Abstract
Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae) is regarded as a pest with a large number of hosts, including crops and weeds. The performance of this whitefly on seven weeds was evaluated in order to identify the most suitable host. The following weeds that are very common in intense agricultural areas in the state of São Paulo, Brazil, were selected for this study: spurge (Euphorbia heterophylla), beggarticks (Bidens pilosa), red tasselflower (Emilia sonchifolia), small-flower galinsoga (Galinsoga parviflora), pigweed (Amaranthus viridis), black nightshade (Solanum americanum), and morning glory (Ipomoea sp.). In free-choice tests, adult preference and oviposition were greatest on spurge. In contrast, morning glory was the least attractive and least oviposited plant. In assays carried out for egg–adult development, egg viability was greater than 87% over all weeds, whereas nymph viability ranged from 74 to 97%. The developmental period from egg to adult ranged from 26.7 to 49.1 days among the hosts under study. The lowest nymph density rate was observed for beggarticks and morning glory. Cluster analysis resulted in a single group formed by spurge, indicating its superiority as a host for B. tabaci biotype B. Even though the parameters evaluated indicate that spurge is the most suitable host among the weeds, all the others allow the reproduction of B. tabaci biotype B. For this reason, they should be observed during cropping and the intercrop period in areas infested by this whitefly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Latin America, Bemisia tabaci (Genn.) is recognized as an important pest and a phytovirus vector (Hilje & Morales 2008, Morales 2011). This species is a complex of biotypes that are morphologically indistinct. These biotypes show differences in phytovirus transmission, in adaptation to hosts, and in their ability to induce physiological anomalies in plant species of economic importance (Brown et al 1995, Hilje & Morales 2008). Twenty-four different populations, of the at least 41 that have been reported, have been classified into biotypes (Perring 2001). However, according to De Barro et al (2011), B. tabaci is a complex of at least 24 morphologically indistinguishable species that can be clearly defined by comparison to consensus sequences and delimited by 3.5% mtCOI sequence pairwise genetic distance divergence.
Bemisia tabaci biotype B is one of the most harmful biotypes to agriculture worldwide. It was introduced to Brazil in the 1990s (Lourenção & Nagai 1994). Direct damages from this biotype are related to the removal of phloem sap, such as changes in vegetative and reproductive plant development. There are also indirect damages, such as the occurrence of sooty mold and phytovirus transmission (Inbar & Gerling 2008, Morales 2011).
Cropping practices are used as preventive measures, and they perform an important role in whitefly management in the agricultural system (Hilje et al 2001). Among the cropping practices for whitefly management, crop rotation with non-host species, residue management, crop-free periods, and weed management have been proven to be effective when used in large areas (Hilje & Morales 2008). There is a wide range of weed species in tropical and subtropical areas, and these species may serve as hosts for many pests and diseases (Arnaud et al 2007). Bemisia tabaci biotype B is a pest with many hosts, including cultivated plants and weeds (Mound & Halsey 1978, Berry et al 2004, Brown 2010).
In the face of the large variety of weeds present in intensively farmed areas of the state of São Paulo, Brazil, the aim of this study was to evaluate the performance of this whitefly on the seven most abundant weed species in these areas, identifying those most suitable for this insect. This knowledge will contribute to crop management, indicating to the farmer which weeds must be monitored and eliminated in crop areas to control B. tabaci biotype B.
Material and Methods
This research was performed at the Experimental Center, Instituto Agronômico (IAC), in Campinas, São Paulo, Brazil, in 2008 and 2009. Two different greenhouses were used: one for obtaining the whitefly colony and another for the experiments. Data on the temperatures inside the greenhouses during the experiments were obtained from a maximum minimum glass thermometer under shade.
Weed selection
First, a survey was carried out to determine the main dicotyledonous weed species found in crop areas in the state of São Paulo. The species selected were spurge (Euphorbia heterophylla), beggarticks (Bidens pilosa), red tasselflower (Emilia sonchifolia), small-flower galinsoga (Galinsoga parviflora), pigweed (Amaranthus viridis), black nightshade (Solanum americanum), and morning glory (Ipomoea sp.). Plants of these species were obtained from seeds collected in the IAC and surrounding areas.
