Abstract
A method for simultaneous analysis of residues of nine organophosphorus pesticides in fruit and vegetables has been developed. It involves matrix solid-phase dispersion (MSPD) for preconcentration before rapid resolution liquid chromatography–tandem mass spectrometry (RRLC–MS–MS). In the MSPD pre-concentration step, the adsorptive performance of multi-walled carbon nanotubes (MWCNT) as MSPD adsorbent and elution with four solvents were investigated; in the LC separation step, a rapid resolution high-throughout LC column was used with gradient elution. The results of the research showed that the linear correlation coefficients (r 2) of the method for the nine target analytes varied between 0.9942 and 0.9996, mean recovery was in the range 71.2–102.8%, with relative standard deviations (RSD) 2.0–11.8%, and limits of detection were all below 0.2 μg kg−1. The method was used for simultaneous analysis of the nine pesticides in eight different fruit and vegetable samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPP) are a class of organic compounds containing C–P or C–O–P, C–S–P, and C–N–P bonds. These pesticides have been used for control of spiders, mites, aphids, beetles, caterpillars, etc., on a wide variety of crops worldwide, because of their advantages of high effectiveness and low toxicity [1]. Because of this widespread use, they have become the most common and persistent contaminants of products of agriculture in developing countries [2]. OPP inhibit acetylcholinesterase, the enzyme that hydrolyzes the neurotransmitter acetylcholine and, thus, terminates the transmission signal on postsynaptic cholinergic receptors [3, 4]. They can reach the food chain in various ways and may, therefore, pose a risk to human health [5, 6]. Thus, the development of analytical methods suitable for their surveillance in food is quite important.
Within the EU, maximum residue levels ranging from 0.01 to 3 mg kg−1 have been established for these pesticides in many vegetables and fruit [7]. This threshold level is also frequently used for testing compliance with guidelines for production of organic food.
For analysis of OPP residues in food, traditional chromatographic analysis, for example gas chromatography (GC) [8–10], gas chromatography–mass spectrometry (GC–MS) [11], high-performance liquid chromatography (LC) [3, 12], and liquid chromatography–mass spectrometry (LC–MS) [13–15], are usually adopted. Traditional LC needs long analysis times to achieve complete separation and quantification of OPP multi-residues. In recent years, with breakthroughs in two core techniques, sub-two-micron particle chromatographic columns and new liquid chromatographic systems, LC–MS technology is entering a new era. Rapid-resolution liquid chromatography–mass spectrometry (RRLC–MS) from Agilent Technologies (Chandler, AZ, USA) is a representative example. This technique is faster and more sensitive than traditional LC–MS [16–18]. These particular advantages will make it widely used in prospective pesticide residue monitoring, although so far there have been few reports of its application.
Effective pretreatment is often required before analysis of trace level pesticide residues in foodstuffs. The methods used frequently include liquid–liquid extraction (LLE) [19], supercritical-fluid extraction (SFE) [20], pressurized liquid extraction (PLE) [21], solid-phase extraction (SPE) [22–24], solid-phase micro-extraction (SPME) [25–27], quick, easy, cheap, effective, rugged, and safe (QuEchERS) [28], and matrix solid-phase dispersion (MSPD) [29, 30]. MSPD, first reported in 1989 [31], is a simple pretreatment method based on dispersion of the sample on an adsorbent which enables simultaneous disruption and homogenization of solid and semi-solid samples, and extraction, fractionation, and clean-up of the analytes in a single step. Its many uses have been extensively reviewed [32–38]. It reduces extraction time and solvent and cartridge consumption, which are the drawbacks of LLE and SPE. In recent years, the technique has become more popular in pesticide analysis and has been introduced in many multi-residue methods for analysis of food [29, 30, 39–41].
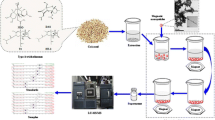
In the work discussed in this paper, MSPD with a new carbon-based nano-material adsorbent, multi-walled carbon nanotubes (MWCNT), has been used to isolate nine OPP, simultaneously, from fruit and vegetables before chromatographic analysis. With large accessible specific surface area and hydrophobic surface, this novel carbonaceous material has large adsorption capacity, and in recent years has been widely used as an effective SPE adsorbent for pre-concentration of pesticides [42–46]. As far as we are aware, however, there have been no reports of use this material as MSPD adsorbent for analysis of organic pollutant residues in different matrixes.
