Abstract
An analysis method based on matrix solid-phase dispersion (MSPD) and gas chromatography tandem mass spectrometry was developed and applied for the analysis for organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in the marketed seafood such as fish, shrimp and shellfish. The parameters of the method including the type and amount of the absorbent, as well as the type and volume of the elution solution were optimized. The recoveries were between 70 and 120% with RSDs less than 20%, and the LODs and LOQs were 0.011–0.046 and 0.037–0.153 ng g−1 under optimized conditions. The results showed that most of the OCPs and PCBs were detectable in the marketed samples with the average concentration range of 0.722–14.206 and 0.034–1.184 ng g−1, respectively. Among the 21 OCPs detected, DDTs had a relatively higher concentration level. And in the PCBs, the concentration of PCB28 was over 45% of the total PCBs determined in all the samples. The developed method was simple, fast and effective, and could successfully be applied for trace amount of OCPs and PCBs determination in seafood matrixes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) are a class of synthetic insecticides which were used worldwide for agriculture and public health since 1940s with characteristics of broad spectrum, high efficiency and low cost [1]. Polychlorinated biphenyls (PCBs) are a group of chlorinated organic compounds derived from biphenyl and widely applied in industry since 1930s as dielectric fluid, lubricating oils and additives in capacitors, transformers, plastics, paints, flame retardants, etc. [2,3,4]. Some of OCPs and PCBs are persistent pollutants which are still widespread in the environment and cause a global threat though banned and restricted for many years [5, 6]. OCPs and PCBs are hydrophobic and chemically stable, making them easily accumulated in the adipose tissues of animals and human causing variety of adverse health effects such as neurological, immunological, reproductive disorders and carcinogenicity [7,8,9].
Seafood is a term of aquatic products for human consumption including aquatic animals and plants from marine or freshwater origin [10]. A variety of contaminants can enter aquatic environment via various ways, resulting in seafood contamination. Most of the animal seafood such as fish, shrimp, and shellfish contain high level of oil matters which makes the pretreatment procedure very difficult for the residue analysis of hydrophobic pollutants such as OCPs and PCBs.
Matrix solid-phase dispersion (MSPD) is a simple and efficient pretreatment method which was introduced by Barker in 1989 [11], and since then it has been widely used for isolation and extraction of environmental contaminants from various complex semi-solid or solid samples such as fruits [12], vegetables [13] and animal tissues [14] because of the superiority that makes tissue homogenization, precipitation, centrifugation, filtration and the sample transfer in one step. In MSPD, the sample was gently grinded with the solid adsorbent (C18, silica gel, etc.), making samples disrupt into tiny fragments scattered on the surface of the solid adsorbent to get better surface area. After the mixture was transferred into an empty SPE column or needle tube, the target compounds were eluted with appropriate solvent. Some traditional methods such as Soxhlet extraction need numerous steps, high volume of solvent and long analytical time [15]. MSPD has many superiorities such as the mild extraction conditions, less operation steps, simple operate procedure, relatively less organic solvent, no need for expensive instrumentation, low cost and suitable for viscous samples [16].
There are some studies on the application of MSPD in the analysis of OCPs and PCBs in seafood, Lott HM developed MSPD method for the determination of 14 OCPs in oysters with recoveries of 66–84% [17]. Lott HM also used MSPD method to detect the concentration of 14 OCPs in crayfish, the recoveries were in the range of 55–118% [18]. Long AR applied MSPD method in the analysis of 9 OCPs in catfish muscle tissue, the recoveries ranged from 82 to 97% [19]. Shen ZL combined MSPD with accelerated solvent extraction (ASE) for the detection of OCPs in fish muscles, with the recoveries ranging from 91 to 104% [20]. Martínez A developed MSPD method for 7 PCBs analysis in turbot fish muscle and dogfish liver with recoveries above 75% [21]. Ling YC applied MSPD method to analyze 4 PCBs in fish with recoveries above 95% [22].
In this paper, a fast, simple, effective analysis method based on MSPD and GC–MS/MS for the detection of OCPs and PCBs in seafood was established. The influencing parameters including the type and amount of absorbent, the type and volume of elution solution were optimized. The developed method was applied to determine the OCPs and PCBs in seafood such as fish, shrimp and shellfish purchased from local markets in Beijing, and the concentration and distribution of OCPs and PCBs were discussed.