Bemisia tabaci biotype B rearing
The colony was established by collecting whitefly adults from a tomato crop in Paulínia, São Paulo, and transferring them to soybean (Glycine max) and kale (Brassica oleracea var. acephala) plants maintained under greenhouse conditions. Soybean and kale plants were used for rearing the whitefly since they are suitable hosts that are easy to obtain and maintain in greenhouses. After a few generations, adults were sent to Dr. Judith K. Brown, University of Arizona, USA, who identified them as B. tabaci biotype B. Recently, adults of the colony were molecularly characterized and confirmed to belong to biotype B (Valle et al 2012b).
Attractiveness to adults and oviposition preference in a free-choice test
The plants were grown in plastic pots (1.8 L) containing an earth-organic compost mixture and Tropstrato® in a 3:1 ratio, with the pH corrected to 6. Five pots were used for each weed species and one plant per pot was kept after thinning. For artificial infestation, pots of tomato plants were placed in the greenhouse containing the whitefly colony for 4 h and subsequently transferred to the greenhouse of the experiment. Each pot of tomato plants, with nearly 300 whitefly adults per plant, was placed in the middle and at equal distance from four pots with the weeds. Adults were counted at 24, 48, and 72 h after infestation on the abaxial surface of two leaves from the upper third of each plant. Counting was performed with the aid of a mirror to avoid disturbing the adults during sampling. On the sixth day after plant infestation, the leaves used in the counting were detached and placed in plastic bags in the refrigerator for further evaluation of the oviposition. A stereoscope with 16× magnification was used to count the eggs found on the abaxial side of the leaf. To obtain the number of eggs per area (cm2), the leaves under evaluation were reproduced on tracing paper and passed through a LI-COR (LI-3100A) leaf area meter.
A randomized block experimental design was used, composed of eight treatments (seven weed species and soybean) with five replicates, for a total of 40 experimental units. Each experimental unit consisted of a pot with a plant. The mean values from counting one pair of leaves per plant provided the values for analysis. The selections made by the adult B. tabaci biotype B of simultaneously offered host plants were compared based on the mean proportions of the adult whitefly selecting one type of host plant over the observation period from 24 to 72 h and the overall mean for the period, as well as the mean number of eggs laid (eggs/cm2). The data were tested under the null hypothesis that no selection behavior implies the expectation of an equal 50:50 ratio. These analyses were performed using a non-parametric procedure, which consisted of the Proc FREQ of SAS (SAS Institute 2002) and interpretation through the χ 2 test at a 5% significance level.
Oviposition and nymph density in a no-choice test
This experiment was carried out with all species of plants used in the adult and oviposition non-preference test. The no-choice test is necessary to complement the free-choice procedures to confirm resistance (Smith et al 1994). When the seven species of weeds and the soybean had two pairs of completely developed leaves, infestation was carried out. Cloth cages made of voile fastened on cylindrical iron frameworks (60 cm height × 35 cm Ø) were used for whitefly confinement on plants. Two hundred adults captured from the colony were placed in each cage, which was sealed at mid-height with cotton string.
A randomized block experimental design was used with eight treatments and five replicates, for a total of 40 experimental units. Each experimental unit consisted of a pot with a plant. The value for each plant was the mean value from the counting performed on a pair of leaves. The analysis was performed using a non-parametric procedure, which consisted of the Proc FREQ of SAS (SAS Institute 2002) and interpretation through the chi-squared test at a 5% significance level.
Sixteen days after starting the experiment, nymph density was estimated through leaf colonization by nymphs. Fully developed leaves were removed from the upper third of the plants and evaluated using a technique based on a visual rating scale that ranged from 0 to 6 in which 0 = leaf with no infestation, 1 = leaf with few eggs and nymphs, and successively up to 6 = leaves totally colonized by eggs and nymphs. This was adapted from a scale proposed by Coelho et al (2009). The final score of each experimental plot was the mean value of two leaves per plant. As the data did not present normality, nymph density was analyzed by the non-parametric test of Friedman, and the mean values were compared through multiple comparisons (p < 0.05) using the SAS statistical program.
