Abstract
Red clover (Trifolium pratense L.) is becoming increasingly important in grassland systems because of its high productivity, protein content and nitrogen-fixing ability, but its use has been constrained by losses due to pests and diseases, and this contributed to the decline of red clover usage allowing white clover (T. repens L.) to become the dominant legume of UK grasslands. One of the major pests of red clover is the clover root weevil (Sitona lepidus Gyllenhal, Coleoptera, Curculionidae) which attacks both the shoots and roots, particularly the N-fixing root nodules, of clover plants. This current work investigates the feeding preferences of S. lepidus with respect to 11 varieties (Pawera, Kuhn, Astra, Norseman, Norseman low, Norseman high, Marcom, Merviot, Milvus, Britta, Sabtoron) and 5 lines (AA30, AA31, AA4493, AA4494 and AA4495) of red clover in order to identify relationships between the variation in attractiveness of different red clover varieties for both adult and larval stages of the weevil. Of those tested Norseman high showed potential resistance, being less favoured by both adults and larvae of the weevil. This may be attributed to the potentially high phyto-oestrogen levels in this variety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Red clover (Trifolium pratense L.) is becoming increasingly important in grassland systems (sheep, beef and dairy) due to its high productivity, protein content and nitrogen (N)-fixing ability which allows lower N fertiliser use and helps to deliver more profitable livestock enterprises. Red clover use has been constrained by the losses due to pests and diseases, and this contributed to the decline of red clover usage in the 1960s (Jones et al. 2003), allowing white clover (T. repens L.) to become the dominant legume of UK grasslands. Whilst there have been a number of studies conducted to identify red clover varieties with high disease (particularly clover rot, Sclerotinia trifoliorum) and nematode (especially stem nematode Ditylenchus dipsaci Kuhn) resistance, little research has been conducted to identify or quantify insect pest resistance.
One of the most important insect pests of clovers is the clover root weevil Sitona lepidus (Gyllenhal. Coleoptera; Curculionidae). S. lepidus is widespread in temperate grasslands in Northern latitudes (Bright 1994; Murray and Clements 1995) and has recently arrived in New Zealand (Barratt et al. 1996) where it has devastated the clover crops. Sitona weevil adults feed on the leaves of the plants and lay their eggs at random. The eggs are washed into the soil by rainfall where they hatch and the first instar larvae feed on the N-fixing root nodules and therefore impair N-fixation (Murray and Clements 1998). As the larvae develop, later instars feed on progressively larger roots. It has been demonstrated that larvae showed a strong preference for root nodules that were fixing N, compared with those that were not (Gerard 2001; Johnson et al. 2005) suggesting that compounds associated with nitrogen fixation may act as location signals and/or be phagostimulatory.
While adult feeding by S. lepidus is known to be highly Trifolium specific (Murray and Clements 1994), Hackell and Gerard (2004) found no difference in nodule preference by first instar S. lepidus larvae between a variety of red clover and white clover. This implies that it is likely that the stimuli that enables the first instar larvae to locate nodules in red and white clover are the same and that larval populations observed in the field are likely to be the outcome of adult distribution, rather than larval behaviour (Hackell and Gerard 2004). Therefore, it might be suggested that S. lepidus damage may be controlled by identifying varieties with high resistance against adult weevils. Murray (1996a, b) demonstrated intraspecific differences in the feeding behaviour of S. lepidus for a range of white clover varieties. Given the wide range of red clover varieties now available from breeding programmes, it should be possible to identify differences in susceptibility to S. lepidus adults. However, a major challenge is to identify differences in the feeding preferences of the larvae below ground. Therefore, this current work investigates the feeding preferences of S. lepidus with respect to 16 varieties of red clover in order to identify relationships between the variation in attractiveness of different red clover varieties for both adult and larval stages of the weevil.