The purpose of this study was to establish an MSPD method using MWCNT as adsorbent for simultaneous analysis of nine OPP in fruit and vegetables by RRLC–MS–MS. Conditions possibly affecting MSPD efficiency and subsequent RRLC–MS–MS analysis were optimized, and are discussed in detail. The proposed method was validated and applied to the analysis of these pesticide residues in eight real samples from a local food market.
Experimental
Reagents and Materials
Standards of the nine pesticides with certified purities of at least 98% were purchased from the Environmental Monitoring Center of the Department of Agriculture (Beijing, China). Formic acid (LC-grade) was purchased from Beijing Chemical Reagents Company (Beijing, China). Ethyl acetate, n-hexane, and acetone (all LC-grade), and dichloroethene and ammonium acetate (both GR-grade) were supplied by Shanghai Chemical Reagents Company (Shanghai, China). Methanol (LC/Spectro-grade) was obtained from Tedia (Fairfield, OH, USA). Acetonitrile (LC/Spectro-grade) was purchased from Burdick and Jackson (Muskegon, MI, USA). A Milli-Q ultra-pure water system from Millipore (Bedford, MA, USA) was used to obtain purified water (>18.3 MΩ). MWCNT (40–60 nm i.d., 40–300 m2 g−1) were obtained from Chengdu AIFA Nanotechport (Chengdu, China).
Individual stock solutions (50.0 mg L−1) of the nine pesticide standards were prepared by dissolving 5.0 mg of each in 100 mL acetonitrile. A multi-component standard solution (1.0 mg L−1) was prepared by mixing 1.0 mL of each individual stock solution and diluting to 50 mL with acetonitrile; this solution was used for optimizing MSPD conditions, for spiking blank sample matrices, for preparing calibration standard solutions, and for studying the linear dynamic range of the RRLC–MS–MS method. Six calibration standard solutions (0.02, 0.1, 0.5, 1.0, 2.0, and 4.0 μg kg−1) were prepared by adding different volumes of the composite standard solution to blank extracts obtained by use of the MSPD procedure. All fresh individual stock solutions were prepared weekly and stored under refrigeration (4 °C) away from light. All other standard multi-component solutions were prepared immediately before use.
Sample Preparation
The samples analyzed (apple, grape, banana, strawberry, tomato, cabbage, spinach, and rape), were obtained from a local market. All the samples were taken from different places throughout the lot (size ca 50 kg). Samples weighed at least 1.0 kg and consisted of at least ten individual fruits or vegetables.
A representative portion of the sample (200 g whole fruit or vegetable) was chopped and homogenized in a plant pulverizer (Shanghai, China). Portions (0.5 g) were then weighed, placed in a glass mortar (50 mL capacity), and gently blended with 1.0 g MWCNT for 5 min using a glass pestle to obtain a homogeneous mixture. This mixture was transferred to a 6-mL SPE tube with a polypropylene frit at the bottom. Another frit was placed on top of the mixture before compression to form a column packing by use of a modified syringe plunger. A 1:1 mixture of acetone and ethyl acetate (20 mL) was used to elute the column at a flow rate of 1 mL min−1. The eluent was collected in a graduated conical tube (15 mL) and evaporated to dryness under a gentle stream of nitrogen. The residue was dissolved in 1:1 acetonitrile–water (2.0 mL) and the pH was adjusted to 5.0 by addition of formic acid. The final extracts were vortex mixed for 2 min and filtered through a 0.45 μm Nylon syringe filter into autosampler vials for analysis.
RRLC–MS–MS Analysis
RRLC analysis was performed on Agilent (USA) 1200 series equipment comprising quaternary pump, micro degasser, high-performance well-plate autosampler, and thermostated column compartment. Samples were separated at 30 °C on an Agilent Zorbax RRHT SB-C18 column (100 mm × 2.1 mm, 1.8-μm particle). The mobile phase was a gradient prepared from an aqueous solution containing 0.1% formic acid and 10 mmol L−1 ammonium acetate (component A) and acetonitrile containing 0.1% formic acid (component B). The gradient conditions were: 0–2.0 min, from 95% A + 5% B to 50% A + 50% B, flow rate 0.6 mL min−1; 2.0–7.0 min, from 50% A + 50% B to 40% A + 60% B, flow rate 0.6 mL min−1; 7.0–8.5 min, from 40% A + 60% B to 15% A + 85% B, flow rate 1.2 mL min−1; 8.5–9.0 min, from 15% A + 85% B back to 95% A + 5% B, flow rate 1.2 mL min−1; 9.0–10.0 min, constant at 95% A + 5% B and flow rate 0.6 mL min−1 to equilibrate the flow. The injection volume was 5.0 μL.
