the Field of Plant Protection, a program funded by the Ministry of Agriculture, Forestry, and Food of Phytosanitary Administration of the Republic Slovenia. Special thanks are given to Anamarija Jagodič and Anita Klobučar for their technical assistance. We would like to thank Gareth Martin (Becker Underwood) for providing the commercial EPN strains.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Laznik, Ž., Trdan, S. Attraction behaviors of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to synthetic volatiles emitted by insect-damaged carrot roots. J Pest Sci 89, 977–984 (2016). https://doi.org/10.1007/s10340-015-0720-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-015-0720-9