Materials and methods
Plants of red clover of the varieties Pawera, Kuhn, Astra, Norseman, Norseman low, Norseman high, Marcom, Merviot, Milvus, Britta, Sabtoron and the lines AA30, AA31, AA4493, AA4494 and AA4495, were established in potting compost in the greenhouse. Norseman high and Norseman low refer to divergent selections for high and low levels of phyto-oestrogens, particularly formononetin. The plants were allowed to grow, watered twice daily at 6:00 and 18:00 hours and were cut at approximately 3 week intervals. A modified N-free Arnon’s Solution (Hewitt 1966; Murray and Hatch 1994) was applied to the plants after each harvest. The plants used did not have any marked physical differences and none of the varieties/lines had a reputation as being particularly susceptible or resistant to pests or diseases.
Collections of S. lepidus adults were made from the same white clover-rich field at IGER North Wyke, between 22 June 2005 and 19 July 2005. Captured weevils were kept in the refrigerator at 5°C until required and adult weevils were sorted into batches of 10. Each batch was held in an egg collection cage which consisted of a plastic pot (430 ml), with mesh top and bottom. Each pot contained a 15 ml vial of water with 10 petioles of freshly-picked white clover and which was plugged with absorbent cotton wool. This arrangement allows eggs to fall through the mesh for collection. The plant materials were replaced daily. The pots were kept under ambient laboratory conditions and eggs were collected daily and stored on moist filter paper in Petri-dishes in a refrigerator at 4°C until required.
Adult feeding test
Morphologically similar leaves of the same physiological age of each variety of red clover were removed from the plants in the glasshouse at random. Each leaf was placed on moist filter paper in a 90 mm diameter plastic Petri-dish. The adult weevils were differentiated into two groups according to their gender, and two adults, one of each gender, chosen at random were introduced into the Petri-dishes, with five replicate dishes of each variety. The Petri-dishes were placed in a controlled environment cabinet at 15 ± 2°C, with a 16 h light: 8 h dark photoperiod, for 42 h. The dishes were then removed from the cabinet and the area of leaf removed by the weevils determined. This was done by placing the leaf under a transparent grid marked in mm squares and estimating the area of leaf removed by the weevil feeding (Murray 1996a). The C:N ratio of the leaf material was determined using leaf material from the same plants as above, and analysed on an elemental analyser (N1500, Carlo Erba, Italy).
Data from the adult feeding test were normalised using a Box-Cox transformation (λ = 0.4) and analysed using the GenStat Procedure ANOVA (GenStat 8th Edition, Lawes Agricultural Trust). A similarity matrix was formed using the mean feeding values for each variety and the varieties clustered using hierarchical cluster analysis (HCLUSTER). The original data was then assigned to the cluster groups and reanalysed using groups/varieties in the ANOVA treatment structure.
Adult longevity test
Longevity tests were carried out for 50 days, under the same conditions as the feeding tests above. The dishes were inspected daily and any dead weevils were removed whilst uneaten plant materials were removed and fresh leaves were replenished every 2 days to avoid fungal infection and starvation which might induce weevil mortality. The number of dead weevils was recorded daily.
The Kaplan–Meier estimation of survivor function (GenStat procedure KAPLANMEIR) for both male and female weevils was calculated for each variety and data for weevils that survived for >50 days was censored, estimates were also made after clustering the data according to the groups formed for feeding preferences.
Larval movement test
When required the Petri-dishes containing eggs were removed from the refrigerator and kept at 25°C for the eggs to hatch. On the day the weevil eggs hatched, red clover plants were destructively sampled, and soil was removed from the roots by washing. The roots were then pat-dried with a paper towel and used within 1 h of detachment. Larvae were used within 24 h of eclosion. Fifty-six 90 mm diameter plastic Petri-dishes, 48 as treatments and 8 as controls were lined with moist filter paper. The dishes were divided into equal quarters, labelled A, B, C and D, in a clockwise rotation. On the moist filter paper in each dish, 102 ± 1.5 mg of root materials of one variety of red clover including a fixed number of 25 nodules was placed in the centre of position A, except in the control dishes where no root material was added. Ten newly hatched first instar larvae, chosen at random were placed, using a fine paintbrush onto the centre of the opposite quarter at position C. All Petri-dishes were placed in dark environment, and the positions of the larvae were recorded after 10 and 30 min. Three replicates for each red cover variety and a root-free control were established. The data were analysed using general linear models (GenStat procedure GLM).