Experimental
Reagents and Standards
Twenty-one OCPs (α-HCH, β-HCH, γ-HCH, δ-HCH, o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, p,p′-DDT, heptachlor, heptachlor epoxide, c, trans-chlordane, aldrin, dieldrin, endrin, endosulfan I, endosulfan II, endosulfan sulfate, methoxychlor) mixed standard stock solution (each OCP at 100 μg mL−1 in isooctane) and seven PCBs (IUPAC NO. 28, 52, 101, 118, 138, 153, 180) mixed standard stock solution (each PCB at 10 μg mL−1 in isooctane) were purchased from AccuStandard (USA). Standard working solution (each analyte at 1 μg L−1) was prepared daily by appropriate dilution of aliquots of the standard stock solutions in acetone.
Mirex (100 μg mL−1 in hexane), 2,4,5,6-tetrachloro-m-xylene (TCMX, 100 μg mL−1 in acetone) and PCB209 (100 μg mL−1 in isooctane) standard solutions were purchased from AccuStandard (USA). TCMX and PCB209 were prepared in acetone as surrogate for all analytes at the concentration of 50 ng mL−1, and mirex was prepared in isooctane as internal standard for all analytes at the concentration of 50 ng mL−1.
Dichloromethane, n-hexane, isooctane, acetone (HPLC grade) were purchased from Merck (Germany). Anhydrous sodium sulfate (analytical grade) was purchased from Beijing chemical works (China), heated at 150 °C for 5 h before use. Florisil (100–200 mesh) and neutral alumina (100–200 mesh) were purchased from Sinopharm Chemical Reagent Co., Ltd (China), heated at 150 °C for 5 h before use. Silica gel (100–200 mesh) was purchased from Qingdao Haiyang Chemical Co., Ltd (China), heated at 120 °C for 2 h before use.
Apparatus
All sample extracts were analyzed by a TRACE GC Ultra gas chromatograph spectrometer (Thermo Fisher Scientific, USA). Capillary column HP-5 MS (30 m × 0.25 mm × 0.25 μm, Agilent Technologies) was used for separation. 10-mL Empty solid-phase extraction columns and polypropylene frits (Agilent Technologies) were used for the MSPD procedure. MTN-2008 Termovap Sample Concentrator (Tianjin Automatic Science Instrument) and IKA T10 high speed homogenate machine (IKA) were used as well.
Sample Preparation
For the fish, shrimp and shellfish samples, 1 g of thawed muscle tissue was placed into a mortar and allowed to stand at room temperature for 10 min after adding 100 μL of surrogate solution (50 ng mL−1). Then, 6 g of neutral alumina was transferred into the mortar, and the mixture was gently blended with glass pestle until homogeneous powder was obtained. The mixture was introduced into a 10-mL empty solid-phase extraction column pre-placed with a polypropylene frit and 1 g of neutral alumina as a co-column adsorbent. Two pieces of round filter paper were placed on the top and plugged with a glass pestle gently. The column was eluted with n-hexane/dichloromethane (50/50, v/v), and 10 mL of the eluate was collected by gravitational force and evaporated to dryness by a gentle nitrogen stream. The residue was dissolved in the mixture solution consisting of 900 μL of isooctane and 100 μL of internal standard solution, and then transferred to a sample vial for gas chromatographic analysis.
Chromatographic analysis
Helium (99.999%) was used as carrier gas and the flow rate was 1.0 mL min−1. The oven temperature was programmed from 90 °C (2 min) to 200 °C at a rate of 15 °C min−1, held for 10 min, then raised to 270 °C at 15 °C min−1, held for 10 min. The temperature of the injector operated in the splitless mode was 270 °C and the injection volume was 1 μL. The temperature of the transfer line was 290 °C.
As for the mass spectra, compounds were fragmented with electron ionization (EI) with the temperature of ion source at 250 °C. Argon (99.999%) was used as collision gas and the pressure was set at 1.0 mTorr. Cycle time was set as 0.2 s. SRM mode was used for the quantitation and other parameters are given in Table 1; the chromatograms of target compounds are shown in Figure S1. The acquisition and processing of data were carried out with Xcalibur™ software.