Mass spectrometric analysis was performed on an Agilent 6410 triple-quadrupole mass spectrometer fitted with an electrospray ionization (ESI) source and controlled by Mass Hunter (Version B.01.03) software. The ESI source conditions were established to furnish average maximum intensity of the precursor ions. The compounds were ionized in the positive-ion polarity mode. The nitrogen nebulizer pressure was 40 psi and the nitrogen drying gas temperature was 350 °C and the flow rate 12 L min−1. The capillary potential was 4,000 V. Quadrupoles Q1 and Q3 were operated at unit resolution. For MS–MS, high-purity nitrogen (>99.99%) was used as collision gas. To optimize the multiple reaction monitoring (MRM) transitions, each individual pesticide at a concentration of 10 mg L−1 in acetonitrile was injected directly. Optimum conditions are summarized in Table 1.
Results and Discussion
Optimization of MSPD Conditions
For efficient isolation of the nine OPP from fruit or vegetable matrixes and evaluation of the analytical potential of MWCNT as MSPD adsorbent, a series of experiments was designed to optimize several important conditions affecting the extraction efficiency, including the type of eluent, the ratio of sample to adsorbent, the volume of eluent, and the flow rate during elution. To determine the optimum ratio of sample to adsorbent, recovery studies were conducted by spiking blank samples (0.5 g) with 10 mg kg−1 of the analyte standards and then blending with 0.5, 1.0, 1.5, or 2.0 g of the appropriate adsorbent. For optimization of the other three conditions, 1.0 g adsorbent was used.
For MSPD extraction, choice of the appropriate eluent is very important. Use of the wrong eluent can result in a long elution time and low recovery of target analytes. Four organic solvents of different polarity, acetone, dichloromethane, ethyl acetate, and n-hexane, were investigated. The experimental results showed that n-hexane did not elute any of the nine analytes fully, and acetone and ethyl acetate did not elute most of the analytes fully. The elution performance of dichloromethane was best for most of the analytes although elution of methamidophos was poor. These results were attributed to the different polarities of the solvents—the dielectric constants of acetone, dichloromethane, ethyl acetate, and n-hexane were 20.70, 8.93, 6.02, and 1.88, respectively. To improve the eluent strength for methamidophos, 1:1 acetone–ethyl acetate was investigated. Its dielectric constant was 13.4, approximately 4.0 higher than that of dichloromethane. The high recoveries obtained suggested that this solvent mixture enabled good elution of all nine analytes. It was therefore chosen for use in subsequent experiments.
The ratio of sample to adsorbent can also affect extraction efficiency. A series of experiments was designed to investigate the effect of different ratios (1:1, 1:2, 1:3, and 1:4) of sample to MWCNT adsorbent on recovery of the nine OPP. The results are shown in Table 2. It is apparent that recovery of most of the analytes was low when the ratio was 1:1. This low recovery could not be improved by increasing the volume of eluent, possibly because the sample matrix could not be dispersed fully in the adsorbent because the volume of adsorbent used was too low. In contrast, recovery was satisfactory when the ratio was 1:2, 1:3, or 1:4. Because a large volume of adsorbent will use more MWCNT and more eluent, a 1:2 ratio of sample to adsorbent was chosen.
After the eluent was chosen, four compounds representing the distribution of polarity of the nine OPP, methamidophos, methidathion, malathion, and ethion, were chosen for optimization of eluent volume and elution flow rate. To assess the effect of eluent volume, a series of experiments was designed in which the eluent volume was changed from 5.0 to 30.0 mL. As shown in Fig. 1, recovery of the target analytes increased with increasing eluent volume from 5.0 to 30.0 mL, although recovery changed very little for eluent volumes >20.0 mL. Thus, to achieve complete elution of the target analytes and save time and solvents, 20.0 mL eluent was selected.