Egg-adult development on plants in the vegetative stage
In the first experiment, which was carried out in the greenhouse in the second half of August 2009, pigweed, black nightshade, and red tasselflower were compared with soybean. When the second leaf of the plants was completely expanded, the pots were transferred to the whitefly colony greenhouse and left there for a 4-h oviposition period to minimize the age difference among the eggs. The potted plants were then taken out of the oviposition greenhouse, and the adults were removed. Then, one area per leaf with 20 eggs and two leaves per plant were marked for a total of 40 eggs per plant for purposes of monitoring. Five plants per weed species were used, for a total of 200 eggs per weed species. After demarcating the areas with an OHP marker (fine tip, 1 mm), the plants were arranged in a greenhouse in a randomized block design. Insect development was observed daily with the aid of a stereoscope. The length and viability of the incubation period and of the nymph stage were determined. After that, in the second half of September 2009, a second experiment was set up and carried out in the same way with morning glory, beggarticks, small-flower galinsoga, and spurge, and these were also compared to soybean.
The data on the incubation and nymph periods were subjected to analysis of variance with no transformation. The viability data were transformed into arc sen √x/100. The mean values were compared using the Tukey’s test (p < 0.05).
Egg–adult development on plants in the reproductive stage
To evaluate the suitability of weeds in the reproductive stage as hosts for B. tabaci biotype B, a third experiment was set up in the second half of April 2009. The experiment was set up, conducted, and evaluated in a way similar to the experiments in the vegetative stage, this time with plants in full flower. The data on the incubation and nymph periods were subjected to analysis of variance with no transformation, and the viability data were transformed into arc sen √x/100. The mean values were compared using the Tukey’s test (p < 0.05).
UPGMA cluster analysis
Cluster analysis was carried out for all the variables in the experiment, except for the biological variables of the B. tabaci biotype B with plants in the vegetative stage. The division of this experiment into two phases made it impossible to use these data for analysis.
Results and Discussion
Attractiveness to adults and oviposition preference in a free-choice test
Spurge was the most attractive plant to B. tabaci biotype B, with a mean of 12.2 adults/cm2, differing from all the other weeds (Table 1). This corroborates with Gachoka et al (2005), who evaluated the attraction of B. tabaci adults belonging to two biotypes, the cassava biotype and okra biotype, to five different weed plant species in Ghana. According to these authors, spurge hosted the greatest number of adult whiteflies. In relation to the less attractive plants, similar results were obtained by Calvitti & Remotti (1998) in Italy, who also noted the low attractiveness of pigweed to B. tabaci.
Regarding oviposition, spurge (171.1 eggs/cm2) and black nightshade (65.2 eggs/cm2) were the weeds that presented the highest mean values, in contrast to beggarticks (22.5 eggs/cm2), morning glory (20.1 eggs/cm2), and small-flower galinsoga (20.0 eggs/cm2), which were the least oviposited. Differences in the intensity of oviposition of B. tabaci biotype B are common in resistance studies in cultivated plants, such as in soybean (McAuslane 1996, Valle et al 2012a), cotton (Torres et al 2007), tomato (Oriani et al 2011), common bean (Oriani et al 2008), melon (Coelho et al 2009), potato (Silva et al 2008), and pumpkin (Alves et al 2005), among others. Therefore, from the present results, a discriminating preference in oviposition of B. tabaci biotype B among the weeds was also noted.
Oviposition and nymph density in a no-choice test
A strong preference of B. tabaci biotype B for oviposition on spurge (Table 1) was corroborated in this experiment, with spurge having the highest mean value of oviposition (59.0 eggs/cm2). Morning glory also confirmed the performance observed in the free-choice experiment; this weed showed the lowest number of eggs (6.7 eggs/cm2), differing from all the other plants.
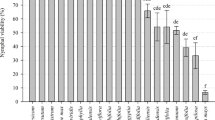
As for nymph density, the lowest numbers were observed in beggarticks and morning glory, with mean scores of 1.2 and 1.0, respectively (Fig 1). These two species had the lowest adult attractiveness, the lowest oviposition in both free- and no-choice tests, and the lowest viability of eggs and nymphs, and were considered the least suitable for this whitefly. In contrast, soybean and spurge showed mean values of 5.8 and 5.6, respectively, which, in light of the other tests carried out, proved to be good hosts for the insect.