The internal standard method was used for quantification, TCMX and PCB209 were used as surrogate for all analytes, and mirex was used as internal standard for all analytes.
Market Sample Collection
To determine the concentration of the OCPs and PCBs in marketed seafood, grass carp (Ctenopharyngodon idellus), spotted silver carp (Aristichthys nobilis), crucian carp (Carassius auratus), little yellow croaker (Larimichthys polyactis), white shrimp (Penaeus chinensis), scallop (Chlamys farreri) and clam (Ruditapes philippinarum) were sampled from local markets or supermarkets in Beijing, and the information of the samples was listed in Table 2. All the samples were moved back to laboratory within 3 h, and the muscle tissues were removed and grinded uniformly by high speed homogenate machine, then stored at −20 °C.
Results and Discussion
Optimization of the MSPD Method
All the optimizing experiments were performed with the muscle tissue sample of crucian carp, and the added concentration of the standard solution was 1.0 ng g−1, three replicates were applied for each parameter, and the RSDs were in range of 0.3–16.9%.
The Type of the Adsorbent
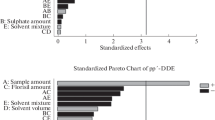
Adsorbent plays an important role in MSPD procedure for supporting, adsorbing, and cleaning-up. The cellular structures of the samples can be disrupted by the adsorbent and the impurities such as water and lipophilic compounds can be solubilized in the adsorbent [23]. Therefore, the choice of an appropriate adsorbent is vital. In this research, florisil, silica gel, and neutral alumina were tested. The mass of adsorbent and sample were 6 and 1 g, the elution solvent was n-hexane/dichloromethane (50/50, v/v) and the volume of collected solution was 10 mL. Figure 1 showed the recoveries using neutral alumina were better than using florisil and silica gel. Moreover, a more homogeneous and dry mixing powder was obtained when using neutral alumina during the blending procedure. Thus, neutral alumina was selected as adsorbent in this MSPD procedure.
The Amount of the Adsorbent
The amount of the adsorbent could influence the extracting and cleaning-up effects as well. When the adsorbent was insufficient, the sample could not be well dispersive with the adsorbent, getting low extracting efficiency and poor purifying effect as well. But if the adsorbent was excessive, the amount of the mixture increased, resulting in slow flow rate and more eluent solvent. In this work, 5, 6 and 7 g of neutral alumina were tested, n-hexane/dichloromethane (50/50, v/v) was used and 10 mL of eluent solvent was collected. As shown in Fig. 2, 6 g was chosen as the amount of the adsorbent for best recovery results and appropriate flow rate.
The Type of the Elution Solvent
For the types of elution solvent, n-hexane/ethyl acetate, n-hexane/acetone and n-hexane/dichloromethane were tested with 6 g of neutral alumina as adsorbent. It was found that n-hexane/dichloromethane had the best clean-up effects for the interferences. Four volume ratios of n-hexane/dichloromethane, 70/30, 60/40, 50/50 and 40/60 were tested. From the results shown in Fig. 3, n-hexane/dichloromethane 40/60 and 50/50 got better recoveries for most target compounds, but n-hexane/dichloromethane 40/60 had more interferences because of higher polarity. In this case, n-hexane/dichloromethane (50/50, v/v) was used as elution solvent.
The Volume of the Elution Solvent
For the volume of elution solution, 6, 8, 10 and 12 mL were tested. From the results shown in Fig. 4, the recoveries were no longer improved when the volume reached 10 mL. Due to the principle of using less organic solvent as far as possible, 10 mL of collected solution was selected eventually.
MSPD Method Validation
The accuracy and precision of the method were evaluated by blank samples (crucian carp, white shrimp and clam) spiked with the OCPs and PCBs at 0.1, 1 and 10 μg L−1 with three replicates for each level, and the results were shown in Table 3. The recoveries for most target compounds were between 70 and 120%, with relative standard deviation less than 20%. Limit of detection (LOD) and limit of quantification (LOQ) were tested with 0.1 μg L−1 spiked samples and calculated by three or ten times of the signal-to-noise ratio. As shown in Table 3, the LODs and LOQs of the OCPs and PCBs ranged from 0.011 to 0.046 and from 0.037 to 0.153 μg L−1 in the seafood samples. The linearity was in the range of 0.1–100 μg L−1, and the results are summarized in Table S1.