The elution flow rate can also affect extraction efficiency. To evaluate the effect of eluent flow rate, a series of experiments was designed in which the elution flow rate was changed from 0.2 to 1.2 mL min−1. In general, recovery increased with decreasing flow rate, and recovery of all analytes was satisfactory when the flow rate was 1.0 mL min−1. To save time and solvents, a flow rate of 1.0 mL min−1 was selected.
Optimization of Mobile Phase, Mode of Elution, and Mobile Phase Flow Rate
It is well known that the mobile phase, mode of elution, and flow rate are three important conditions affecting chromatographic separation of target analytes. A series of experiments was designed to investigate the effect of mobile phase on the chromatographic separation. The mobile phases investigated were different mixtures of acetonitrile, methanol, and water (of different pH). The experimental results showed that a mixture of water containing 0.1% formic acid and 10 mmol ammonium acetate with acetonitrile containing 0.1% formic acid as mobile phase was a good choice. Because of their similar chemical structures and polarity, it is not easy to achieve complete chromatographic separation of the nine target analytes by isocratic elution, so gradient elution was adopted. The gradient selected resulted in resolution of the nine target analytes in nine min with ideal separation and good peak shape.
Optimization of the Conditions Used for Mass Spectrometry
The mobile phase used in LC–MS is important because it can affect the response of the mass spectrometer to the target analytes. In ESI, transfer of the ion to the gas phase from solution using the appropriate solvent not only increases the efficiency of generation of the gas phase ion but can also enhance detection sensitivity by converting the neutral target analyte molecule into an ion in the testing solution. The MS response to slightly alkaline compounds in the acidic solution is high in positive-ion scanning mode whereas that to slightly acidic compounds is poor, as might be expected on the basis of the theory of solution chemistry [47]. A series of experiments was designed to investigate the effect of solvent on MS response to the target analytes. Except for methamidophos which is slightly alkaline, the other eight analytes are all nearly neutral. When an acidic solvent was used, a good MS response was obtained for methamidophos in positive-ion scanning mode because the acidic solvent greatly promotes pre-formation of its ion in the sample solution, further improving the sensitivity of the method. Acidic or alkaline mobile phases had little effect on other eight analytes, however, because of their nearly neutral properties. Four representative compounds, methamidophos, methidathion, malathion, and ethion, were investigated in this experiment. With 1:1 acetonitrile–water containing 10 mmol L ammonium acetate as mobile phase with the volatile formic acid or sodium acetate as the regulator of acidity or alkalinity, the MS response to the target analytes was investigated under acidic or alkaline conditions in the pH range from 1.0 to 9.0 in positive-ion scanning mode. pH was measured with precision pH test paper. In Fig. 2, four detailed curves show the trend of MS response to the four analytes with varying pH. It is apparent that the experimental results support the previous presumption. The optimum pH was confirmed to be 5.0 because of the highest MS response to the four analytes.
The classical ESI interface usually requires a low mobile phase flow rate to promote desolvation and thus improve ionization efficiency. Use of a low flow rate can, however, sacrifice the high separation efficiency of the sub-two-micron particle chromatographic column, prolong the retention time of the compounds, and further reduce the sensitivity of the method. Compared with the classical ESI interface, with the development of high-desolvation-efficiency technology, the ESI interface on the Agilent 6410 QQQ mass spectrometer has been improved to enable mobile phase to pass through at a high flow rate without affecting the ionization efficiency. Drying gas temperature is usually 325–350 °C. Within this temperature range the relationship between mobile phase flow rate and nebulizer pressure or drying gas flow rate was investigated with the requirement that good ESI efficiency was achieved. According to the experimental results, to achieve good ESI both nebulizer pressure and drying gas flow rate must be increased when the mobile phase flow rate is increased. This may be because incomplete ionization can result if nebulizer pressure and drying gas flow rate are too low, destroying the insulating property of the spray chamber. High drying gas temperature can usually improve the desolvation of the high-flow-rate solution. The optimum conditions were finally confirmed to be 0.6–1.2 mL min−1 mobile phase flow rate, 40 psi nebulizer pressure, 12 L min−1 drying gas flow rate, and 350 °C drying gas temperature.