Rating scale (scores varying from 0 (no infestation) to 6 (leaf completely infested by eggs and nymphs), according to Coelho et al (2009)) determined for densities of nymphs of Bemisia tabaci biotype B infesting seven weeds as compared to soybean in greenhouse (n = 5) by using a visual rating technique.
The data obtained in the present study corroborate the results from two other studies. Kiill et al (1998) observed a high level of infestation of Bemisia sp. in spurge, characterized by having over 20% of the leaf area infested by eggs, nymphs, and adults. Villas Bôas et al (2003) also noted high infestation of adults and nymphs in spurge when evaluating weed species exposed to B. tabaci biotype B.
Egg–adult development on plants in the vegetative stage
In the first experiment, the incubation period was not affected by the plants on which the eggs were placed, presenting mean values ranging from 13.3 to 13.7 days for soybean, black nightshade, red tasselflower, and pigweed (Table 2). In the egg stage, the insect absorbs water and possibly solutes through the pedicel (Walker et al 2010), and, for this reason, great changes are not expected in the duration of this stage due to the effects of the plant. However, temperature has a significant effect on the time of development in all stages of B. tabaci (Butler-Junior et al 1983). Thus, the long incubation period in this study, when compared to data from experiments in which temperature control was used in ranges more favorable to the insect (25–30°C), may have been due to the time of year in which this experiment was carried out, that is, in lower temperature months. In this experiment, during the egg stage, the mean temperature was 20.8°C (with range of 12.8 to 28.4°C), which was lower than the ideal temperature for the insect. Albergaria & Cividanes (2002) observed an 11.9-day incubation period of B. tabaci biotype B at 20°C, close to the result found in this research.
The viability of the eggs remained above 94% for all treatments except pigweed, which had a mean value of 87.5% (Table 2). Nava-Camberos et al (2001) also found viabilities over 90% for the egg stage in many cultivated plants. Similarly, Campos et al (2009) found values from 97 to 100% when evaluating the biological parameters of this insect in cotton genotypes.
Bemisia tabaci biotype B had a shorter nymph stage when reared on black nightshade (18.6 days), red tasselflower (19.0 days), and soybean (20.0 days) than on pigweed (22.2 days). The shorter duration of the nymph stage indicates better suitability of these three weeds for the whitefly since the presence of resistance type antibiosis in plants can prolong development periods in the immature stages (Smith 1989).
Considering the egg–adult cycle, the differences observed for the duration of the nymph stage were consistent, showing that both black nightshade (32.0 days) and red tasselflower (32.5 days) led to development times similar to soybean (33.3 days), which is considered an excellent host for the insect (Valle et al 2012a). The cycle in pigweed, however, was the longest, reaching 35.7 days, and it differed from all the others.
Concerning nymph viability (Table 2), the greatest mean value was induced by soybean (95.0%), while pigweed induced the lowest rate (75.0%). Similar to the egg viability, Gachoka et al (2005) found nymph viabilities below 20% for the weed plants tested. Nymph viability on cultivated plants in this study was very similar to those in studies by Coelho et al (2009), in which adult emergence rates ranged from 68.2 to 90.9%. In contrast to these results, Mizuno & Villas Bôas (1997) obtained a nymph viability percentage of 50% on cabbage and 47.5% on tomato at 25°C.
Differences in the egg stage were found in the second experiment as the eggs deposited on soybean were the first to originate nymphs (8.2 days), whereas the ones placed on morning glory took 9.1 days (Table 2). When comparing the incubation periods of this experiment with those in the first experiment, there was a difference in mean time, which was 13.5 days in the first study and 8.6 in this study. The differences in the time for nymphs to hatch are due to the increase in temperature in this second experiment, in which the mean temperature was 21.15°C, with a range of 15.1 to 30.0°C in the egg stage and 12.4 to 30.6°C in the nymph stage. There was no difference among the treatments for egg viability (Table 2), which ranged from 90.5% (beggarticks) to 97.5% (soybean), showing values that are similar to the ones obtained in the first experiment.