The Concentration and Distribution of OCPs and PCBs in Real Samples
From the results shown in Table 4, most of the OCPs and PCBs could be detected in the samples. The residual concentration of ∑OCPs was 1.216–4.248 μg L−1 in grass carp, 1.403–15.918 μg L−1 in spotted silver carp, 1.432–8.9 μg L−1 in crucian carp, 1.988–14.206 μg L−1 in little yellow croaker, 0.986–4.021 μg L−1 in white shrimp, 0.722–1.444 μg L−1 in scallop and 2.583–8.853 μg L−1 inclam, and the concentration of ∑PCBs was 0.166–0.374 μg L−1 in grass carp, 0.034–0.658 μg L−1 in spotted silver carp, 0.24–0.529 μg L−1 in crucian carp, 0.27–1.184 μg L−1 in little yellow croaker, 0.193–0.353 μg L−1 in white shrimp, 0.174–0.224 μg L−1 in scallop and 0.306–0.543 μg L−1 inclam. Among the OCPs, DDTs (o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, p,p′-DDT) had apparently high concentration in all the samples, and ∑DDTs in the samples followed the order: little yellow croaker (6.549 ng g−1) > spotted silver carp (4.408 ng g−1) > clam (4.263 ng g−1) > crucian carp (2.662 ng g−1) > grass carp (1.418 ng g−1) > white shrimp (1.117 ng g−1) > scallop (0.600 ng g−1). In the fish samples, the concentration of ∑DDTs in marine fish was apparently higher than that in freshwater fish, and the concentration in predacious fish was far higher than that in omnivorous and herbivorous fish. The DDTs concentration in little yellow croaker, spotted silver carp and clam samples was apparently higher than in other samples. The Other OCPs were in a relative low level in all the samples.
From Fig. 5, the relative abundances of DDT congeners (to ∑DDTs) were similar in fish samples from freshwater (grass carp, spotted silver and crucian carp), and followed the sequence of p,p′-DDE > o,p′-DDE > p,p′-DDD > o,p′-DDT ~ p,p′-DDT > o,p′-DDD. p,p′-DDE was the predominant DDT congener in grass carp, spotted silver, crucian carp and little yellow croaker, ranged from 33.0 to 68.6%. While o,p′-DDE was the predominant in white shrimp and scallop samples.
The ratio of (p,p′-DDE + p,p′-DDD) to p,p′-DDT was used to evaluate the source of DDTs [24]. When the ratio was greater than 1, indicating that the metabolites of DDTs were mainly exist in seafood without fresh inputs, and when the ratio was less than 1, it means that the environment had new source of DDTs. In this study, the ratios were 9.9 for grass carp, 44.4 for spotted silver carp, 21.0 for crucian carp, 1.7 for little yellow croaker, 3.7 for white shrimp, 0.9 for scallop and 3.5 for clam, indicating that almost none freshness of exposure occurred recently except scallop, but historical inputs of dicofol.
As for the relative abundances of HCH congeners (to ∑HCHs) were not significantly different among seafood species with different habitats. As shown in Fig. 6, β-HCH was predominant HCH congener in grass carp, spotted silver, crucian carp and white shrimp, ranged from 38.2 to 59.6%. While δ-HCH was predominant in little yellow croaker and scallop.
The ratio of β-/(α + γ)-HCH was used to identify the source of HCH [25]. If the ratio was less than 0.06, it means that new inputs of lindane or technical HCH occurred. If the ratio was greater than 0.06, indicating the historical inputs of lindane or technical HCH. In this study, the ratios were 2.4 for grass carp, 1.8 for spotted silver carp, 2.0 for crucian carp, 0.6 for little yellow croaker, 0.8 for white shrimp, 0.5 for scallop and 0.7 for clam. From the results, no fresh exposure existed recently.