Results
Adult feeding test
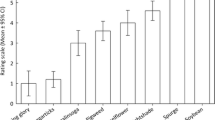
There were no significant differences in the amount of leaf material consumed by the weevils. However, the cluster analysis identified three varietal groups (Fig. 1) with a significant (P < 0.001) difference in the mean consumption between group 1 (32.3 ± 2.95 mm2) and group 3 (5.9 ± 2.42 mm2). There was no significant difference in the C:N ratio of the leaf material taken from the different varieties (mean 9.65 ± 0.305). No mortality of weevils was found during the test period.
Adult longevity test
The estimated mean survival time for 50% of the female population was 15, 14 and 12 days for groups 1,2 and 3, respectively, and 14, 12 and 6 days for the males of the three groups. When the Kaplan–Meier curves (Fig. 2) were compared using the Wilcoxon test there were no significant differences between the curves for individual varieties. However, when the data were clustered using the groups in Fig. 3, there were significant differences between group 3 and groups 1 and 2 for both females (P = 0.031) and males (P = 0.003). There was no significant difference between males and females in the overall survival time, although 20% of males lived longer than 50 days, compared with 8% of females.
Larval movement test
Larvae were observed after 10 and 30 min exposure to root material from the different red clover varieties. There were no significant differences in the numbers of larvae that moved to segments which had no root material present (segments B and D). However, there were significant differences in the numbers of larvae that moved towards the root material of the different varieties (Fig. 3). After 10 min (Fig. 3a), the larvae showed significant (P = 0.022) responses to the presence of root material of most varieties when compared with the control treatment, exceptions being Kuhn, Britta and Milvus. However after 30 min (Fig. 3b) all except Britta showed a significant increase in the numbers of larvae moving to section A (P = 0.001) compared with the control.
Figure 4 shows the relationship between adult feeding (amount eaten of each variety) and larval attraction (percentage of larvae moving to segment A after 30 min). There was a large group of varieties where there was a similar level of adult feeding and larval attraction, however, there were four outliers: Britta where there was a high degree of adult feeding, but low larval attraction; Norseman high where there was low adult and larval feeding and AA4494 and Norseman where there was moderate adult feeding, but a high degree of larval attraction.
Discussion
Red clover has been shown to be vulnerable to damage by S. lepidus adults (Murray 1996b; Gerard et al. 2005). The results from this study indicate differences in adult feeding preferences between varieties of red clover under test. The C:N ratio of the foliage can be an indicator of nutritional quality of the plant. In this experiment there were no significant differences in the ratio between the varieties, and therefore it may be assumed that differences seen result from other factors, such as secondary compounds. The challenge is to determine the mechanism of resistance in these varieties. Hackell and Gerard (2004) demonstrated that S. lepidus larvae showed a distinct preference for active nodules and suggested that volatiles associated with N-fixation may stimulate the attraction. Formononetin is an important signalling molecule in the clover/Rhizobium symbiosis and is therefore necessary for good plant performance. Cook et al. (1995) suggested that formononetin acted as a defensive against infestation by the stem nematode D. dipsaci, and has been implicated in deterring adult S. lepidus feeding (Gerard et al. 2005).
The varieties can be separated into three groups on the basis of their feeding preference (Fig. 2) with the third group consisting solely of the variety Norseman high. Interestingly, the Norseman and Norseman low lines are also in different groups. It seems possible that divergent selection for altered level of phyto-oestrogens has also, directly or indirectly, led to differences in attractiveness to S. lepidus. This confirms the work of Gerard et al. (2005) who used similar high, low and parent Pawera selections for phyto-oestogens. Although the formononetin content of the plants was not assessed in the present study, previous work (Gosden et al. 1984) showed concentrations of 0.12, 0.60 and 1.27% in the low, unselected and high lines of Norseman, respectively.