The nymph stage differed among all treatments, with the longest duration observed in beggarticks (26.2 days) and the shortest in spurge (18.3 days). These results suggest that when B. tabaci biotype B colonizes spurge in the field, more generations occur in a certain period of time than for the other weeds tested. Nymph viability ranged from 75.5% for morning glory to 93% for soybean, with this leguminous plant differing from spurge (91.5%) and beggarticks (89.5%).
Considering the egg–adult cycle, the tendencies observed in the nymph stage were the same, such that spurge showed the fastest development (26.7 days) and beggarticks showed the slowest (35 days), differing from the other weeds tested.
Egg–adult development on plants at the reproductive stage
The incubation period (Table 3) was not affected by the plants evaluated. In this period, the mean temperature was 20.8°C, with a range of 12.8 to 28.4°C. The mean values, which did not differ among themselves, varied from 12.3 days in black nightshade, morning glory, and spurge to 13.0 days in pigweed, and these values are very close to the range of 13.3 to 13.7 days obtained in the first experiment with plants in the vegetative stage (Table 2). When examining the mean viability of eggs, it was noted that soybean and red tasselflower induced 100% viability, differing from pigweed with 92%. As for the other treatments, the viabilities reached 99% (beggarticks), 98% (spurge and small-flower galinsoga), 97.5% (black nightshade), and 96.5% (morning glory), which are within the values normally found for this stage of the insect and similar to studies by Albergaria & Cividanes (2002), who found egg viability ranging from 58.6 to 97.7% in soybean.
As for the duration of the nymph period, in which the main temperature was 20.2°C (11.6–29.2°C), mean values from 24.0 to 26.2 days were found for black nightshade, morning glory, spurge, soybean, and red tasselflower, the plants that promoted the fastest development in this stage, and 30.7 and 36.1 days for beggarticks and pigweed, respectively, the weeds that induced the slowest development. Thus, the last two were the least suitable to the insect. Smith (1989) asserts that when a plant provides unsuitable nutrition to an insect, the insect’s development may be harmed, prolonging its life cycle and/or interfering in the viability of one or more life cycle stages. This nutritional inadequacy may have occurred with nymphs that were fed with pigweed and beggarticks, in which a longer nymph stage was observed.
Nymph viability also differed among plants; pigweed and beggarticks (74%) and morning glory (80.5%) had the lowest, while the highest were obtained in soybean and spurge (97%), red tasselflower (93.5%), and small-flower galinsoga (92%), indicating that they are the most suitable plants for nymph development.
For plants in the vegetative stage, nymphs presented viabilities similar to the nymphs reared on plants in the reproductive stage, since pigweed, morning glory, and beggarticks presented the lowest viabilities for both. Similar to the values obtained in the reproductive stage, it was noted that spurge and soybean in the vegetative stage were the most suitable hosts for the development of the insect. Thus, it can be inferred that in both the vegetative and the reproductive stages, these weeds provide similar conditions for B. tabaci biotype B development.
The egg–adult period differed among treatments, ranging from 36.3 (black nightshade) to 49.1 (pigweed). Pigweed and beggarticks thus prolonged the cycle of the insect, whereas the lowest values were found for red tasselflower, soybean, spurge, morning glory, and black nightshade, which induced faster development.
UPGMA clustering analysis
UPGMA analysis yielded three distinct groups, as shown in the dendrogram (Fig 2). The outermost distinct group is formed by spurge, which is different from all of the others. This confirms that this weed is the best host for B. tabaci biotype B. The second group is formed by black nightshade, red tasselflower, and soybean, plants considered to be intermediate hosts for the insect. The third group, represented by small-flower galinsoga, beggarticks, pigweed, and morning glory, was considered to be the group least preferred by this whitefly.
Hierarchical classification of weeds as hosts of Bemisia tabaci biotype B by Euclidean distance calculated from the variables “adult attractiveness (adults/cm2),” “egg–adult development,” “eggs and nymphal viability on plants at reproductive stage,” “free-choice and no-choice oviposition experiments,” and “nymphal density,” by UPGMA clustering.