As shown in Fig. 7, the concentration of ∑PCBs was much lower than that of ∑OCPs. The composition of the PCBs in samples is shown in Fig. 8. Among the seven PCBs, PCB28 was the predominant component.
Compared with the maximum residue limit (MRL, 0.5 mg kg−1 for ∑DDTs, 0.1 mg kg−1 for ∑HCHs, 0.5 mg kg−1 for ∑PCBs) of seafood legislated by China, the residue concentration of ∑DDTs, ∑HCHs and ∑PCBs determined in this study was far below the limits. The MRLs in muscles from other animal origin food fixed by European Union was 1.0, 0.2 and 0.1 mg kg−1 for ∑DDTs, α-HCH and β-HCH, respectively. All the limited concentration was significantly higher than that detected in the present study. Furthermore, many literatures reported the residue levels of OCPs and PCBs in seafood. For instance, 11 OCPs in edible fish from Wuhan (China) were in the range of 2.04–189.04 ng g−1 [26] and the OCPs in four fish species from Lake Ziway (Ethiopia) was between 1.41 and 63.8 ng g−1 [27]. The concentration of 15 PCBs in marketed shrimp in San Francisco ranged from 0.11 to 2.40 ng g−1 [28]. It was also found OCPs and PCBs in the fish and shellfish collected from Dalian, Tianjin and Shanghai in China with concentration of 0.01–145 and 0.83–11.4 ng g−1 [29]. Comparing to the reported levels of residue, the concentration of OCPs and PCBs in the present study was relatively low.
Comparison with Other Published Methods
The performance characteristics of the developed method in this work were compared with that in other published methods which obtained to extract OCPs and PCBs in seafood samples. The published methods including Soxhlet extraction, pressurized liquid extraction (PLE), selective pressurized liquid extraction (SPLE) and microwave-assisted extraction (MAE) were compared in aspects of recovery, adsorbent amount, solvent consumption and whether need to heat. As shown in Table 5, the developed method in this work got satisfactory results, simple operation, less solvent and energy consumption.
As given in Table 5, compared to other published method using MSPD for determination of OCPs and PCBs in seafood, the method in this work has many advantages such as abundant target compounds, large number and wide range of matrix samples and acceptable results.
Conclusion
A simple, rapid and effective method based on MSPD and GC–MS/MS was established for the detection of 21 OCPs and 7 PCBs in marketed seafood. The influencing parameters including the type and amount of the absorbent, the type and volume of the elution solution were optimized. Under the optimized conditions, satisfying recovery, LODs and LOQs were obtained. The developed method was successfully applied to detect the residue of OCPs and PCBs in the marketed seafood. The results showed the concentrations of the OCPs and PCBs were at relatively low levels.
References
Shoiful A, Fujita H, Watanabe I, Honda K (2013) Concentrations of organochlorine pesticides (OCPs) residues in foodstuffs collected from traditional markets in Indonesia. Chemosphere 90(5):1742–1750. doi:10.1016/j.chemosphere.2012.10.022
Milun V, Lusic J, Despalatovic M (2016) Polychlorinated biphenyls, organochlorine pesticides and trace metals in cultured and harvested bivalves from the eastern Adriatic coast (Croatia). Chemosphere 153:18–27. doi:10.1016/j.chemosphere.2016.03.039
Zheng J, Yu L-H, Chen S-J, Hu G-C, Chen K-H, Yan X, Luo X-J, Zhang S, Yu Y-J, Yang Z-Y, Mai B-X (2016) Polychlorinated biphenyls (PCBs) in human hair and serum from E-waste recycling workers in Southern China: concentrations, chiral signatures, correlations, and source identification. Environ Sci Technol 50(3):1579–1586. doi:10.1021/acs.est.5b04955