In the present study, the Norseman high tended to have low attractiveness for the larvae, this appears to be contra indicatory to the work of Johnson et al. (2005) who showed formononetin to be attractive to larvae when presented as a single compound. The reasons for this are not clear, and there may be interaction with other attractance cues. It has been postulated that it is the Rhizobia rather than the plant that determines the larval feeding preference (Hackell and Gerard 2004). However, the results of the present study indicate differences in attraction between varieties of red clover which would only be expected if it is the plant that was conferring the preference. Compounds such as amino acids, may have a role in determining feeding preference. For example, Havlíčková (1980) showed that the amino acid tyrosine inhibited adult S. lineatus feeding on peas, but that simple sugars increased feeding. Barratt and Byers (1992) suggested phyto-alexins along with other secondary chemicals may impart resistance. It has been also suggested that larvae are attracted to nodules because of the concentration of amino acids, which provide a high quality food source, found in them (Hackell and Gerard 2004).
Although there are a plethora of studies that investigate feeding preferences in insect/plant relationships, there are fewer studies on the potential preferences of root feeding larvae. This is mainly due to difficulties in visualising the interaction in the soil. Recent advances in technologies, for example X-Ray Tomography (Johnson et al. 2004), have enabled us to determine the movement of insects in intact soil cores, However, such techniques are not cost effective for rapid screening of a large number of samples. Therefore, in this study we used a simple Petri-dish arena bioassay. This allowed us to quickly observe host plant sensing by the larvae and their relative movement towards potential hosts. We recorded little focussed movement of the larvae in the plantless control arenas, even after 30 min. The position of the larvae after 10 min gives a good indication of attractance, as this more immediate measurement and reduces the number of chance encounters that may occur as time advances and reduces the possible desensitisation of the larvae which may occur if the arena becomes flooded by volatiles given off by the plant. In the present study, even after 30 min, there was significantly less attraction of the larvae to Norseman high and Britta which may suggest that there may be an actual deterrent mechanism.
The results from the feeding groups follow through in the study of survival times of adults fed a single variety with the Norseman high having a significantly shorter survival time than adults feeding on the other two preference groups. These survival times compare favourably with an earlier study (Murray 1996b) where adults feeding in on a single variety of red clover had a mean LT50 time of 26 days. Clearly, this shows the importance of considering genetic variation when investigating insect feeding and attraction or plant resistance to insect feeding.
Overall, this study identifies possible targets for breeding plant resistance in red clover; Fig. 4 shows that there is generally a strong relationship between adult and larval feeding. There are, however, a number of significant observations; Norseman high has the best overall potential with low levels of both adult feeding and larval attraction and may be expected to allow good establishment and survival of the plant. Alternatively, Britta shows low attraction to larval feeding, but may be susceptible to adult feeding.
These experiments assess the relative resistance of different varieties of red clover to insect damage by comparison of the consumption of the leaves, and attraction of the larvae to roots in no-choice tests. This may indicate that the variety which is eaten the least is the most resistant; conversely, it may indicate that the variety meets the nutritional requirements of the insect more effectively (Murray 1996a), although both mechanisms reduce the level of damage to the plant. Other forms of resistance may be of greater importance in the field, for example, tolerance of the plant to initial weevil attack, or antibiosis, particularly impacts on oviposition. Whilst it is not possible to extrapolate the findings of this study to the field, it can be seen that there is a basis for more detailed evaluation of resistance in these plants and therefore presents opportunities for plant breeders.