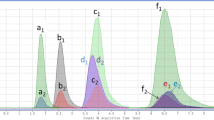
MRM mode is usually used for quantitative analysis of organic compounds at trace levels by mass spectrometry. To optimize performance and sensitivity, the fragmentor potential was selected to produce the highest signal intensity for the precursor ion. The collision energy was then adjusted to produce the highest intensity for the main fragment. To select the proper fragmentor potential, flow injections of 10 mg L−1 of each individual standard solution were performed with the fragmentor potential varied from 50 to 130 V in steps of 10 V. From the total ion chromatogram (TIC), the corresponding [M + H]+ for ESI+ was used to obtain the extracted ion chromatograms (EIC) for the different fragmentor potentials; the optimum fragmentor potentials were then selected. With the optimum fragmentor potential, another flow injection experiment was conducted to optimize the collision energy. Each time segment of the analysis was performed by varying the collision energy from 5 to 40 V in steps of 5 V. The optimum collision potential was defined as that generating the highest signal for the main fragment ion. The optimum conditions for each MRM transition are summarized in Table 1. Two MRM transitions were chosen for each analyte. The transition “parent ion → daughter ion (p1)” was used for quantification and the transition “parent ion → daughter ion (p2)” was chosen for confirmation. TIC chromatograms obtained from a blank banana sample and a blank banana sample fortified by addition of mixed standard solution (2.0 μg L−1), and EIC of the nine confirmed monitoring quantitative ions are shown in Fig. 3.
TIC obtained from blank and fortified banana sample, and EIC of nine quantitative ions: a blank sample (TIC), b blank sample fortified by addition of mixed standard solution (TIC), c m/z 94.0 ion for methamidophos (EIC), d m/z 127.1 ion for monocrotophos (EIC), e m/z 193.0 ion for mevinphos (EIC), f m/z 85.0 ion for methidathion (EIC), g m/z 232.1 ion for parathion-methyl (EIC), h m/z 127.1 ion for malathion (EIC), i m/z 236.0 ion for parathion-ethyl (EIC), j m/z 169.0 ion for diazinon (EIC), and k m/z 171.2 ion for ethion (EIC)
Validation of the Method
To validate the method, the calibration plot, detection limit (LOD), quantification limit (LOQ), recovery, and repeatability were studied.
Calibration plots were established by spiking 0.5 g blank banana samples with solutions containing the nine target analytes at six different concentrations, subjecting them to the optimized MSPD procedure, and analyzing the six extracts by the method described above. The linearity of the calibration plots was calculated for all the pesticides; the linear ranges and correlation coefficients are listed in Table 3. It is apparent there is a good linear relationship between response and concentration over the range selected, with correlation coefficients ranging from 0.9942 to 0.9996. This indicates the method developed in this work performed well in quantification of the nine target analytes.
Analytical method detection limits for the pesticides were calculated as the amounts for which the signal-to-noise ratio was three (S/N = 3) in MRM mode, and LOQs were calculated as the amounts for which S/N = 10. LODs were in the range 0.06 to 0.15 μg kg−1 and LOQs were in the range 0.2–0.5 μg kg−1. The results are summarized in Table 3.
To evaluate the accuracy of the method, recovery was studied by spiking 0.5 g blank banana samples with appropriate volumes of the composite working standard solution to furnish concentrations in the final extract of 0.2 (0.5), 100.0, and 200.0 μg kg−1 (low, medium, and high concentration in the linear range). Recovery and precision of the method were investigated by analysis of three replicate samples as described above. The results, listed in Table 4, show that the mean recovery of the nine pesticides at three levels from blank samples are satisfactory, ranging from 71.2 to 102.8%, which indicates the method meets requirements stipulated for methods used for such analyses (recovery from 70 to 110%). The precision of the method was evaluated by study of repeatability and reproducibility, expressed as relative standard deviations (RSD). Repeatability was assessed by extraction and analysis of 0.5 g blank samples spiked at each recovery level on the same day; reproducibility was investigated by testing these same samples on the next day. Replicate (n = 6 for each concentration) samples were analyzed and RSD was calculated for each pesticide (Table 4). The method was shown to be precise, with RSD values ranging from 1.8 to 13.2% for all the pesticides studied at all spiking levels, which again meets requirements stipulated for such methods (RSD ≤ 20%).
Application to Real Samples
To show the applicability of the method to real samples it was used for analysis of residues of the nine pesticides in five fruits (apple, grape, banana, strawberry, and tomato) and three vegetables (cabbage, spinach, and rape) purchased from a local food market in April 2009. The results are listed in Table 5. Six of the pesticides were detected in different amounts in four fruit and three vegetables, and the other three pesticides were not detected. According to Commission Directive 2000/24/EC established by the European Union to stipulate maximum levels of organophosphorus pesticide residues in fruit and vegetables, concentrations of malathion in apple and grape exceeded the specified values.