Wang Y, Hu J, Lin W, Wang N, Li C, Luo P, Hashmi MZ, Wang W, Su X, Chen C, Liu Y, Huang R, Shen C (2016) Health risk assessment of migrant workers’ exposure to polychlorinated biphenyls in air and dust in an e-waste recycling area in China: indication for a new wealth gap in environmental rights. Environ Int 87:33–41. doi:10.1016/j.envint.2015.11.009
Bajwa A, Ali U, Mahmood A, Chaudhry MJ, Syed JH, Li J, Zhang G, Jones KC, Malik RN (2016) Organochlorine pesticides (OCPs) in the Indus River catchment area, Pakistan: status, soil-air exchange and black carbon mediated distribution. Chemosphere 152:292–300. doi:10.1016/j.chemosphere.2016.01.024
Stagakis M, Costopoulou D, Vassiliadou I, Karavoltsos S, Sakellari A, Kalogeropoulos N, Leondiadis L (2015) Determination of polychlorinated biphenyls in Aegean fish and seafood. Anal Lett 49(7):1114–1126. doi:10.1080/00032719.2015.1070161
Khwaja S, Mushtaq R, Mushtaq R, Yousuf M, Attaullah M, Tabbassum F, Faiz R (2013) Monitoring of biochemical effects of organochlorine pesticides on human health. Health 05(08):1342–1350. doi:10.4236/health.2013.58182
Mahmood A, Malik RN, Li J, Zhang G (2014) Human health risk assessment and dietary intake of organochlorine pesticides through air, soil and food crops (wheat and rice) along two tributaries of river Chenab, Pakistan. Food chem toxicol int j publ Br Ind Biolog Res Assoc 71:17–25. doi:10.1016/j.fct.2014.05.008
Toan VD, Quy NP (2015) Residues of polychlorinated biphenyls (PCBs) in sediment from CauBay River and their impacts on agricultural soil, human health risk in KieuKy Area, Vietnam. Bull Environ Contam Toxicol 95(2):177–182. doi:10.1007/s00128-015-1581-x
NDA EFSA (2014) Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA J 12(7):3761. doi:10.2903/j.efsa.2014.3761
Barker SA (2000) Matrix solid-phase dispersion. J Chromatogr A 885(1–2):115–127. doi:10.1016/S0021-9673(00)00249-1
Ramos JJ, Rial-Otero R, Ramos L, Capelo JL (2008) Ultrasonic-assisted matrix solid-phase dispersion as an improved methodology for the determination of pesticides in fruits. J Chromatogr A 1212(1–2):145–149. doi:10.1016/j.chroma.2008.10.028
Lin Q-B, Shi H-J, Xue P (2010) MSPD–GC–MS–MS determination of residues of 15 organic nitrogen-containing pesticides in vegetables. Chromatographia 72(11–12):1143–1148. doi:10.1365/s10337-010-1773-9
Cheng J, Liu M, Yu Y, Wang X, Zhang H, Ding L, Jin H (2009) Determination of pyrethroids in porcine tissues by matrix solid-phase dispersion extraction and high-performance liquid chromatography. Meat Sci 82(4):407–412. doi:10.1016/j.meatsci.2008.09.011
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: past and present panacea. J Chromatogr A 1217(16):2383–2389. doi:10.1016/j.chroma.2009.11.027
Garcia-Lopez M, Canosa P, Rodriguez I (2008) Trends and recent applications of matrix solid-phase dispersion. Anal Bioanal Chem 391(3):963–974. doi:10.1007/s00216-008-1898-y
Lott HM, Barker SA (1993) Matrix solid-phase dispersion extraction and gas chromatographic screening of 14 chlorinated pesticides in oysters (Crassostrea virginica). J AOAC Int 76(1):67–72
Lott HM, Barker SA (1993) Extraction and gas chromatographic screening of 14 chlorinated pesticides in crayfish (Procambarus clarkii) hepatopancreas. J AOAC Int 76(3):663–668
Long AR, Crouch MD, Barker SA (1991) Multiresidue matrix solid phase dispersion (MSPD) extraction and gas chromatographic screening of nine chlorinated pesticides in catfish (Ictalurus punctatus) muscle tissue. J Assoc Off Anal Chem 74(4):667–670
Shen Z-L, Yuan D, Zhang H, Hu M, Zhu J-H, Zhang X-Q, Suc Q-D (2011) Matrix solid phase dispersion-accelerated solvent extraction for determination of OCP residues in fish muscles. J Chin Chem Soc 58(4):494–502. doi:10.1002/jccs.201190012