The families with the greatest number of host species of B. tabaci are Fabaceae, Compositae, Malvaceae, Solanaceae, and Euphorbiaceae (Mound & Halsey 1978). In the last family, these authors listed 24 hosts (most of the Euphorbia genus) for this species of whitefly, including spurge and Euphorbia pulcherrima (Willd.), commonly known as poinsettia. These two Euphorbiaceae are generally used in rearing this insect for experiments in IAC due to their fast egg–adult development and the high viability of the immature stages. Soybean (second group) belongs to Fabaceae, a family that has the greatest number of known host plants for B. tabaci, 56 species (Mound & Halsey 1978). The second group also includes red tasselflower, from Compositae, with 33 known host species, and black nightshade, belonging to Solanaceae, with 27 known host species (Mound & Halsey 1978).
The least suitable plants for B. tabaci biotype B were classified into the third group: small-flower galinsoga and beggarticks, from Compositae; pigweed, from Amaranthaceae; and morning glory, from Convolvulaceae. Few species are listed as hosts of B. tabaci in Amaranthaceae; Mound & Halsey (1978) cite seven species, including two weeds (Amaranthus retroflexus and Gomphrena globosa), but neither with economic impact as weeds.
Among the weeds evaluated, spurge was found to be the most suitable host for B. tabaci biotype B. However, all of the weeds evaluated allowed insect reproduction, thus showing an ability to maintain B. tabaci biotype B populations under field conditions. This should be considered with regard to the cropping time or during the intercrop period in areas where there are high populations of this whitefly and susceptible crops.
References
Albergaria NMMS, Cividanes FJ (2002) Exigências térmicas de Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotrop Entomol 31:359–363
Alves AC, Lourenção AL, Melo AMT (2005) Resistência de genótipos de aboboreira a Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotrop Entomol 34:973–979
Arnaud LSEP, Santos CDG, Lima JAA, Feitosa FAA (2007) Predominância de begomovírus em tomateiros na região produtora da Ibiapaba, Ceará, e sua detecção natural em plantas daninhas. Fitopatol Bras 32:241–246
Berry SD, Fondong VN, Rey C, Rogan D, Fauquet CM, Brown JK (2004) Molecular evidence for five distinct Bemisia tabaci (Homoptera: Aleyrodidae) geographic haplotypes associated with cassava plants in sub-Saharan Africa. Ann Entomol Soc Am 97:852–859
Brown JK (2010) Phylogenetic biology of the Bemisia tabaci sibling species group. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, Dordrecht, pp 31–67
Brown JK, Frohlich DR, Rosell RC (1995) The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Ann Rev Entomol 40:511–534
Butler-Junior GD, Henneberry TJ, Clayton TE (1983) Bemisia tabaci (Homoptera: Aleyrodidae): development, oviposition and longevity in relation to temperature. Ann Entomol Soc Am 76:310–313
Calvitti M, Remotti PC (1998) Host preference and performance of Bemisia argentifolii (Homoptera: Aleyrodidae) on weeds in Central Italy. Environ Entomol 27:1350–1356
Campos ZR, Boiça-Junior AL, Lourenção AL, Campos AR (2009) Parâmetros biológicos de Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae) em genótipos de algodoeiro. Bragantia 68:1003–1007
Coelho SAMP, Lourenção AL, Melo AMT, Schammass EA (2009) Resistência de meloeiro a Bemisia tabaci biótipo B. Bragantia 68:1025–1035
De Barro PJ, Liu S, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Ann Rev Entomol 56:1–19
Gachoka KK, Obeng-Ofori D, Danquah EY (2005) Host suitability of two Ghanaian biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on five common tropical weeds. Int J Trop Insect Sci 25:236–244
Hilje L, Morales FJ (2008) Whitefly bioecology and management in Latin America. In: Capinera J (ed) Encyclopedia of entomology. Springer, Heidelberg, pp 4250–4260
Hilje L, Costa HS, Stansly PA (2001) Cultural practices for managing Bemisia tabaci and associated viral diseases. Crop Prot 20:801–812
Inbar M, Gerling D (2008) Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu Rev Entomol 53:431–438
Kiill LHP, Haji FNP, Lima PCF (1998) Avaliação do grau de infestação de mosca branca (Bemisia spp.) em plantas invasoras em áreas de frutíferas irrigadas. In: Encontro Latino-Americano e do Caribe sobre Mosca-branca e Geminivirus (Recife, BR).