Conclusion
An MSPD–RRLC–MS–MS method has been developed for simultaneous analysis of trace levels of nine OPP residues in fruit and vegetables, and has been applied successfully. The results show that use of MWCNT as MSPD adsorbent results in efficient pre-concentration of the nine analytes from fruit and vegetables. Results from analysis of eight real fruit and vegetable samples revealed the method is highly suitable for routine monitoring of low levels of OPP multi-resides in fruit and vegetables, with the advantages of ease of operation, high precision and selectivity, and low limit of detection. It can be used to detect low levels of OPP multi-residues in other foods.
References
Tomlin CDS (1997) The pesticide manual, 11th edn. British Crop Protection Council, Surrey
Chen J, Duan CF, Guan YF (2010) J Chromatogr B 878:1216–1225. doi:10.1016/j.jchromb.2010.02.031
Buonasera K, D’Orazio G, Fanali S, Dugo P, Mondello L (2009) J Chromatogr A 1216:3970–3976. doi:10.1016/j.chroma.2009.03.005
Li Q, Nagahara N, Takahashi H, Takeda K, Okumura K, Minami M (2002) Toxicology 172:181–190. doi:10.1016/S0300-483X(02)00004-5
Bosgra S, Voet HVD, Boon PE, Slob W (2009) Regul Toxicol Pharm 54:124–133. doi:10.1016/j.yrtph.2009.03.004
Rahimi R, Abdollahi M (2007) Pestic Biochem Phys 88:115–121. doi:10.1016/j.pestbp.2006.10.003
http://ec.europa.eu/food/plant/protection/resources/mrl_crop.pdf
Kim DH, Heo GS, Lee DW (1998) J Chromatogr A 824:63–70. doi:10.1016/S0021-9673(98)00629-3
Simplício AL, Boas LV (1999) J Chromatogr A 833:35–42. doi:10.1016/S0021-9673(98)00941-8
Patel K, Fussell RJ, Macarthur R, Goodall DM, Keely BJ (2004) J Chromatogr A 1046:225–234. doi:10.1016/j.chroma.2004.06.063
Zambonin CG, Quinto M, Vietro ND, Palmisano F (2004) Food Chem 86:269–274. doi:10.1016/j.foodchem.2003.09.025
Sanz CP, Halko R, Ferrera ZS, Rodríguez JJS (2004) Anal Chim Acta 524:265–270. doi:10.1016/j.aca.2004.06.024
Mol HGJ, Dam RCJV, Steijger OM (2003) J Chromatogr A 1015:119–127. doi:10.1016/S0021-9673(03)01209-3
Liu M, Hashi Y, Song YY, Lin JM (2005) J Chromatogr A 1097:183–187. doi:10.1016/j.chroma.2005.10.022
Liu M, Hashi Y, Song YY, Lin JM (2006) Chinese J Anal Chem 34:941–945. doi:10.1016/S1872-2040(06)60045-5
Yoshida T, Majors RE (2006) J Sep Sci 29:2421–2432. doi:10.1002/jssc.200600267
Klejdus B, Vacek J, Benešová L, Kopecký J, Lapčík O, Kubáň V (2007) Anal Bioanal Chem 389:2277–2285. doi:10.1007/s00216-007-1606-3
Díaz L, Llorca-Pórcel J, Valor I (2008) Anal Chim Acta 624:90–96. doi:10.1016/j.aca.2008.06.053
Pirard C, Widart J, Nguyen BK, Deleuze C, Heudt L, Haubruge E, De Pauw E, Focant JF (2007) J Chromatogr A 1152:116–123. doi:10.1016/j.chroma.2007.03.035
Zuin VG, Yariwake JH, Bicchi C (2003) J Chromatogr A 985:159–166. doi:10.1016/S0021-9673(02)01400-0
Blasco C, Font G, Picó Y (2005) J Chromatogr A 1098:37–43. doi:10.1016/j.chroma.2005.08.037
Liu PY, Liu QX, Ma YS, Liu JW, Jia X (2006) Chinese J Chromatogr 24:228–234. doi:10.1016/S1872-2059(06)60011-4
Economou A, Botitsi H, Antoniou S, Tsipi D (2009) J Chromatogr A 1216:5856–5867. doi:10.1016/j.chroma.2009.06.031