Martínez A, Ramil M, Montes R, Hernanz D, Rubí E, Rodríguez I, Torrijos RC (2005) Development of a matrix solid-phase dispersion method for the screening of polybrominated diphenyl ethers and polychlorinated biphenyls in biota samples using gas chromatography with electron-capture detection. J Chromatogr A 1072(1):83–91. doi:10.1016/j.chroma.2004.12.034
Ling YC, Huang IP (1995) Multiresidue-matrix solid-phase dispersion method for determining 16 organochlorine pesticides and polychlorinated biphenyls in fish. Chromatographia 40(5):259–266. doi:10.1007/BF02290355
Loveland PM, Reddy AP, Pereira CB, Field JA, Bailey GS (2001) Application of matrix solid-phase dispersion in the determination of dibenzo[a, l]pyrene content of experimental animal diets used in a large-scale tumor study. J Chromatogr A 932(1–2):33–41. doi:10.1016/S0021-9673(01)01207-9
Yang L, Xia X, Liu S, Bu Q (2010) Distribution and sources of DDTs in urban soils with six types of land use in Beijing, China. J Hazard Mater 174(1–3):100–107. doi:10.1016/j.jhazmat.2009.09.022
He W, Qin N, He Q-S, Wang Y, Kong X-Z, Xu F-L (2012) Characterization, ecological and health risks of DDTs and HCHs in water from a large shallow Chinese lake. Ecolog Inform 12:77–84. doi:10.1016/j.ecoinf.2012.05.008
Cui L, Ge J, Zhu Y, Yang Y, Wang J (2015) Concentrations, bioaccumulation, and human health risk assessment of organochlorine pesticides and heavy metals in edible fish from Wuhan, China. Environ Sci Pollut Res Int 22(20):15866–15879. doi:10.1007/s11356-015-4752-8
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2014) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake-Lake Ziway, Ethiopia. Ecotoxicol Environ Saf 106:95–101. doi:10.1016/j.ecoenv.2014.04.014
Fillos D, Scott LL, De Sylor MA, Grespin M, Luksemburg WJ, Finley B (2012) PCB concentrations in shrimp from major import markets and the United States. Environ Toxicol Chem 31(5):1063–1071. doi:10.1002/etc.1803
Yang N, Matsuda M, Kawano M, Wakimoto T (2006) PCBs and organochlorine pesticides (OCPs) in edible fish and shellfish from China. Chemosphere 63(8):1342–1352. doi:10.1016/j.chemosphere.2005.09.029
Suchan P, Pulkrabová J, Hajšlová J, Vr Kocourek (2004) Pressurized liquid extraction in determination of polychlorinated biphenyls and organochlorine pesticides in fish samples. Anal Chim Acta 520(1–2):193–200. doi:10.1016/j.aca.2004.02.061
Choi M, Lee IS, Jung RH (2016) Rapid determination of organochlorine pesticides in fish using selective pressurized liquid extraction and gas chromatography-mass spectrometry. Food Chem 205:1–8. doi:10.1016/j.foodchem.2016.02.156
Wilkowska AM, Biziuk M (2010) Rapid method for the determination of organochlorine pesticides and PCBs in fish muscle samples by microwave-assisted extraction and analysis of extracts by GC-ECD. J AOAC Int 93(6):1987–1994
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Weijia Li declares that she has no conflict of interest. Donghui Liu declares that she has no conflict of interest. Jindong Li declares that he has no conflict of interest. Jing Gao declares that she has no conflict of interest. Chuntao Zhang declares that she has no conflict of interest. Peng Wang declares that he has no conflict of interest. Zhiqiang Zhou declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All animal experiments in the present study were carried out in accordance with institutional and national guidelines for the care and use of laboratory animals.
Funding
This study was funded by National Natural Science Foundation of China (Contract Grants 21337005, 21277171).
Informed consent
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Liu, D., Li, J. et al. Matrix Solid-Phase Dispersion Combined with GC–MS/MS for the Determination of Organochlorine Pesticides and Polychlorinated Biphenyls in Marketed Seafood. Chromatographia 80, 813–824 (2017). https://doi.org/10.1007/s10337-017-3282-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3282-6