Lourenção AL, Nagai H (1994) Surtos populacionais de Bemisia tabaci no Estado de São Paulo. Bragantia 53:53–59
McAuslane HJ (1996) Influence of leaf pubescence on ovipositional preference of Bemisia argentifolii (Homoptera: Aleyrodidae) on soybean. Environ Entomol 25:834–841
Mizuno ACR, Villas Bôas GL (1997) Biologia da mosca-branca (Bemisia argentifolii) em tomate e repolho. Available at: http://www.cnph.embrapa.br/pa/pa01.html. Accessed 15 Mar 2010
Morales FJ (2011) Interaction between Bemisia tabaci, begomoviruses, and plant species in Latin America and the Caribbean. In: Thompson WMO (ed) The whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) interaction with geminivirus-infected host plants. Springer, London, pp 15–49
Mound LA, Halsey SH (1978) Whitefly of the world: a systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. British Museum (Natural History). Wiley, Chichester, 340 p
Nava-Camberos U, Riley DG, Harris MK (2001) Temperature and host plant effects on development, survival, and fecundity of Bemisia argentifolli (Homoptera: Aleyrodidae). Environ Entomol 30:55–63
Oriani MAG, Vendramim JD, Brunherotto R (2008) Aspectos biológicos de Bemisia tabaci biótipo B (Hemiptera: Aleyrodidae) em seis genótipos de feijoeiro. Neotrop Entomol 37:191–195
Oriani MAG, Vendramim JD, Vasconcelos CJ (2011) No-choice ovipositional nonpreference of Bemisia tabaci (Gennadius) B biotype on tomato genotypes. Sci Agric 68:147–153
Perring TM (2001) The Bemisia tabaci species complex. Crop Prot 20:725–737
SAS Institute (2002) SAS/STAT User’s guide, version 9.0, TS level 00MO. SAS Institute Inc, Cary
Silva MS, Lourenção AL, Souza-Dias JAC, Miranda-Filho HS, Ramos VJ, Schammass EA (2008) Resistance of potato genotypes (Solanum spp.) to Bemisia tabaci biotype B. Hortic Bras 26:221–226
Smith CM (1989) Plant resistance to insects—a fundamental approach. Wiley, New York, pp 53–74
Smith CM, Khan ZR, Pathak MD (1994) Techniques for evaluating insect resistance in crop plants. Lewis, Boca Raton, pp 17–114
Torres LC, Souza B, Amaral BB, Tanque RL (2007) Biologia e não-preferência para oviposição por Bemisia tabaci (Gennadius) biótipo B (Hemiptera: Aleyrodidae) em cultivares de algodoeiro. Neotrop Entomol 36:445–453
Valle GE, Lourenção AL, Pinheiro JB (2012a) Adult attractiveness and oviposition preference of Bemisia tabaci biotype B in soybean genotypes with different trichome density. J Pest Sci 85:431–442
Valle GE, Lourenção AL, Zucchi MI, Pinheiro JB, Abreu AG (2012b) MtDNA variability in whitefly (Bemisia tabaci) populations in Brazil. Genet Mol Res 10:2155–2164
Villas Bôas GL, Inoue-Nagata AK, Lima RS, Pereira W, Giordano LB (2003) Avaliação de plantas daninhas como possíveis hospedeiras de mosca-branca. Hortic Bras 21:344–347
Walker GP, Perring TM, Freeman TP (2010) Life history, functional anatomy, feeding and mating behavior. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, New York, pp 109–160
Acknowledgments
The authors would like to thank Dr. Jorge Braz Torres for the helpful comments to improve this manuscript, Dr. Robert Deuber for his comments on the weeds, the CAPES Foundation for the financial support, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research productivity fellowship to the second author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Jorge B Torres – UFRPE
Rights and permissions
About this article
Cite this article
Sottoriva, L.D.M., Lourenção, A.L. & Colombo, C.A. Performance of Bemisia tabaci (Genn.) Biotype B (Hemiptera: Aleyrodidae) on Weeds. Neotrop Entomol 43, 574–581 (2014). https://doi.org/10.1007/s13744-014-0238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-014-0238-5