Albero B, Sánchez-Brunete C, Tadeo JL (2005) Talanta 66:917–924. doi:10.1016/j.talanta.2004.12.046
Sagratini G, Mañes J, Giardiná D, Damiani P, Picó Y (2007) J Chromatogr A 1147:135–143. doi:10.1016/j.chroma.2007.02.066
Ravelo-Pérez LM, Hernández-Borges J, Borges-Miquel TM, Rodríguez-Delgado MÁ (2008) Food Chem 111:764–770. doi:10.1016/j.foodchem.2008.04.020
Cortés-Aguado S, Sánchez-Morito N, Arrebola FJ, Frenich AG, Vidal JLM (2008) Food Chem 107:1314–1325. doi:10.1016/j.foodchem.2007.09.033
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) J AOAC Int 86:412–431
de Llasera MPG, Reyes-Reyes ML (2009) Food Chem 114:1510–1516. doi:10.1016/j.foodchem.2008.11.006
Chu XG, Hu XZ, Yao HY (2005) J Chromatogr A 1063:201–210. doi:10.1016/j.chroma.2004.12.003
Barker SA, Long AR, Short CR (1989) J Chromatogr 475:353–31
Barker SA (2007) J Biochem Biophys Methods 70:151–162. doi:10.1016/j.jbbm.2006.06.005
Gabriela K, Eva B, Miroslava L (2003) Czech J Food Sci 21:219–234
Barker SA (2000) J Chromatogr A 885:115–127
Alfred B, Jan J (2002) Wiad Chem 56:1035–1052
Ahmed FE (2001) Trends Anal Chem 20:649–661
Zhong WK, Hao J, Fan YB, Wang MJ (2000) Chinese J Anal Chem 28:904–910
Barker SA (2000) J Chromatogr A 880:63–68
Fernandez-Alvarez M, Llompart M, Lamas JP, Lores M, Garcia-Jares C, Cela R, Dagnac T (2009) J Chromatogr A 1216:2832–2842. doi:10.1016/j.chroma.2008.10.026
Radišić M, Grujić S, Vasiljević T, Laušević M (2009) Food Chem 113:712–719. doi:10.1016/j.foodchem.2008.07.103
Hu YY, Zheng P, He YZ, Sheng GP (2005) J Chromatogr A 1098:188–193. doi:10.1016/j.chroma.2005.09.093
Du D, Wang MH, Zhang JM, Cai J, Tu HY, Zhang AD (2008) Electrochem Commun 10:85–89. doi:10.1016/j.elecom.2007.11.005
El-Sheikh AH, Sweileh JA, Al-Degs YS, Insisi AA, Al-Rabady N (2008) Talanta 74:1675–1680. doi:10.1016/j.talanta.2007.09.005
El-Sheikh AH, Insisi AA, Sweileh JA (2007) J Chromatogr A 1164:25–32. doi:10.1016/j.chroma.2007.07.009
Wang S, Zhao P, Min G, Fang GZ (2007) J Chromatogr A 1165:166–171. doi:10.1016/j.chroma.2007.07.061
Yu ZG, Ding WM, Chen YH, You J, Liu YY, Wang H, Yang ZD (2010) Chromatographia 72:33–38. doi:10.1365/s10337-010-1632-8
Pramanik BN, Ganguly AK, Gross ML (2005) Applied electrospray mass spectrometry. Chemical Industry Press, Beijing
Acknowledgments
We greatly appreciate financial support from Heilongjiang Province Natural Scientific Fund (E2007-35, 11531003), National Natural Scientific Fund (51078120), Doctor-degree Program New Teacher Fund of the Ministry of Education (20092303120004) and High Education Fund (11511082) of Heilongjiang Province of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, Sx., Yu, Zg., Yu, Hn. et al. Multi-Walled Carbon Nanotubes as Matrix Solid-Phase Dispersion Extraction Adsorbent for Simultaneous Analysis of Residues of Nine Organophosphorus Pesticides in Fruit and Vegetables by Rapid Resolution LC–MS–MS. Chromatographia 73, 33–41 (2011). https://doi.org/10.1007/s10337-010-1840-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-010-1840-2