References
Barratt BIP, Barker GM, Addison PJ (1996) Sitona lepidus Gyllenhal (Coleoptera: Curculioniodae), a potential clover pest to New Zealand. N Z Entomol 19:22–30
Barratt BIP, Byers RA (1992) Legume seedling preferences of adult Sitona hispidulus (F.) (Coleoptera: Curculionidae). Environ Entomol 21:103–106
Bright DE (1994) Revision of the Genus Sitona (Coleoptera, Curculionidae) of North-America. Ann Entomol Soc Am 87:277–306
Cook R, Tiller SA, Mizen KA, Edwards R (1995) Isoflavonoid metabolism in resistant and susceptible cultivars of white clover infected with the stem nematode Ditylenchus dipsaci. J Plant Physiol 146:348–354
Gerard PJ (2001) Dependence of Sitona lepidus (Coleoptera: Curculionidae) larvae on abundance of white clover Rhizobium nodules. Bull Ent Res 91:149–152
Gerard PJ, Crush DL, Hackell DL (2005) Interaction between Sitona lepidus and red clover lines selected for formononetin content. Ann Appl Biol 147:173–181
Gosden AF, Davies WE, Hughes L, Jones R (1984) Breeding for reduced formononetin in red clover. In: Thompson DJ (ed) Forage legumes. BGS Occasional Symposium 16, Burchett’s Green, Maidenhead, UK, 12–23 February, pp166–167
Hackell DL, Gerard PJ (2004) Nodule preference by first instar clover root weevil. N Z Plant Prot 57:319–322
Havlíčková H (1980) Causes of different feeding rates of pea leaf weevil Sitona lineatus on three pea cultivars. Entomol Exp Appl 27:287–292
Hewitt E J (1966) Sand and water culture methods used in the study of plant nutrition. Technical Communication. Commonwealth Agricultural Bureau of Horticultural and Plantation Crops: 22
Johnson SN, Gregory PJ, Greenham JR, Zhang XX, Murray PJ (2005) Attractive properties of an isoflavonoid found in white clover root nodules on the clover root weevil. J Chem Ecol 31:2223–2229
Johnson SN, Read DB, Gregory PJ (2004) Tracking larval insect movement within soil using high resolution X-ray microtomography. Ecol Entomol 29:117–122
Jones R, Abberton MT, Weller R (2003) Enhancing the Role of Red Clover for sustainable UK Agriculture. IGER Innov 7:36–39
Murray PJ (1996a) Evaluation of a range of varieties of white clover for resistance to feeding by weevils of the genus Sitona. Plant Var Seeds 9:9–14
Murray PJ (1996b) Influence of food source on feeding, longevity and fecundity of Sitona flavescens, a major pest of white clover in the UK. In: Frame J (ed) Recent research and development on white clover in Europe. Saku Jogeva Taru, Estonia, 28–31 August 1995, pp 118–121
Murray PJ, Clements RO (1998) Transfer of nitrogen between clover and wheat: Effect of root herbivory. Eur J Soil Biol 34:25–30
Murray PJ, Clements RO (1995) Distribution and abundance of three species of Sitona (Coleoptera: Curculionidae) in grassland in England. Ann Appl Biol 127:229–237
Murray PJ, Clements RO (1994) Investigations of the host feeding Preferences of Sitona weevils found commonly on white clover (Trifolium repens) in the UK. Entomol Exp Appl 71:73–79
Murray PJ, Hatch DJ (1994) Sitona weevils (Coleoptera, Curculionidae) as agents for rapid transfer of nitrogen from white clover (Trifolium repens L) to perennial ryegrass (Lolium perenne L). Ann Appl Biol 125:29–33
Acknowledgments
IGER is supported by the UK Biotechnology and Biological Sciences Research Council. The authors would like to thank Mr M. S. Dhanoa for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jürgen Gross.
Rights and permissions
About this article
Cite this article
Murray, P.J., Cheung, A.K.M. & Abberton, M.T. Intraspecific variation in Trifolium pratense: impact on feeding and host location by Sitona lepidus (Coleoptera, Curculionidae). J Pest Sci 80, 51–57 (2007). https://doi.org/10.1007/s10340-006-0153-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-006-0153-6